
Neurodevelopmental outcome in hypoplastic left heart syndrome after hybrid procedure
Introduction
Improvements in prenatal diagnosis, birth, perinatal, and perioperative management in specialized pediatric heart centers have raised survival rates up to 86% even for the most complex forms of congenital heart disease (CHD), like the hypoplastic left heart syndrome (HLHS) (1). However, this is paralleled by only slight improvement of neurodevelopmental outcome (2). Neurological impairments and intellectual problems remain recognized challenges among long-term survivors with HLHS (3). Endeavor in the care of this growing high-risk population has shifted from reduction of mortality to prevention of long-term morbidity, improvement of health-related quality of life (HRQoL) and neurodevelopmental sequelae (4).
Antenatal brain maturational delay has been described to play an important role in this altered cerebral development (5). In fetuses with HLHS, abnormal brain growth, especially of the white matter structures, becomes detectable as early as the second trimester and reduced retrograde blood flow via the hypoplastic aortic arch has been hypothesized to be a culprit factor (6,7). It is well described that term infants with HLHS show disproportionately reduced head circumferences at birth (7-9) and a delay of approximately one month in structural brain development (10), constituting a risk factor for consequent brain injury and developmental delay (11,12). It has been postulated that prevalence and severity of developmental disabilities further increase, if open-heart surgery is required during the neonatal period, since hypoxic/ischemic conditions, coagulation imbalances, inflammatory processes, and anesthetic drugs related to surgeries may further delay brain maturation and increase the risk of secondary acquired structural brain injury (13).
After nearly two decades of experience with the Hybrid procedure (surgical bilateral pulmonary banding and catheter-guided stenting of patent arterial duct, as well as interventional atrial septum manipulation on demand) as an alternative option to the conventional strategy to treat neonates with HLHS (14,15), the aim was to examine midterm outcome, pathological cerebral MRI findings, brain volumes and HRQoL of HLHS hybrid patients. We hypothesized that children with HLHS undergoing the hybrid procedure and receiving their cardiac staged surgeries and intervention within the last 6 years, where neuroprotective strategies were in focus, will have better outcome and a higher HRQoL.
Methods
Study design
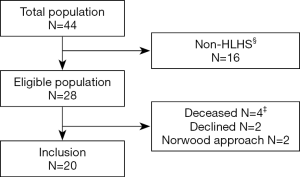
Twenty patients with HLHS and hybrid approach were prospectively enrolled at the University pediatric heart center of Giessen, Germany and examined prior Fontan surgery between October 2012 and July 2016. Patients were enrolled out of a cohort of 44 HLHS/HLHC (hypoplastic left heart complex) patients without known chromosomal abnormalities, born between April 2010 and May 2014 (Figure 1). Sixteen had HLHC morphology and were excluded for subsequent analysis. Of the 28 HLHS patients, 2 patients received a Norwood Stage I operation, in 2 patients, the parents decided for palliative care, and 2 parents rejected participation. The remaining 20 HLHS patients followed the hybrid approach and were all included into the study. The demographic data and clinical parameters were prospectively collected at admission and during the hospital stay for each operation or intervention. Perinatal data were retrospectively extracted from patients file. Growth data were transformed into z-scores based on WHO Child Growth Standards (Anthro Software, Version 3.2.2 from www.who.int/childgrowth/). Small-for-gestational age was defined as standardized weight (z-score weight; WTZ) and/or standardized height (z-score height, HTZ) <10th percentile. As measure of disproportionate head growth, the difference between standardized measures of HC (z-score HC; HCZ) and WTZ as well as HTZ was calculated, and relative head growth as HC change in cm by weight gain in kg (HC/weight change) and by height growth in cm (HC/height change) was analyzed. Socioeconomic status was evaluated with a demographic questionnaire (16). The control cases included children admitted to the pediatric neurology department and who received a cerebral MRI for headache [2], afebrile, generalized epileptic seizures [2], suspected epilepsy, not confirmed in follow-up [1], and recurrent vomiting [1]. All control patients had a cerebral MRI scan without pathological findings and a normal neurocognitive development. There were no significant differences of birth history between HLHS patients and the control patients. The study protocol concurred to the ethical guidelines of the 1975 declaration of Helsinki as reflected in a prior approval by the local ethics committee and written consent provided by parents or caregivers.
