The evolution of blood-spot newborn screening
Introduction
The objective of medical screening tests is to identify clinically significant disorders from a target population, using a reliable method. Certain inborn errors of metabolism and other metabolic disorders can be detected pre-symptomatically in apparently healthy babies using appropriate laboratory tests. Screening is only appropriate if there is: a demonstrable benefit from early diagnosis; a suitable test with high specificity and sensitivity; a system of confirmation of results, counselling, treatment and follow-up; and the balance of harms and benefits is positive. Over the last 50 years the concept of screening asymptomatic babies to identify severe disorders has been realised. The field of newborn screening has changed dramatically over that period of time, embracing advances in technology, which have been incorporated into various programmes today.
Phenylketonuria (PKU)
Robert Guthrie developed a simple test in 1961 that could be used for mass screening of infants for PKU (1). In 1962 he received a grant to perform a study of 400,000 infants and thus by 1963 population screening began. By 31st December 1963, 29 US states and Puerto Rico had contributed data for the study. Thus 2013 marks the 50th anniversary of the publication by Guthrie and Susi of the method used for population based screening for PKU using a bacterial inhibition assay (BIA) (2). This elegant method utilises elevated levels of phenylalanine in the blood of infants soaked into disks of filter paper, to overcome β-2-thienylalanine inhibition of growth of Bacillus subtilis, on an agar culture. As a consequence, patients with PKU can be easily recognised on gels with florid growth of these bacteria around the blood disk and approximate levels were determined by comparison with growth obtained from a known concentration calibration curve (Figure 1). Although the method was used on a small scale in a single hospital to start with, with increasing success over a relatively short period of time, it was quickly adopted by all states within USA and many other countries. The success of this technique was built upon the improvement noted by an effective therapy in children as described by Bickel H et al. (3) ten years prior to Guthrie’s (2) publication. Both individuals subsequently acknowledged that both a reliable and cheap test and an effective disease modifying treatment were essential to ensure success of a PKU screening programme. The data that conclusively demonstrated that this was indeed the case took many years to come to light (4,5). It became increasingly clear with time that the management of this rare inborn error of metabolism was not just about restricting phenylalanine in the diet but also about managing all aspects of diet, ensuring effective phenylalanine restriction (by regular monitoring), and providing a healthy nutritionally complete diet, to ensure normal growth. Family support was also required in order to achieve a good outcome. These observations with the first disorder that was systematically screened for, have been replicated in different ways in disorders that have been subsequently added to screening panels. It is very clear, therefore, that the infrastructure to reliably diagnose and manage a screening result needs to be in place before a population based programme is implemented.
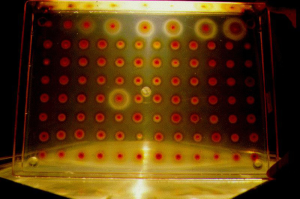
Following the success of PKU screening, the principle of an inhibition assay was used to develop tests for other disorders such as homocystinuria and maple syrup urine disease (6,7). However, despite the successes of the BIA methods, disorders such as PKU are now detected using other technologies [see tandem mass spectrometry (MSMS) screening below].
Congenital hypothyroidism (CH)
The merits for screening for CH had been realised by the early 1970s (8). Like classical PKU, this disorder has an insidious onset with permanent severe neuro-disability becoming apparent before the end of the first year of life. Initially radiological screening tests were proposed but by 1973 Dussault and Laberge determined that blood filter paper samples could be used to measure the low level of thyroxine (T4) in CH (9). Using measurement of T4 there was significant overlap between affected and unaffected and a significant percentage of infants required further testing including the measurement of a pituitary hormone, TSH, to ensure cases were identified. In 1974 and 1975, methodology using radio-immunoassay on blood filter paper samples for TSH and T4 respectively had been developed (10,11). Various programmes around the world subsequently developed these different methodologies, leading to different screening strategies around the world including T4 only, TSH only, or a combination of T4 and TSH. The physiological surge in TSH within the first 24 hours after birth led to a high rate of false positives and the need to use age specific ranges. Unlike BIA screening, these assays provided quantitative results for analytes rather than semi quantitative. This has consequently led to an ongoing debate about what cases can be identified by screening and were associated with the inclusion of quality control and proficiency testing programs. Over time, the analytical sensitivity of assays has been improved and reported missed cases from screening have also been identified. These issues have provided fuel for the debate of where to set a cut-off for either free T4 or TSH. It seems that most cases of thyroid gland aplasia would be identified by either assay but that detailed assessment of the assay and associated confirmatory testing protocols are required in order to ascertain other causes such as dyshormonogenesis and pituitary insufficiency.
