Cerebral palsy in children: a clinical overview
Introduction
Cerebral palsy (CP) is primarily a neuromotor disorder that affects the development of movement, muscle tone and posture (1-3). The underlying pathophysiology is an injury to the developing brain in the prenatal through neonatal period (1-3). Although the initial neuropathologic lesion is non-progressive, children with CP may develop a range of secondary conditions over time that will variably affect their functional abilities (4,5).
Based on an international consensus, a generally agreed upon definition of CP is as follows:
CP describes a group of permanent disorders of movement and posture, causing activity limitation, that are attributed to nonprogressive disturbances that occurred in the developing fetal or immature brain. The motor disorders of CP are often accompanied by disturbances of sensation, perception, cognition, communication, and behavior, by epilepsy, and by secondary musculoskeletal problems (1).
CP is characterized by heterogeneity in risk factors, underlying specific etiology, clinical features, severity of functional limitations, associated and secondary conditions, treatment options, and evolution of the condition over the lifespan of the individual (6-8). Shevell [2019], has explored the argument for a consideration to view CP as a spectrum disorder rather than a discrete unitary clinical condition (9).
The prevalence of CP for all live births ranges from 1.5 to 3 per 1,000 live births, with variation between high income and low to middle income countries and geographic region (2-5,7,10-12). Because, in many infants and children, abnormal neuromotor findings tend to resolve within the first few years, especially during the first 2–5 years, of life, the reported prevalence of CP tends to be higher during infancy. Although prematurity and low birthweight are main risk factors for CP, multiple other factors are also associated with or potentially increase the risk for CP (Table 1) (2-5,7,11,13-15). Multiple epidemiological studies report that half of the children who develop CP were born at term without any identified risk factor (3,7,8,11,12,15). Although in most cases, CP is a result of an injury to the fetal or neonatal brain, post-neonatal onset CP has been recognized. Postneonatal CP results from an injury to the brain after neonatal period and before 5 years of age (5,11,12,15). The most common causes of postneonatal CP are traumatic brain injury, near-drowning, and meningitis (11).

Full table
Diagnosis
Novak et al. [2017], based on their systematic review of literature published between 1988 and 2016, contend that it is possible to accurately diagnose CP in early infancy (8). They emphasized early diagnosis to optimize long term functional outcomes on the basis of positively modulating their impact on neuroplasticity. Novak et al. [2017] reported that early and accurate diagnosis of CP is possible based on a combination of findings from medical history, neuroimaging, and standardized individually administered neurological and motor assessment tools (8). Standardized tools should be administered and interpreted by medical professionals with specific training and experience in their use. Based on their review, Novak et al. found that:
- For infants ≤5 months corrected age, the most predictive tools for detecting risk for CP are term-age magnetic resonance imaging (MRI) (86–89% sensitivity), the Prechtl Qualitative Assessment of General Movements (98% sensitivity), and the Hammersmith Infant Neurological Examination (90% sensitivity) (8).
- For infants ≥6 months corrected age, the most predictive tools for detecting CP risk are MRI (86–89% sensitivity), the Hammersmith Infant Neurological Examination (90% sensitivity), and the Developmental Assessment of Young Children (83% C index) (8).
Novak et al. [2017] proposed that when a diagnosis of CP cannot be made with certainty in young infants, an interim clinical diagnosis of ‘high risk of CP’ should be made, so that CP specific early interventions can be initiated (8). A diagnosis of high risk for CP requires motor dysfunction and either an abnormality on MRI scan and/ or a clinical history indicating risk for CP (8).
Although it is likely that based on a meticulous clinical history, findings on MRI scan and standardized neuromotor assessment, CP can be accurately diagnosed in early infancy by those specifically trained and experienced in using the tools, a specific diagnosis of CP in most primary care or pediatric practice settings is difficult to make with certainty during first 1–2 years of life (1-3,5,6,11,16). Approximately half of the infants who develop CP have identifiable high-risk factors, which allows for early screening and early diagnosis (8). Those infants without any known risk factor at birth first come to medical attention when parents notice delayed or atypical neuromotor developmental progression, when CP is suspected (8). A diagnosis of CP is primarily based on clinical findings and is generally more reliable after 2 years of age, because early signs and symptoms suggestive of CP may in fact be a normal variation or developmental lag and tend to resolve in many infants (1-6,11,16,17). In some children, clinical findings suggestive of CP may continue to evolve up to 4–5 years of age (1-6,11,16,17).