Surgical approach and standard care
The 20 patients were palliated with the Hybrid approach as stage I procedure, which includes surgical bilateral pulmonary banding and catheter-guided stenting of patent arterial duct, with interventional atrial septum manipulation on demand, without use of cardiopulmonary bypass (CPB). After stage I we aim to admit the neonate to a normal neonatal unit as soon as possible, where it has close contact to its parents in a less stressful ambience and where often (partial) breastfeeding is possible. After discharge, the patients are seen weekly at our outpatient clinic to estimate the right date for comprehensive stage II operation. All patients received comprehensive stage II, which combines the Norwood stage I and II in one step: removal of the bilateral pulmonary bands, removal of the PDA stent and PDA closure, aortic arch reconstruction, and anastomosis of the superior vena cava to the right pulmonary artery (Glenn procedure) (14). All CPB surgeries were performed under moderate hypothermia, and “beating-heart” condition, with selective head perfusion (n=20, 100%). During comprehensive stage II operation selective antegrade head perfusion has a flow rate of 35–50 mL/KG/BW), MAP (right arm) is kept ≥40 mmHg, cerebral SO2 is kept >65% and hemoglobine is kept >10 g/dL. After disconnection of the CPB hemoglobine is strived between 12–15 g/dL by hemofiltration, milrinone (0.5 µg/KG/min) is started, nitrite oxide given on 20 ppm, and noradrenaline is administrated on 0.1–0.3 µg/KG/min. Continuous perioperative brain monitoring with near infrared spectroscopy monitoring as well as modified ultrafiltration at the end of CPB was performed in all patients. We also target to extubate 2 to 6 hours after comprehensive stage II surgery on the pediatric cardiac ICU.
Neurodevelopmental assessments
All HLHS patients were assessed with the Bayley-III by a trained developmental pediatrician. The Bayley-III measures cognition (Cognitive Composite Score, CCS), language (Language Composite Score, LCS) and motor function (Motor Composite Score, MCS). Normative composite scores were calculated by age for each scale and compared to American test norms: mean 100±15 points, cut-off points for moderate and severe developmental delay of <85 points (1 SD below normative mean) and <70 points (2 SD below normative mean) (17). All study patients received a detailed examination by a pediatric neurologist or a developmental pediatrician. Depending on severity they were divided into different groups: normal, milder reflex or tone abnormality, distinct reflex and tone abnormality or cerebral palsy (18).
Brain MRI and brain volumetry
Brain MRI scans were performed on a 3.0 Tesla Magnetom Verio B17 scanner (Siemens Medical Systems, Erlangen, Germany) under sedation and were rated by a single pediatric neuroradiologist. The volumes of each tissue compartment were calculated with the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) from high resolution 3D T1-weighted images acquired with a magnetization prepared rapid acquisition gradient echo (MP-RAGE) scan (TR, 1,900 ms; TI, 900 ms; FOV, 35.0 mm × 26.3 mm; matrix 256×256; flip angle 9; sagittal plane; slice thickness 1.2 mm; 112 slices) (19). Total brain volume was defined as sum of gray, deep gray and white matter, and total intracranial volume included total brain volume and CSF.
QoL questionnaires
QoL by proxy report at the time of Bayley examination was assessed with the TNO-AZL Preschool Children Quality of Life (TAPQOL). The questionnaire contains 43 items divided over 12 multi-item scales that contain physical, social, cognitive and emotional functioning domains (20).
At 3 to 4 years of life, the parents’ perception of HRQoL was assessed with the German version of the Preschool Pediatric Cardiac Quality of Life (P-PCQLI) questionnaire (21). This 51-item questionnaire measures the frequency of disease related problems and severity of negative emotions about these on five scales (physical, emotional, social, therapeutic impact, functional development). The total score (range, 51 to 255) is calculated as the average score of the five scales.
Statistical analyses
Statistical analyses were carried out with IBM SPSS Statistics for Macintosh, version 24 (IBM Corp., Armonk, NY, USA) and R (Vienna, Austria; http://www.R-project.org/). With the respective measures of dispersion, descriptive statistics are presented as mean, SD or median, IQR for continuous variables, and as frequency and percentage for categorical variables. A Shapiro-Wilk test was used to test normality. ANOVA or Kruskal Wallis and post hoc students t-test or Mann-Whitney-U test were used to calculate differences between groups. Correlations of brain volume ratios with growth data and neurologic outcome were calculated with Pearson’s correlation for normally distributed variables and Spearman’s rho for non-normally distributed data. Linear regression was used to assess for impact of growth on outcome. A significance level of 0.05 was applied.