The use of radioimmunoassay for other disorders was soon considered and immunoassay methods were developed for CAH and cystic fibrosis (CF) screening (12,13). The implementation of newborn screening for hypothyroidism rapidly identified several severe congenital cases with a vastly improved outcome. In this regard, the programmes undoubtedly were a success. As the number of babies tested and the complexity of testing increased as well as the need to optimise the quality of results, programmes required increasing automation. Instruments were developed to provide consistent punched samples and automated processing for immunoassays. The automation of processes facilitated high throughput screening to a wider population.
Cystic fibrosis (CF)
The implementation of screening for CF also required a paradigm shift in terms of the conceptual basis for screening. The improvement in health outcome was not based on a single therapy such as using thyroxine or with-holding phenylalanine. The management of CF required rigorous multi-disciplinary management incorporating improving respiratory function, prompt treatment of infections and optimising nutrition. Moreover, some patients could be relatively well over the first year of life with some severe complications only becoming apparent later in childhood. Because of this, there was some debate about the merits of a population based screening approach in terms of the effectiveness of early intervention versus the later intervention of clinically ascertained cases. This debate has led to the later implementation of CF screening programmes in countries such as the UK. The outcome in terms of survival has taken many years to ascertain, but it is now clear that cases presenting clinically with respiratory infections or steatorrhoea are very vulnerable long-term (14,15). The benefit in early diagnosis of CF prevents such presentations and delays the onset of co-morbidities and thus survival.
In the early 1960s screening newborns for CF was made possible through the measurement of meconium albumin levels (16). However, false negative results were obtained due to the samples being stored at room temperature or CF patients with normal pancreatic activity resulting in decreased albumin content (17,18). In addition, premature newborns with low pancreatic activity and those babies with intestinal atresia could produce a false positive result (17). In 1979 another conceptual shift in CF screening occurred with the development of an assay using a biomarker of disease [immunoreactive trypsin (IRT)] rather than a direct metabolite such as phenylalanine which could be quantitated from dried blood spot samples already being collected by newborn screening programmes (13).
It was apparent even from early publications that a single IRT test is poorly predictive of CF. IRT levels may be increased in cases of perinatal stress, congenital infection, renal failure or trisomy (13,18) and a falsely elevated level of IRT may be found in babies 2-5 days of age (19). It has also been demonstrated that IRT levels decline with age (13). Therefore age appropriate cut off values should be used to interpret results. To improve the specificity of CF screening protocols various approaches have been developed that use two samples collected at different ages. Furthermore, the availability of confirmatory testing, with a reliable diagnostic test, such as the sweat test was vital for the effectiveness of this test as a newborn screening tool. Since the 1990s a second tier approach of looking for cystic fibrosis transmembrane conductance regulator (CFTR) mutations has been used by several programmes. This has become more important as mutation specific therapies are developed. The use of IRT/DNA protocols for CF detection has enabled faster turnaround and minimal additional cost (20).
A more controversial problem is identification of a small number of babies who are healthy CF carriers but this seems more than balanced out by reduction of anxious families due to false-positive results obtained through employing the IRT alone protocol. In turn, this allows for informing parental reproductive decisions and information for other family members (21).