Persistence of primitive reflexes or primary motor pattern beyond the expected age is a key clinical characteristic of CP (11-14,16). Persistence of primitive reflexes prevents or delays typical progression of motor development and sequential acquisition of higher level neuromotor skills. A diagnosis of CP is first suspected when there is a failure to attain certain key milestones at expected age (Table 2) (4).
The neurologic impairment of motor system in children who have CP is characterized, in order of frequency, by spasticity, dyskinesia, hypotonia, and ataxia (4,6,12,13). Mixed presentations are not uncommon. Hypotonia, with or without associated spasticity—generally truncal hypotonia and spasticity of extremities, are also seen. Based on clinical findings, CP is generally classified as spastic, dyskinetic, and hypotonic or mixed (3,12-14,16).
Thirty-five percent of children with CP have spastic diplegia, which is the most common clinical phenotype of CP (3,4,11,12,15). Spastic diplegia is due to damage to the immature oligodendroglia between 20 and 34 weeks of gestation (2,3,15). The most common neuropathologic finding seen on neuroimaging is periventricular leukomalacia (2,3,15). In spastic diplegia, both the motor corticospinal and the thalamocortical pathways are affected (2,3). Most children with spastic diplegia have normal cognitive function and good prognosis for independent ambulation. Spastic quadriplegia comprises 20% of children with CP and this clinical phenotype is associated with premature birth and neuroimaging shows severe periventricular leukomalacia and multicystic cortical encephalomalacia (3,4,11,12,15). Spastic quadriplegia is associated with significant functional limitations, cognitive deficit, epilepsy, visual impairment and other associated conditions (2,3,12-14). Children with spastic quadriplegia have poor prognosis for independent ambulation. Twenty-five percent of children with CP have spastic hemiplegia (3,4,11,12,15). Spastic hemiplegia is most commonly seen in infants born at term and most cases are due to in utero or perinatal stroke (15). Most children with spastic hemiplegia have normal cognitive abilities, are able to maintain independent ambulation and a high level of functional abilities (2,3,10,14,16). Extrapyramidal CP comprises choreoathetotic, dystonic or dyskinetic clinical phenotypes and comprises 15% cases of CP (3,4,11,12,15). Most cases are seen in infants born at term and associated with hypoxic-ischemic encephalopathy, kernicterus, neurometabolic or neurogenetic disorder (2,3,15). Children with extrapyramidal CP have a higher incidence of associated conditions—cognitive deficit, seizures, behavioral problems, sleep disturbances, visual impairment or hearing impairment (3,4,10,12,13,16).
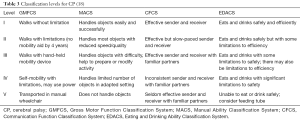
Four functional classification systems (Table 3) are used in persons with CP to allow for a standardized way to assess support and therapy needs of the individual (18-23). The Gross Motor Function Classification System (GMFCS) is used to describe gross motor function, especially the ability to walk, for children from 2 to 18 years of age (19,20). GMFCS is used to describe self-initiated movements as well as movements assisted by devices such as walkers, crutches, canes or wheelchairs (19,20). The Manual Ability Classification System (MACS) is used to describe the typical use of both hands and upper extremities for children from 4 to 18 years of age (21). The Communication Function Classification System (CFCS) is used to describe the ability of persons with CP for daily routine communication (sending or receiving a message) (22). The CFCS considers all methods of communication including vocalizations, manual signs, eye gaze, pictures, communication boards or speech generating devices (22). The Eating and Drinking Ability Classification System (EDACS) is used to describe the eating and drinking function for children 3 years and older (23). The EDACS assesses the eating and drinking safety (risk for aspiration or chocking), and eating and drinking efficiency (the amount food lost and the time taken to eat) (23).