Results
Patient data and cardiac diagnoses
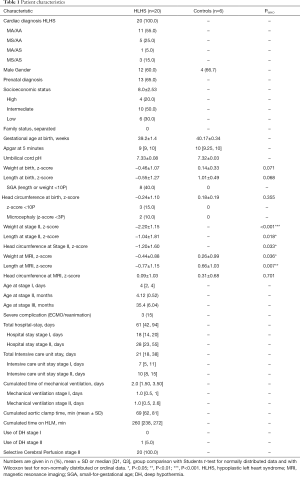
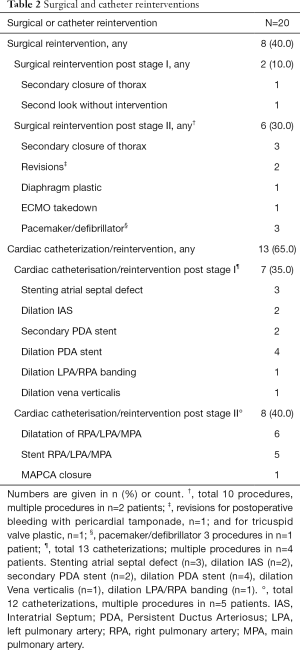
Characteristics of the 20 HLHS patients and control children are listed in Table 1. Cardiac subtypes of HLHS are shown in Table 1. All patients received the hybrid procedure at stage I without use of cardiopulmonary bypass (median age of 4 days), and the subsequent comprehensive stage II operation at 4.1±0.5 months. The average length of intensive care unit stay and discharge time at stage I and stage II was 8.4±5.3 days (range, 1.0 to 24.0), and 16.5±14.0 days (range, 7.0 to 49.0), respectively. The median discharge time after stage I and II was after 17.5 days (range, 8 to 32) and 27.5 days (range, 17 to 116), respectively. Two patients who were not discharged between the two surgeries had a total hospital stay of 145 and 228 days. Eight (40%) patients after Hybrid stage I and 13 (65%) after stage II had a complicated course with need for reintervention. Surgical reinterventions and cardiac catheterizations were needed in 2 and 7 patients after stage I, and in 6 and 8 patients after stage II, respectively (Table 2).
Full table
Full table
Growth
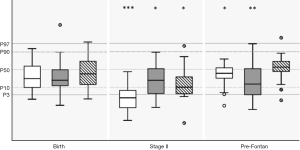
Growth parameters were in the lower normal range at birth with mean values for weight, length and head circumference at 32th percentile (P=0.07), 29th percentile (P=0.06) and 40th percentile, respectively (P=0.35) (Figure 2). One patient was microcephalic (head circumference below 3rd percentile) and two patients (10%) had head circumferences below the 10th percentile. The rate of small-for-gestational age newborns was 40%. After a significant drop in all growth parameters until stage II (average weight: 2nd percentile; average height: 15th percentile; average head circumference: 12th percentile), we found catch-up growth in head circumference (53th percentile, P=0.701) until follow-up at 2–3 years of age prior to Fontan surgery. However, both weight (32th percentile, P=0.036) and height (22nd percentile, P=0.007) remained below normative values before Fontan surgery, with a slight increase in stunting (HC to weight: P=0.24, HC to length: P=0.19) (Figure 2).