Tandem mass-spectrometry (MSMS) screening
Until the 1990s, one test was used to provide information about one disorder. However, electrospray MSMS with automated sample injection is used to provide high throughput screening to identify a wide range of metabolites (amino acids and acyl carnitines) that are observed in a range of inborn errors of metabolism (22-25). MSMS could provide information on disorders with one significant analyte for example measurement of phenylalanine in PKU. But the strength of this technique was that a metabolic profile could be created, representing the status of various amino acids and acylcarnitines at the time the blood-spot was collected (Figure 2). From this blood profile, as well as PKU various pathological disorders could be reliably identified such as medium chain acyl CoA dehydrogenase deficiency, classical maple syrup urine disease, type II tyrosinaemia and mitochondrial trifunctional protein deficiencies (incorporating LCHAD). However, again the implementation of this as a screening tool was another paradigm shift as the assay was a metabolic profile that changed depending on environmental influences (including age at collection of the sample, feeding regimen, parental nutrition, temperature and time of storage). As such, the sensitivity and specificity of the screen varied depending on the analyte and condition being considered. Some analytes such as citrulline or octanoylcarnitine are elevated in different conditions and hence the utility of the test is most enhanced when the profile is considered in its entirety. As the world wide experience of this test has grown, it has become apparent that each centre needs to have rigorous algorithms that define various elements to testing:
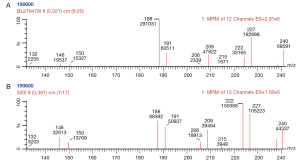
MSMS based screening protocols appear to have changed the landscape of newborn screening in the dynamic complexity of interpretation of many simultaneous results (31). However, most developed economies would consider such programmes as a necessary cost-effective tool to identify a range of severe disorders.
Other disorders
The conditions so far described are included on all Australian bloodspot newborn screening programmes. Evaluation of test suitability should include the following assessments: there is a demonstrated benefit or likely benefit from early diagnosis; there are suitable tests and treatment; follow-up services are available. The benefit may or may not be balanced against financial and other costs depending on the available technology, the frequency of the disorder in the region and other factors. Screening is possible for the following conditions depending on local circumstances.
Screening is currently not recommended in Australia for the following conditions where screening tests are not available, or, tests are available but proof of advantage from early diagnosis is absent or uncertain, or the test is unsuitable or does not detect those cases in which there might be an advantage (32). New knowledge about screening and screening outcome in these conditions should be monitored regularly.
Future directions
In 2006, The American College of Medical Genetics and Genomics published an evaluation scheme for assessing various disorders for consideration on the expanded newborn screening panel (33). This rigorous process systematically evaluated the indications for screening by asking a selection of specialists to assess per individual disease: the burden of untreated disease, the throughput and cost of testing strategy, the availability of confirmatory testing, the simplicity of diagnosis and treatment, the benefit of early intervention to the individual, family and society and the prevention of mortality. Using a standardised scoring system, the various disorders were ranked with MCADD, PKU and CH not surprisingly being ranked amongst the highest. There had been some discussion about screening for lysosomal storage disorders but in this paper, conditions such as Fabry disease, Krabbe disease and Pompe disease scored amongst the lowest from all the conditions being considered. It was acknowledged that the seminal publications indicating efficacy of therapy for infantile-onset Pompe disease were yet to occur and this condition in particular would score higher in future years (34,35). However, for Fabry and Krabbe diseases, the reliability of classical case definition and the evidence base for efficacy of early intervention remained elusive (36-38). By multiplexing some of the lysosomal storage disorders together, the cost of screening can be reduced, but the difficult question of how to manage results needs to be worked through for individual disorders (39,40). SCID is the latest condition to have reached the national screening threshold for USA after a systematic approach of evaluating treatment and testing strategies was undertaken (41-44).
As more and more technologies become available, it will open up opportunities to look at all types of conditions and risk factors. Whilst it is technically possible to screen for nutritional deficiencies such as vitamin B12 deficiency, or infections such as CMV or congenital disorders such as Duchene muscular dystrophy or fragile X syndrome, a strategy needs to be in place to manage the results in terms of counselling and intervention. The availability of read-through technology for certain disorders does provide a real indication for genetic targeted screening (45-47). Some are considering the panacea of whole genome screening, but this will provide risk factors for conditions yet to come and identify disorders that are yet to be described. Genomic screening may also identify risks for disease that would never manifest in the lifetime of the individual so it is imperative that any information acquired from whole genome or exome analysis is explained adequately to the end-user (48). Massively parallel sequencing (MPS) could be implemented with agreed disease targeted gene panels, lowering the risk of incidental findings and increasing depth of coverage. Countries with very large populations, spread over huge distances such as in India and China now embarking on screening programmes need to consider what conditions are prevalent, what treatments are available and how they may be delivered to the affected individual wherever they are. This may indeed be the biggest challenge.