Although, in most cases of CP, no treatable underlying etiology is identified, an etiological evaluation should be considered to identify certain treatable conditions associated with disturbances of neuromotor development. MRI scan is recommended when CP is suspected and in 90% of cases of CP, MRI scan of brain shows abnormal findings, which include a range of brain malformations, in utero stroke, or white matter loss (3,7,8,11,14,15,17). Abnormal neuromotor development, loss of motor skills, unexplained hypoglycemia, recurrent vomiting or seizures should prompt consideration of an inborn error of metabolism (14,15,17). A family history of unexplained neurologic symptoms or infant deaths may also suggest possibility of neurometabolic disorder (11,14,15,17). Laboratory investigations for possible inborn errors of metabolism and neurogenetic disorders should be considered in consultation with specialists in neurometabolic and neurogenetic disorders.
Prevention and treatment interventions
Prevention of premature and low birthweight births is the most significant consideration in reducing the overall incidence of CP. Antenatal administration of magnesium sulfate, when premature birth is imminent before 32 weeks of gestation, has been shown to confer neuroprotection and reduce the risk of CP development in neonates (11,24). Therapeutic hypothermia has been shown to reduce the risk of CP in term and late preterm infants with hypoxic-ischemic encephalopathy (11,25,26). Therapeutic hypothermia is initiated within 6 hours after birth with the aim of lowering the body and/or head temperature by 2 °C for 48 hours (11,25,26).
Novak et al. [2017], based on their systematic review have emphasized the importance of early diagnosis so that CP specific interventions can be initiated early to optimize their impact on the developing brain’s neuroplasticity (8). Examples of CP specific early interventions found to be effective in improving neuromotor function include the use of constraint-induced movement therapy in hemiplegic CP, and early, intense, enriched, task-specific training-based interventions at home (8).
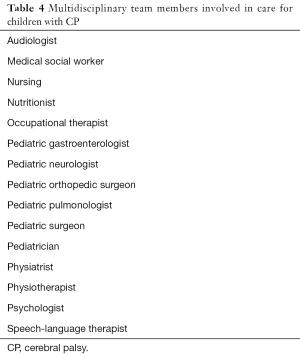
A multidisciplinary team (Table 4) approach provides the best model for medical care of children and adults with CP across their lifespan to manage various associated and secondary conditions as well as address support system and psychosocial needs (6,10,12-14). It is not within the scope of this review to describe management of all conditions in detail; this section provides an overview of selected interventions used in the management of CP.
Full table
General medical conditions
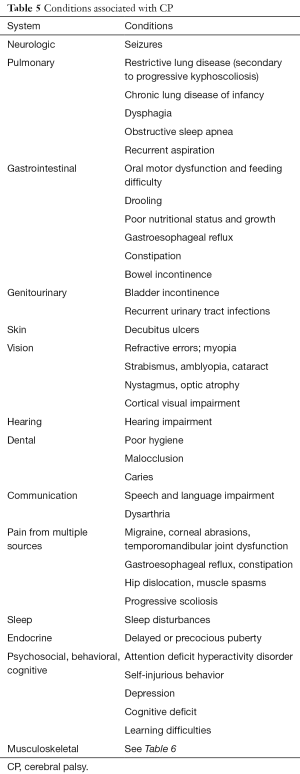
Children with CP also have many associated conditions (Table 5) that require general medical care and are best managed in the primary care setting based on standard practice guidelines (6,10,12-14,27).
Full table
Full table
Treatment of spasticity
The degree or severity of spasticity in CP varies depending upon the stage of arousal of the child at the time and the duration since the inciting event that lead to spasticity (11,16,28-32). Muscle spasticity in a child with CP may interfere with certain functions as well as may serve to facilitate certain functions. Therefore, reduction of spasticity should be considered within the context of its functional impact and multiple factors (Table 7) need careful consideration (11,16,28-32).

Full table
Different treatment interventions (Table 8) have been used to treat spasticity in children with CP (11,16,28-32). The decision to use any particular treatment intervention is guided by the goal of the treatment. In some cases, the goal may be to reduce focal spasticity, whereas in others it may be to reduce generalized spasticity. Also, the risks and benefits of any particular intervention should be carefully considered. Physiotherapy and occupational therapy by age 4–5 years of age have been shown to be relatively more effective than if started at a later age (33,34). Botulinum toxin injection is used to treat focal spasticity with optimal effectiveness between 1 and 6 years of age for the treatment of lower extremity spasticity and between 5 and 15 years of age for spastic hemiplegia (11,16,28-32). Spasticity management is best guided by a physician with expertise and experience with the use of different treatment interventions.