Neurodevelopmental outcome
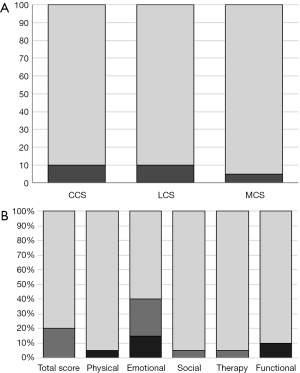
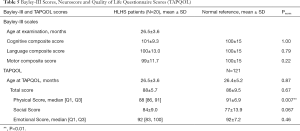
Mean age at follow-up was 26.5±3.6 months. The CCS, LCS, and MCS average scores were 101.5±9.3 (range, 80 to 120, P=0.48), 100.3±13.0 (range, 74 to 124), and 98±11.7 (range, 70 to 121); and thus were comparable to normative data (Table 3). Mild/moderate developmental delay (score <85) was noted in 2/20 patients (10%) in each the CCS and LCS, and in 1/20 of the patients (5%) in the MCS: no subject scored <70 in these scales (Figure 3). Stunting (relative head circumference to weight and height) was not associated with any of the Bayley-III scales (all P>0.05). Neurological assessment showed mild abnormalities such as muscular hypotonia or motor coordination deficits in 2/20 participants (10%). No major neurological handicap like cerebral palsy, deafness or blindness was noted.
Full table
MRI findings
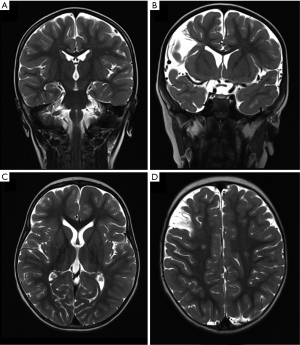
Out of 19 patients undergoing brain MRI scans, 4 (21%) had at least one abnormality, with two patients presenting with multiple findings (Table 3, Figure 4). In one patient cerebral MRI could not be performed due to a MR-incompatible pacemaker. Most common findings were late sequelae of old strokes present in 3 patients (16%), all smaller than one third of the cerebral artery supply territory. In one of these patients associated focal enlargement of supratentorial outer liquor spaces was visible. Patients with focal lesions did not show poorer outcome compared to patients with normal MRI scans (data not shown).
Compared to controls, white matter volume was lower (WM/ICV ratio P=0.014), and CSF volumes (CSF/ICV ratio P=0.042) were higher in HLHS patients (Table 4). HCZ at birth (r: 0.47; P=0.044), at stage II (r: 0.69; P=0.009) and at the time of the brain MRI scan (r: 0.61; P=0.005) correlated with total brain matter volume. Interestingly, HCZ change from birth to stage II was related to WM volume (r: 0.58, P=0.009) and explained 30% of its variability, whereas head growth between stage II and pre Fontan was not. Brain volumes were not associated with neurodevelopmental outcome (all P>0.05).
Full table
Quality of life questionnaires (TAPQOL/PCQLI)
The average TAPQOL total score in our cohort was 88±5.7, which was comparable to the median total score for normal controls (86±9.5, P=0.67), showing an overall well discernible QoL evaluated by parent as proxy report (Table 5). The median physical items were lower compared to healthy controls (P=0.007). However, HRQoL by proxy report assessed at 39.7±3.9 months with the PCQLI was comparable to the reference data of patients with biventricular CHD in all subscales (Figure 3B, Table 6).
Full table

Full table
Discussion
An increase in survival rate of children with HLHS over the past decade and recognition of neurodevelopmental disabilities in this growing population has set the focus on innovations for protecting the brain. This study investigated outcome of HLHS hybrid patients before Fontan surgery, receiving their palliative correction between 2010 and 2014, where potential neuroprotective interventions like moderate hypothermia, selective head perfusion, “beating-heart” operation and brain monitoring constitute the perioperative standard protocol at the Pediatric Heart Center Giessen, Germany. Measurements of the Bayley-III Scales and HRQoL were favorable for the majority of patients, with median outcome scales comparable to the norm. Notably, only 10% (2/20) of the patients showed mild developmental delay in at least one domain at 2 to 3 years of age.
These findings are encouraging compared to significantly lower motor and cognitive outcome scores for children receiving heart surgeries and interventions between 1996 and 2011 (2,22-24); although one has to keep in mind that some of these studies used the Bayley-II, which in comparison to the Bayley-III reports lower developmental scores (25,26).