Over the last 50 years, newborn screening using simple spots of blood has evolved using the most contemporary technology of the time, such that it now has the potential to identify a huge range of conditions. Each country needs to be aware of the potential and limitations of each technology and how they may apply these to treat their own population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Guthrie R. Blood Screening for Phenylketonuria. JAMA 1961;178:863.
- Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963;32:338-43. [PubMed]
- Bickel H, Gerrard J, Hickmans EM. Influence of phenylalanine intake on phenylketonuria. Lancet 1953;265:812-3. [PubMed]
- Smith I, Beasley MG, Ades AE. Intelligence and quality of dietary treatment in phenylketonuria. Arch Dis Child 1990;65:472-8. [PubMed]
- Saudubray JM, Rey F, Ogier H, et al. Intellectual and school performances in early-treated classical PKU patients. The French collaborative study. Eur J Pediatr 1987;146:A20-2. [PubMed]
- Efron ML, Young D, Moser HW, et al. A simple chromatographic screening test for the detection of disorders of amino acid metabolism. a technic using whole blood or urine collected on filter paper. N Engl J Med 1964;270:1378-83. [PubMed]
- Naylor EW, Guthrie R. Newborn screening for maple syrup urine disease (branched-chain ketoaciduria). Pediatrics 1978;61:262-6. [PubMed]
- Taranger J, Berglund G, Claesson I, et al. Screening for congenital hypothyroidism in the newborn. Lancet 1973;1:487. [PubMed]
- Dussault JH, Laberge C. Thyroxine (T4) determination by radioimmunological method in dried blood eluate: new diagnostic method of neonatal hypothyroidism? Union Med Can 1973;102:2062-4. [PubMed]
- Dussault JH, Coulombe P, Laberge C, et al. Preliminary report on a mass screening program for neonatal hypothyroidism. J Pediatr 1975;86:670-4. [PubMed]
- Klein AH, Agustin AV, Foley TP Jr. Successful laboratory screening for congenital hypothyroidism. Lancet 1974;2:77-9. [PubMed]
- Pang S, Hotchkiss J, Drash AL, et al. Microfilter paper method for 17 alpha-hydroxyprogesterone radioimmunoassay: its application for rapid screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab 1977;45:1003-8. [PubMed]
- Crossley JR, Elliott RB, Smith PA. Dried-blood spot screening for cystic fibrosis in the newborn. Lancet 1979;1:472-4. [PubMed]
- Dijk FN, McKay K, Barzi F, et al. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch Dis Child 2011;96:1118-23. [PubMed]
- Sims EJ, Mugford M, Clark A, et al. Economic implications of newborn screening for cystic fibrosis: a cost of illness retrospective cohort study. Lancet 2007;369:1187-95. [PubMed]
- Murray J, Cuckle H, Taylor G, et al. Screening for cystic fibrosis. Health Technol Assess 1999;3:i-iv, 1-104. [PubMed]
- Stephan U, Busch EW, Kollberg H, et al. Cystic fibrosis detection by means of a test-strip. Pediatrics 1975;55:35-8. [PubMed]
- Eggermont E. Enzymic activities in meconium from human foetuses and newborns. Biol Neonat 1966;10:266-80. [PubMed]
- Hammond KB, Abman SH, Sokol RJ, et al. Efficacy of statewide neonatal screening for cystic fibrosis by assay of trypsinogen concentrations. N Engl J Med 1991;325:769-74. [PubMed]
- Farrell PM, Aronson RA, Hoffman G, et al. Newborn screening for cystic fibrosis in Wisconsin: first application of population-based molecular genetics testing. Wis Med J 1994;93:415-21. [PubMed]
- Green NS, Dolan SM, Murray TH. Newborn screening: complexities in universal genetic testing. Am J Public Health 2006;96:1955-9. [PubMed]
- Millington DS, Kodo N, Norwood DL, et al. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis 1990;13:321-4. [PubMed]
- Chace DH, Millington DS, Terada N, et al. Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin Chem 1993;39:66-71. [PubMed]
- Rashed MS, Ozand PT, Bucknall MP, et al. Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr Res 1995;38:324-31. [PubMed]
- Wiley V, Carpenter K, Wilcken B. Newborn screening with tandem mass spectrometry: 12 months' experience in NSW Australia. Acta Paediatr Suppl 1999;88:48-51. [PubMed]