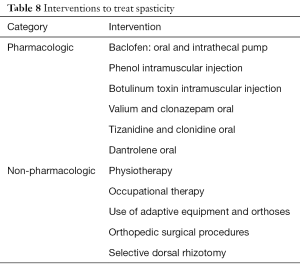
Full table
Orthopedic surgical procedures
Children with CP develop multiple secondary, often progressive, musculoskeletal conditions (Table 9) that may require orthopedic surgical interventions that are best managed by orthopedic surgeons with experience and expertise in these surgical procedures (6,10,13,16,33,34). The type and severity of these conditions vary depending upon the type and severity of CP. A number of factors are considered in planning any of these surgical interventions that include the age of the child, severity and progressive or nonprogressive nature of the condition, support system for post-operative and long term follow-up and care, potential for functional improvement, and potential for amelioration or prevention of complications. A common practice consideration is to perform most procedures as a single event multiple level surgery (SEMLS) to avoid multiple exposure to anesthesia risk and other operative risks (16,33). Also, this approach allows for a planned course of post-operative rehabilitation.
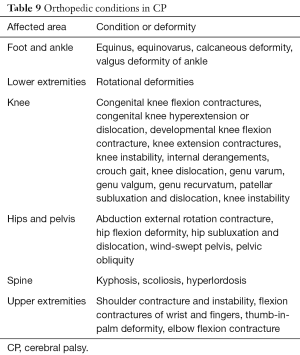
Full table
Physiotherapy
Physiotherapy has been shown to improve muscle strength, local muscular endurance and joint range of movement in children with CP (35,36). Physiotherapy exercises are used to prevent or reduce joint contractures; this is achieved by passive gentle range of motion exercises and stretches across major joints. Increased muscle strength is achieved by performing regularly scheduled progressively increasing resistive exercises involving all major muscle groups. Low resistance, high repetition exercises of major muscle groups improve local muscular endurance. Specific physiotherapy exercises are designed to improve balance, postural control, gait, and assist with mobility and transfers (for example from bed to wheelchair).
Functional strength training combined with plyometric exercises and balance training have been used to improve function in individuals with CP (31,35-38). Plyometric exercise improves muscle power, which includes strength and speed (35,36). In regards to functional strength training, studies have shown that targeting specific muscles is most effective in muscle activation (35,36,38). A study has shown that 12 weeks of an adaptive bungee trampoline program improved lower limb muscle strength (39). This bungee trampoline program included bouncing, hopping, heel jumps, jumping with eyes closed, practicing a sequence of jumps, and games such as dodgeball.
The use of constraint-induced therapy centers on the idea of selective upper extremity strengthening in children with CP (8,12-14,40,41). The intervention focuses on having the child use the affected limb, while simultaneously restraining the use of normally functioning limb. Prolonged restraint and disuse of the normally functioning upper limb may result in disuse weakness.
The application of conductive education based on the concept that children with or without motor deficits learn the same way (14,30,31,35,42). The conductive education specialist attempts to unify developmental areas including emotional, cognitive, motor and communicative domains in order to improve integration and global functionality in participants (14,30,31,35,42). The effectiveness of conductive education (CE) in improving functional capabilities of children with CP has not been clearly established (14,30,31,35,42).
Treadmill training
Treadmill training as an intervention for children with CP aims at improving balance as well as lower extremity symmetry (10,30,31,35,43-46). It provides important measures in developing an understanding of how to walk independently. Specific methods of treadmill training vary, with protocols demonstrating differences in training speeds (varied based on age at intervention), use of or lack of body weight support techniques (support under the arms or with utilization of a harness on the patient while on the treadmill), and frequency or duration of the training (10,30,31,35,43-46). Studies have demonstrated that 3–4 sessions per week over a period of 3–4 months of treadmill training in children under 6 years of age with ambulatory capability have led to improvement in gait velocity and enhancement of stepping movements, as well as independence with walking (10,30,31,35,43-46).
Treadmill training is time and labor intensive. Robotic gait training has been shown to reduce the time and labor burden associated with traditional treadmill training (47). Robotic assisted device can harness the child appropriately and can be programmed to simulate normal gait. Studies have shown increased walking speed and endurance with the use of robotic gait training. Randomized controlled trials have shown effectiveness of robot assisted gait training in improving gait velocity, spatiotemporal station, and endurance in children with CP (47).