It has been postulated that the Hybrid procedure might be beneficial in terms of protecting the premature brain (27). Nevertheless, the type of first stage procedure in respect to timing of the CBP surgery might be a discriminating factor. Brain immaturity has been identified as a risk factor for consequent brain injury and developmental delay (11). Therefore, one possible reason could be the impact of early use of heart-lung machine and the inevitable invasive intra- and postoperative management during an immature developmental stage when the brain is more vulnerable. A short postoperative care after hybrid approach, early extubation after placement of the pulmonary artery bandings and fast transfer to a normal ward, where the neonate can be close to its parents, and less feeding problems seem to be not so invasive and immobilizing for the neonate. In fact, adequate caloric intake during either pre- and post-surgical period, was suggested to improve outcome of CHD patients (28). In contrast, failure to thrive and poor head growth in childhood have been related to mid-term and long-term cognitive delay (e.g., attention deficit disorders, aggressive behavior and poor social and emotional development) (7,29-31). We found a decline in all growth parameters before stage II in our cohort, with a complete catch-up only for HCZ pre-Fontan, and thus increased stunting at follow-up compared to birth. Neither HC growth, nor stunting was associated with neurodevelopmental outcome. Lack of association between asymmetric head growth and neurodevelopmental outcome was previously described by Miller et al., and it has been suggested that the brain-sparing mechanism is not applicable to SV CHD. Multiple contributors to abnormal head growth in HLHS, such as cerebral ischemia and reperfusion injury, combined with impaired autoregulation of the brain vessles, infection/inflammatory processes and analgesic/sedative drugs increase the complexity of neonatal brain injury in HLHS (32,33). These insults may lead to oxidative stress and apoptotic cell death of immature brain cells, especially immature white matter and subplate neurons (34,35). Delay in brain maturation of approximately 1 month at term (10) and propensity for the critically ill neonate to exhibit a pressure-passive circulation related to a disturbance of cerebral auto-regulation (36) underscore the importance for early postnatal brain-protection. Postponement of the time of cardiopulmonary bypass surgery from the more susceptible neonatal time period towards 4 to 6 months of age may lead to higher brain maturity and may contribute to a reduced vulnerability of these structures towards perioperative cerebral damage.
In fact, head growth and in particular extent of head growth restriction before stage II was predictive of WM volume at 2–3 years of age, pointing to early vulnerability of the maturing brain. WM volumes were reduced and CSF volumes were increased in patients compared to norms, as previously described (6,37). Still, need for staged palliation puts these children at risk for repeated chronic hypoxic conditions, intracerebral hemorrhages, strokes and inflammatory episodes (38). Detection of old brain lesions indicating previous insults in 16% (3/19) of our patients, and association with poorer language development highlights the importance of neuroimaging for risk stratification in this high-risk population.
While the favorable outcome data from our participants seem to be emboldening, caretakers of these patients have to keep in mind that children with HLHS who performed well within the norm in early childhood, might show impairments in more complex functions such as language, memory function (39), and also in fine motor and visuomotor skills when they reach school age (40). In early childhood, motor development is more affected than cognition, with lower motor scores than cognitive scores (41). Hence, these patients need to be re-assessed longitudinally when they reach school age, which is part of an ongoing clinical study.
Assessment of HRQoL using the TAPQOL, a generic questionnaire and the PCQLI, a tool for disease related problems showed an overall good QoL of HLHS hybrid patients reported by parents. However, the median physical items of patients were lower compared to healthy controls (P=0.007). This is consistent with the finding of a study examining HRQoL in 1-year-old infants with CHD and open-heart surgery, that also found a significant reduction of parental-reported physical functioning. When these children were examined at four years, parents reported poorer communication but better social functioning and similar physical functioning compared to healthy controls (42). In contrast to our expectations, children showed an overall comparable HRQoL to a normative cohort of biventricular CHD. This good QoL can be explained by the disability paradox (43), as perceived QoL is a function of overall burden and parents may overlook difficulties, because parents are relieved that their children have survived the critical first months of life. It might also be due to the finding that behavior problems occur more frequently in older children, being confronted with more difficulties when interacting with older peers (44).
Our study is limited by the small size of our patient cohort. Therefore, some significant correlations might have occurred by chance. We used a cross-sectional study model, which impeded a longitudinal assessment of volumetric changes over time. Consequently, the effects of reduction of brain volumes on neurodevelopment, executive functioning and behavior later in life remain unclear. Therefore, a multicenter follow-up study with a longitudinal design is already running.