- Van Hove JL, Zhang W, Kahler SG, et al. Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: diagnosis by acylcarnitine analysis in blood. Am J Hum Genet 1993;52:958-66. [PubMed]
- Alodaib AN, Carpenter K, Wiley V, et al. Homocysteine measurement in dried blood spot for neonatal detection of homocystinurias. JIMD Rep 2012;5:1-6. [PubMed]
- McHugh D, Cameron CA, Abdenur JE, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genet Med 2011;13:230-54. [PubMed]
- Marquardt G, Currier R, McHugh DM, et al. Enhanced interpretation of newborn screening results without analyte cutoff values. Genet Med 2012;14:648-55. [PubMed]
- Wilcken B, Wiley V, Hammond J, et al. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med 2003;348:2304-12. [PubMed]
- Wilcken B. Medicine. Newborn screening: gaps in the evidence. Science 2013;342:197-8. [PubMed]
- Joint Royal Australasian College of Physicians and Human Genetics Society of Australasia Committee on newborn screening. Newborn bloodspot testing policy. Human Genetics of Australasia website: HGSA and RACP; 2011 [cited 2014 18/3/14]; Available online: http://www.hgsa.org.au/about/hgsa-committees/joint-hgsaracp-newborn-screening-committee
- Newborn screening: toward a uniform screening panel and system. Genet Med 2006;8 Suppl 1:1S-252S. [PubMed]
- Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 2007;68:99-109. [PubMed]
- Yang CF, Liu HC, Hsu TR, et al. A large-scale nationwide newborn screening program for Pompe disease in Taiwan: towards effective diagnosis and treatment. Am J Med Genet A 2014;164A:54-61. [PubMed]
- Ross LF. Newborn screening for lysosomal storage diseases: an ethical and policy analysis. J Inherit Metab Dis 2012;35:627-34. [PubMed]
- Lantos JD. Dangerous and expensive screening and treatment for rare childhood diseases: the case of Krabbe disease. Dev Disabil Res Rev 2011;17:15-8. [PubMed]
- Kemper AR, Knapp AA, Green NS, et al. Weighing the evidence for newborn screening for early-infantile Krabbe disease. Genet Med 2010;12:539-43. [PubMed]
- Sista RS, Wang T, Wu N, et al. Multiplex newborn screening for Pompe, Fabry, Hunter, Gaucher, and Hurler diseases using a digital microfluidic platform. Clin Chim Acta 2013;424:12-8. [PubMed]
- Fletcher J, Wilcken B. Neonatal screening for lysosomal storage disorders. Lancet 2012;379:294-5. [PubMed]
- Calonge N, Green NS, Rinaldo P, et al. Committee report: Method for evaluating conditions nominated for population-based screening of newborns and children. Genet Med 2010;12:153-9. [PubMed]
- Janik DK, Lindau-Shepard B, Comeau AM, et al. A multiplex immunoassay using the Guthrie specimen to detect T-cell deficiencies including severe combined immunodeficiency disease. Clin Chem 2010;56:1460-5. [PubMed]
- Lipstein EA, Vorono S, Browning MF, et al. Systematic evidence review of newborn screening and treatment of severe combined immunodeficiency. Pediatrics 2010;125:e1226-35. [PubMed]
- Gerstel-Thompson JL, Wilkey JF, Baptiste JC, et al. High-throughput multiplexed T-cell-receptor excision circle quantitative PCR assay with internal controls for detection of severe combined immunodeficiency in population-based newborn screening. Clin Chem 2010;56:1466-74. [PubMed]
- Davies J, Sheridan H, Bell N, et al. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med 2013;1:630-8. [PubMed]
- Barry PJ, Plant BJ, Nair A, et al. Effects of Ivacaftor in cystic fibrosis patients carrying the G551D mutation with severe lung disease. Chest 2014. [Epub ahead of print]. [PubMed]
- Ho G, Reichardt J, Christodoulou J. In vitro read-through of phenylalanine hydroxylase (PAH) nonsense mutations using aminoglycosides: a potential therapy for phenylketonuria. J Inherit Metab Dis 2013;36:955-9. [PubMed]
- Tucker EJ, Mimaki M, Compton AG, et al. Next-generation sequencing in molecular diagnosis: NUBPL mutations highlight the challenges of variant detection and interpretation. Hum Mutat 2012;33:411-8. [PubMed]