Occupational therapy
Occupational therapy is an integral component in the interdisciplinary treatment of individuals with CP, with various studies demonstrating its long-term effects on promoting improvement in fine motor functionality (35). A major focus of occupational therapy is to improve fine motor function of upper extremities to assist the child in performing activities of daily living more efficiently. Occupational therapist also works in organization of child’s play areas, providing adaptive equipment for self-care and learning and to modify child’s learning environment to facilitate attention and information processing (35).
Orthotics, adaptive equipment, and assistive technology
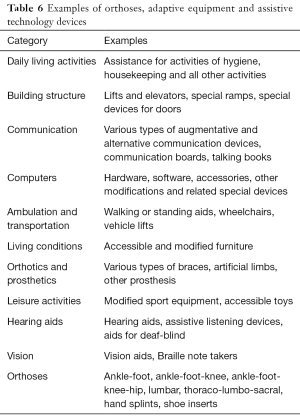
In the long-term management of children with CP, it is important to determine how much assistance is required on a daily basis for optimal functioning. Orthoses, adaptive equipment and assistive technology devices (Table 6) are used to improve child’s functional abilities and facilitate activities of daily living (13,14,32-34,48-50).
Assistive technology plays an important role in the management of persons who have CP and other developmental disorders. According to the United States Individuals with Disabilities Education Act (IDEA), the term “assistive technology device” means any item, piece of equipment, or product system, whether acquired commercially off the shelf, modified, or customized, that is used to increase, maintain, or improve functional capabilities of a child with a disability (48). The term does not include a medical device that is surgically implanted, or the replacement of such device. The term “assistive technology service” means any service that directly assists a child with a disability in the selection, acquisition, or use of an assistive technology device. A significant amount of variability exists within adaptive technology for children with CP, as these devices are ideally tailored to the individual’s existing muscle constraints.
Other interventions
Many other specific interventions or intervention approaches have been used in the treatment of CP; however, the evidence for effectiveness and recommendation for routine use of such interventions is equivocal and limited. Some of these interventions or approaches include acupuncture, neurodevelopmental training, sensory integration, electrical stimulation, suit therapy, hippotherapy, music therapy, video game therapy, and stem cell therapy (51-66).
Outcomes
With early intervention and appropriate medical care and ongoing support services, most children with CP grow up to be adults; overall survival of all children with CP until the age of 20 years is 90% (2,3,5-7,14,67-69). It is important to recognize that many age-related changes and diseases occur earlier in persons with CP. Of adults who have CP, most are over the age of 45; deaths attributed to CP per se are rare; 95% of children with diplegia and 75% of children with quadriplegia survive until the age of 30 years; and 95%of children who have CP with mild cognitive deficit and 65% of children with severe cognitive deficit survive until the age of 38 years (2,3,6,10,67-69). With appropriate support and intervention, 2 our of 3 individuals with CP are ambulatory with or without assistance, 3 out of 4 have ability to speak, and 1 out of 2 have normal cognitive abilities (2,3,6,10,67-69).
Conclusions
CP is the most common cause of motor abnormalities seen in infants and children. The reported incidence of CP has remained steady over past several decades. Although prematurity and low birthweight are important risk factors, about half of all children who develop CP were born at term, normal birthweight, with no identified risk factors. A specific underlying etiology can be identified only in a very small percentage of cases. The diagnosis of CP is based mainly on findings on history and physical examination. Most children with CP live to be adults. Because of multiple associated conditions and complexities of support needed, management of CP is best done by a multidisciplinary team.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 2007;109:8-14. [PubMed]
- Colver A, Fairhurst C, Pharoah PO. Cerebral palsy. Lancet 2014;383:1240-9. [Crossref] [PubMed]
- Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers 2016;2:15082. [Crossref] [PubMed]
- National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Data and statistics for cerebral palsy. Available online: https://www.cdc.gov/ncbddd/cp/data.html
- National Institute for Health and Care Excellence (UK). Cerebral palsy in under 25s: assessment and management. Available online: https://www.ncbi.nlm.nih.gov/books/NBK419326/pdf/Bookshelf_NBK419326.pdf
- Patel DR, Greydanus DE, Calles JL Jr, et al. Developmental disabilities across the lifespan. Dis Mon 2010;56:304-97. [Crossref] [PubMed]
- Stavsky M, Mor O, Mastrolia SA, et al. Cerebral palsy-trends in epidemiology and recent development in prenatal mechanisms of disease, treatment, and prevention. Front Pediatr 2017;5:21. [Crossref] [PubMed]
- Novak I, Morgan C, Adde L, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr 2017;171:897-907. [Crossref] [PubMed]
- Shevell M. Cerebral palsy to cerebral palsy spectrum disorder: time for a name change? Neurology 2018. [Epub ahead of print]. [PubMed]
- Himmelmann K. Children and youth with complex cerebral palsy: care and management. Edited by Laurie J. Glader, Richard D. Stevenson. London: Mac Keith Press, 2019, pp 384. ISBN: 978-1-909962-98-9. Acta Paediatr 2019. [Epub ahead of print].