In conclusion, we found that HLHS patients following the Hybrid approach show favorable Bayley-III Scales and good QoL for the majority of patients, with median outcome scales that were not significantly different from the norm. Approximately, one quarter showed disease related pathological findings at cerebral MRI and, compared to controls, white matter volumes were reduced and CSF volumes were increased in HLHS patients. Extended neuroprotective strategies during perioperative management, improvement of nutrition and growth are important tools to further improve outcome. Routine neurodevelopmental follow-up assessments of performance at school age will elucidate the long-term clinical impact of these early imaging findings.
Acknowledgements
Special thanks go to the MRI staff, in particular to Ali Rad, Dr. Kerstin Gummel, and Dr. Jürgen Bauer (Giessen) for their data acquisition and help with the database.
Kinderherzen e.V., Bonn, Germany. The sponsor had no influence on study design, the collection, analysis, and interpretation of data, the writing of the paper, and the decision to submit the paper for publication.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics committee (Justus-Liebig University, Faculty of Medicine) (No. 42/12) and informed consent was taken from all the patients.
References
- Wernovsky G. The paradigm shift toward surgical intervention for neonates with hypoplastic left heart syndrome. Arch Pediatr Adolesc Med 2008;162:849-54. [Crossref] [PubMed]
- Gaynor JW, Stopp C, Wypij D, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 2015;135:816-25. [Crossref] [PubMed]
- Hansen JH, Rotermann I, Logoteta J, et al. Neurodevelopmental outcome in hypoplastic left heart syndrome: Impact of perioperative cerebral tissue oxygenation of the Norwood procedure. J Thorac Cardiovasc Surg 2016;151:1358-66. [Crossref] [PubMed]
- Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 2012;126:1143-72. [Crossref] [PubMed]
- Ruiz A, Cruz-Lemini M, Masoller N, et al. Longitudinal changes in fetal biometry and cerebroplacental hemodynamics in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2017;49:379-86. [Crossref] [PubMed]
- McQuillen PS, Goff DA, Licht DJ. Effects of congenital heart disease on brain development. Prog Pediatr Cardiol 2010;29:79-85. [Crossref] [PubMed]
- Miller TA, Zak V, Shrader P, et al. Growth Asymmetry, Head Circumference, and Neurodevelopmental Outcomes in Infants with Single Ventricles. J Pediatr 2016;168:220-5.e1. [Crossref] [PubMed]
- Shillingford AJ, Ittenbach RF, Marino BS, et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young 2007;17:189-95. [Crossref] [PubMed]
- Turan S, Rosenbloom JI, Hussein M, et al. Longitudinal analysis of head and somatic growth in fetuses with congenital heart defects. J Clin Ultrasound 2017;45:96-104. [Crossref] [PubMed]
- Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg 2009;137:529-36; discussion 536-7. [Crossref] [PubMed]
- Beca J, Gunn JK, Coleman L, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation 2013;127:971-9. [Crossref] [PubMed]
- Sun L, Macgowan CK, Sled JG, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015;131:1313-23. [Crossref] [PubMed]
- International Cardiac Collaborative on Neurodevelopment I. Impact of Operative and Postoperative Factors on Neurodevelopmental Outcomes After Cardiac Operations. Ann Thorac Surg 2016;102:843-9. [Crossref] [PubMed]
- Yerebakan C, Valeske K, Elmontaser H, et al. Hybrid therapy for hypoplastic left heart syndrome: Myth, alternative, or standard? J Thorac Cardiovasc Surg 2016;151:1112-21, 1123.e1-5.
- Schranz D, Bauer A, Reich B, et al. Fifteen-year single center experience with the "Giessen Hybrid" approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol 2015;36:365-73. [Crossref] [PubMed]
- Largo RH, Pfister D, Molinari L, et al. Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Dev Med Child Neurol 1989;31:440-56. [Crossref] [PubMed]
- Bayley N. Bayley Scales of Infant and Toddler Development Manual 3rd Edition. San Antonio, TX: The Psychological Corporation, 2006.