- Michael-Asalu A, Taylor G, Campbell H, et al. Cerebral palsy: diagnosis, epidemiology, genetics, and clinical update. Adv Pediatr 2019;66:189-208. [Crossref] [PubMed]
- Johnston MV. Cerebral palsy. In: Kliegman RM, St Geme III JW, Blum NJ, et al. editors. Nelson textbook of pediatrics. 21st ed. Philadelphia: Elsevier, 2020:3168-72.
- Johnson TL, Chin EM, Hoon AH. Cerebral palsy. In: Batshaw ML, Roizen NJ, Pellegrino L. editors. Children with disabilities. 8th ed. Baltimore: Paul Brookes, 2019:423-56.
- Liptak GS, Murphy NA. Council on Children With Disabilities. Providing a primary care medical home for children and youth with cerebral palsy. Pediatrics 2011;128:e1321-9. [Crossref] [PubMed]
- Korzeniewski SJ, Slaughter J, Lenski M, et al. The complex aetiology of cerebral palsy. Nat Rev Neurol 2018;14:528-43. [Crossref] [PubMed]
- Oskoui M, Shevell MI, Swaiman KF. Cerebral palsy. In: Swaiman KF, Ashwal S, Ferriero DM, et al. editors. Pediatric neurology: principles and practice. 6th ed. Philadelphia: Elsevier, 2017:e1660-72.
- Ashwal S, Russman BS, Blasco PA, et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2004;62:851-63. [Crossref] [PubMed]
- Paulson A, Vargus-Adams J. Overview of four functional classification systems commonly used in cerebral palsy. Children (Basel) 2017. [Crossref] [PubMed]
- Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214-23. [Crossref] [PubMed]
- Rosenbaum PL, Palisano RJ, Bartlett DJ, et al. Development of the Gross Motor Function Classification System for cerebral palsy. Dev Med Child Neurol 2008;50:249-53. [Crossref] [PubMed]
- Eliasson AC, Krumlinde-Sundholm L, Rösblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol 2006;48:549-54. [Crossref] [PubMed]
- Hidecker MJ, Paneth N, Rosenbaum PL, et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev Med Child Neurol 2011;53:704-10. [Crossref] [PubMed]
- Sellers D, Mandy A, Pennington L, et al. Development and reliability of a system to classify the eating and drinking ability of people with cerebral palsy. Dev Med Child Neurol 2014;56:245-51. [Crossref] [PubMed]
- Shepherd E, Salam RA, Middleton P, et al. Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2017;8:CD012077. [PubMed]
- Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349-58. [Crossref] [PubMed]
- Shepherd E, Salam RA, Middleton P, et al. Neonatal interventions for preventing cerebral palsy: an overview of Cochrane Systematic Reviews. Cochrane Database Syst Rev 2018;6:CD012409. [PubMed]
- Dodge NN. Medical management of cerebral palsy. In: Patel DR, Greydanus DE, Omar HA, et al. editors. Neurodevelopmental disabilities: clinical care for children and young adults. Dordrecht: Springer Science, 2011:227-48.
- Patel DR, Soyode O. Pharmacologic interventions for reducing spasticity in cerebral palsy. Indian J Pediatr 2005;72:869-72. [Crossref] [PubMed]
- Nahm NJ, Graham HK, Gormley ME Jr, et al. Management of hypertonia in cerebral palsy. Curr Opin Pediatr 2018;30:57-64. [Crossref] [PubMed]
- Novak I, McIntyre S, Morgan C, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol 2013;55:885-910. [Crossref] [PubMed]
- Feferman H, Harro J, Patel DR, et al. Therapeutic interventions in cerebral palsy. Int J Child Adolesc Health 2011;4:333-9.
- Blumetti FC, Wu JCN, Barzi F, et al. Orthopaedic surgery in dystonic cerebral palsy. J Pediatr Orthop 2019;39:209-16. [Crossref] [PubMed]
- Horstmann HM, Beck EE. Orthopaedic management in cerebral palsy. 2nd ed. London: Mac Keith Press, 2007:1-46;120-211.
- Bovid KM, Patel DR. Orthopaedic considerations. In: Rubin IL, Merrick J, Greydanus DE, et al. editors. Healthcare for people with intellectual and developmental disabilities across the lifespan. Cham: Springer International Publishing, 2016:1107-22.
- Dod KJ, Imms C, Taylor NF. editors. Physiotherapy and occupational therapy for people with cerebral palsy. London: Mac Keith Press, 2010:73-281.
- Das SP, Ganesh GS. Evidence-based approach to physical therapy in cerebralpalsy. Indian J Orthop 2019;53:20-34. [Crossref] [PubMed]
- Dewar R, Love S, Johnston LM. Exercise interventions improve postural control in children with cerebral palsy: a systematic review. Dev Med Child Neurol 2015;57:504-20. [Crossref] [PubMed]
- Kaya Kara O, Livanelioglu A, Yardımcı BN, et al. The effects of functional progressive strength and power training in children with unilateral cerebral palsy. Pediatr Phys Ther 2019;31:286-95. [Crossref] [PubMed]
- Germain AM, Blackmore AM, Gibson N, et al. Effects of adaptive bungee trampolining for children with cerebral palsy: a single-subject study. Pediatr Phys Ther 2019;31:165-74. [Crossref] [PubMed]
- Jamali AR, Amini M. The effects of constraint-induced movement therapy on functions of cerebral palsy children. Iran J Child Neurol 2018;12:16-27. [PubMed]
- Chiu HC, Ada L. Constraint-induced movement therapy improves upper limb activity and participation in hemiplegic cerebral palsy: a systematic review. J Physiother 2016;62:130-7. [Crossref] [PubMed]
- Myrhaug HT, Odgaard-Jensen J, Jahnsen R. The long-term effects of conductive education courses in young children with cerebral palsy: a randomized controlled trial. Dev Neurorehabil 2019;22:111-9. [Crossref] [PubMed]
- Mall V. Treadmill therapy in cerebral palsy. Eur J Paediatr Neurol 2019;23:543. [Crossref] [PubMed]
- Valentín-Gudiol M, Mattern-Baxter K, Girabent-Farrés M, et al. Treadmill interventions in children under six years of age at risk of neuromotor delay. Cochrane Database Syst Rev 2017;7:CD009242. [PubMed]
- Chrysagis N, Skordilis EK, Stavrou N, et al. The effect of treadmill training on gross motor function and walking speed in ambulatory adolescents with cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil 2012;91:747-60. [Crossref] [PubMed]
- Valentin-Gudiol M, Mattern-Baxter K, Girabent-Farrés M, et al. Treadmill interventions with partial body weight support in children under six years of age at risk of neuromotor delay. Cochrane Database Syst Rev 2011.CD009242. [PubMed]
- Carvalho I, Pinto SM, Chagas DDV, et al. Robotic gait training for individuals with cerebral palsy: a systematic review and meta-analysis. Arch Phys Med Rehabil 2017;98:2332-44. [Crossref] [PubMed]
- Individuals with Disabilities Act. Statute and regulations. Available online: https://sites.ed.gov/idea/statuteregulations/
- Desch LW. The spectrum of assistive and augmentative technology for individuals with developmental disabilities. In: Accardo PJ. editor. Capute and Accardo’s neurodevelopmental disabilities in infancy and childhood. 3rd ed. Baltimore: Paul H. Brookes, 2008:691-720.
- Cusack SR. Assistive technology. In: Rubin IL, Crocker AC. editors. Medical care for children and adults with developmental disabilities. 2nd ed. Baltimore: Paul H. Brookes, 2008:548-56.