- Latal Hajnal B, Sahebkar-Moghaddam F, Barnwell AJ, et al. Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol 1999;21:788-93. [Crossref] [PubMed]
- Mayer KN, Latal B, Knirsch W, et al. Comparison of automated brain volumetry methods with stereology in children aged 2 to 3 years. Neuroradiology 2016;58:901-10. [Crossref] [PubMed]
- Fekkes M, Theunissen NC, Brugman E, et al. Development and psychometric evaluation of the TAPQOL: a health-related quality of life instrument for 1-5-year-old children. Qual Life Res 2000;9:961-72. [Crossref] [PubMed]
- Niemitz M, Seitz DC, Oebels M, et al. The development and validation of a health-related quality of life questionnaire for pre-school children with a chronic heart disease. Qual Life Res 2013;22:2877-88. [Crossref] [PubMed]
- Ravishankar C, Zak V, Williams IA, et al. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr 2013;162:250-6.e2. [Crossref] [PubMed]
- Newburger JW, Sleeper LA, Bellinger DC, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation 2012;125:2081-91. [Crossref] [PubMed]
- Mussatto KA, Hoffmann RG, Hoffman GM, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics 2014;133:e570-7. [Crossref] [PubMed]
- Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr 2012;161:222-8.e3. [Crossref] [PubMed]
- Jary S, Whitelaw A, Walloe L, et al. Comparison of Bayley-2 and Bayley-3 scores at 18 months in term infants following neonatal encephalopathy and therapeutic hypothermia. Dev Med Child Neurol 2013;55:1053-9. [Crossref] [PubMed]
- Sakamoto T. Current status of brain protection during surgery for congenital cardiac defect. Gen Thorac Cardiovasc Surg 2016;64:72-81. [Crossref] [PubMed]
- Mangili G, Garzoli E, Sadou Y. Feeding dysfunctions and failure to thrive in neonates with congenital heart diseases. Pediatr Med Chir 2018;40. [Crossref] [PubMed]
- Black MM, Dubowitz H, Krishnakumar A, et al. Early intervention and recovery among children with failure to thrive: follow-up at age 8. Pediatrics 2007;120:59-69. [Crossref] [PubMed]
- Dykman RA, Casey PH, Ackerman PT, et al. Behavioral and cognitive status in school-aged children with a history of failure to thrive during early childhood. Clin Pediatr (Phila) 2001;40:63-70. [Crossref] [PubMed]
- Rotermann I, Logoteta J, Falta J, et al. Neuro-developmental outcome in single-ventricle patients: is the Norwood procedure a risk factor? Eur J Cardiothorac Surg 2017;52:558-64. [Crossref] [PubMed]
- McPherson C, Haslam M, Pineda R, et al. Brain Injury and Development in Preterm Infants Exposed to Fentanyl. Ann Pharmacother 2015;49:1291-7. [Crossref] [PubMed]
- Walters JL, Paule MG. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol 2017;60:2-23. [Crossref] [PubMed]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110-24. [Crossref] [PubMed]
- Kichev A, Rousset CI, Baburamani AA, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling and cell death in the immature central nervous system after hypoxia-ischemia and inflammation. J Biol Chem 2014;289:9430-9. [Crossref] [PubMed]
- Menke J, Michel E, Hillebrand S, et al. Cross-spectral analysis of cerebral autoregulation dynamics in high risk preterm infants during the perinatal period. Pediatr Res 1997;42:690-9. [Crossref] [PubMed]
- Owen M, Shevell M, Donofrio M, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr 2014;164:1121-7.e1. [Crossref] [PubMed]
- Fogel MA, Li C, Elci OU, et al. Neurological Injury and Cerebral Blood Flow in Single Ventricles Throughout Staged Surgical Reconstruction. Circulation 2017;135:671-82. [Crossref] [PubMed]
- Miatton M, De Wolf D, Francois K, et al. Neurocognitive consequences of surgically corrected congenital heart defects: A review. Neuropsychol Rev 2006;16:65-85. [Crossref] [PubMed]
- Liamlahi R, von Rhein M, Buhrer S, et al. Motor dysfunction and behavioural problems frequently coexist with congenital heart disease in school-age children. Acta Paediatr 2014;103:752-8. [PubMed]
- Latal B. Neurodevelopmental Outcomes of the Child with Congenital Heart Disease. Clin Perinatol 2016;43:173-85. [Crossref] [PubMed]
- Werner H, Latal B, Valsangiacomo Buechel E, et al. Health-related quality of life after open-heart surgery. J Pediatr 2014;164:254-8.e1. [Crossref] [PubMed]
- Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med 1999;48:977-88. [Crossref] [PubMed]
- Karsdorp PA, Everaerd W, Kindt M, et al. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol 2007;32:527-41. [Crossref] [PubMed]