- Salazar AP, Pagnussat AS, Pereira GA, et al. Neuromuscular electrical stimulation to improve gross motor function in children with cerebral palsy: a meta-analysis. Braz J Phys Ther 2019;23:378-86. [Crossref] [PubMed]
- Sánchez-Kuhn A, Pérez-Fernández C, Cánovas R, et al. Transcranial direct current stimulation as a motor neurorehabilitation tool: an empirical review. Biomed Eng Online 2017;16:76. [Crossref] [PubMed]
- Bosques G, Martin R, McGee L, et al. Does therapeutic electrical stimulation improve function in children with disabilities? A comprehensive literature review. J Pediatr Rehabil Med 2016;9:83-99. [Crossref] [PubMed]
- Karadağ-Saygı E, Giray E. The clinical aspects and effectiveness of suit therapies for cerebral palsy: a systematic review. Turk J Phys Med Rehabil 2019;65:93-110. [Crossref] [PubMed]
- Şahin S, Köse B, Aran OT, et al. The effects of virtual reality on motor functions and daily life activities in unilateral spastic cerebral palsy: a single-blind randomized controlled trial. Games Health J 2020;9:45-52. [PubMed]
- Tekin F, Kavlak E, Cavlak U, et al. Effectiveness of neuro-developmental treatment (Bobath concept) on postural control and balance in cerebral palsied children. J Back Musculoskelet Rehabil 2018;31:397-403. [Crossref] [PubMed]
- Martín-Valero R, Vega-Ballón J, Perez-Cabezas V. Benefits of hippotherapy in children with cerebral palsy: a narrative review. Eur J Paediatr Neurol 2018;22:1150-60. [Crossref] [PubMed]
- Pavão SL, Rocha NACF. Sensory processing disorders in children with cerebral palsy. Infant Behav Dev 2017;46:1-6. [Crossref] [PubMed]
- Jing G. Summary of curative effect of scalp acupuncture exercise therapy on spastic cerebral palsy. Ann Phys Rehabil Med 2018;61:e322.
- Li LX, Zhang MM, Zhang Y, et al. Acupuncture for cerebral palsy: a meta-analysis of randomized controlled trials. Neural Regen Res 2018;13:1107-17. [Crossref] [PubMed]
- Kułak-Bejda A, Kułak P, Bejda G, et al. Stem cells therapy in cerebral palsy: a systematic review. Brain Dev 2016;38:699-705. [Crossref] [PubMed]
- Boruczkowski D, Pujal JM, Zdolińska-Malinowska I. Autologous cord blood in children with cerebral palsy: a review. Int J Mol Sci 2019. [Crossref] [PubMed]
- Novak I, Walker K, Hunt RW, et al. Concise review: stem cell interventions for people with cerebral palsy: systematic review with meta-analysis. Stem Cells Transl Med 2016;5:1014-25. [Crossref] [PubMed]
- Section On Complementary And Integrative Medicine, Council on Children with Disabilities, American Academy of Pediatrics, et al. Sensory integration therapies for children with developmental and behavioral disorders. Pediatrics 2012;129:1186-9. [Crossref] [PubMed]
- Alves-Pinto A, Turova V, Blumenstein T, et al. The case for musical instrument training in cerebral palsy for neurorehabilitation. Neural Plast 2016;2016:1072301. [Crossref] [PubMed]
- Tarakci D, Ozdincler AR, Tarakci E, et al. Wii-based balance therapy to improve balance function of children with cerebral palsy: a pilot study. J Phys Ther Sci 2013;25:1123-7. [Crossref] [PubMed]
- Tosi LL, Maher N, Moore DW, et al. Adults with cerebral palsy: a workshop to define the challenges of treating and preventing secondary musculoskeletal and neuromuscular complications in this rapidly growing population. Dev Med Child Neurol 2009;51 Suppl 4:2-11. [Crossref] [PubMed]
- Rapp CE Jr, Torres MM. The adult with cerebral palsy. Arch Fam Med 2000;9:466-72. [Crossref] [PubMed]
- Haak P, Lenski M, Hidecker MJ, et al. Cerebral palsy and aging. Dev Med Child Neurol 2009;51 Suppl 4:16-23. [Crossref] [PubMed]