
Growth failure in pediatric onset inflammatory bowel disease: mechanisms, epidemiology, and management
Introduction
Impairment of growth is recognized as one of the most serious complications of inflammatory bowel disease (IBD) in pediatric patients (1,2). Growth failure was reported to affect 15–40% of patients with pediatric onset Crohn’s disease (CD). In contrast, only 3–10% of children with ulcerative colitis (UC) may present with reduced height velocity at the time of diagnosis (3). Growth impairment has been defined in the Paris classification (Table 1) (4).
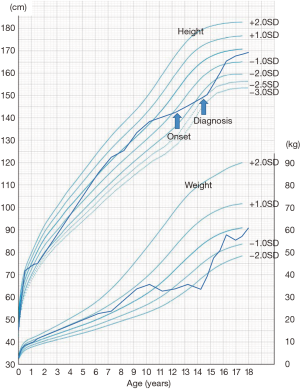
Several factors can contribute to growth failure in patients with IBD. It is well known that growth velocity is influenced by nutrition status, some treatment methods [e.g., corticosteroids (CS) administration], and by the disease itself. Some reports suggest that growth failure develops prior to the diagnosis of IBD, which means that malnutrition and hypercytokinemia due to the disease occur before other symptoms develop in pediatric patients. Figure 1 shows a typical case of pediatric onset CD with growth failure. Therefore, pediatric gastroenterologists should pay attention to patient’s growth not only during treatment, but also at the time of diagnosis. This review describes the epidemiology of growth failure in pediatric onset IBD and how IBD treatment affects the growth of these patients.
Mechanisms of growth failure in pediatric IBD
Growth failure is highly associated with malnutrition, which occurs secondary to decreased food intake due to abdominal pain and other symptoms, malabsorption due to intestinal mucosal damage, increased energy requirement due to inflammation, or drug-nutrient interactions. But it also occurs independently from malnutrition, by GH resistance due to inflammation (5).
Ballinger et al. (6) have shown increased release of serotonin (hydroxytryptamine, 5-HT) from the hypothalamus in rats with experimental colitis, and that this was associated with anorexia. A reduced hypothalamic activity and its association with poor appetite has also been detected in patients with lung cancer (7). Interleukin (IL)-1 was reported to affect the hypothalamic activity (7-9).
Gryboski et al. (10) reported that children with CD have delayed gastric emptying, whereas children with UC had normal emptying. In this study, 12 out of 15 patients with CD showed upper gastrointestinal symptoms, such as nausea and anorexia, and five of them had evidence of growth retardation. As for malabsorption, protein losing enteropathy was frequently reported in patients with active CD (11).
GH resistance was reported to play a critical role in the growth failure that affects patients with IBD (5,12-14). The GH-insulin like growth factor (IGF)-1 axis regulates linear growth, and circulating IGF-1 levels were decreased in patients with active CD (15). The cause of low IGF-1 concentrations in children with CD is thought to be multifactorial, as IGF-1 concentrations may depend on several interrelated factors, such as nutritional status, disease activity, and circulating plasma cytokine levels, including levels of tumor necrosis factor (TNF) and IL-1.
Genetic background may be also associated with growth failure in patients with IBD. Lee et al. (16) investigated 951 subjects with IBD, including 317 patients with CD, and reported that a polymorphism in the dymeclin gene DYM was significantly associated with growth impairment. D’Mello et al. (17) suggested that GM-CSF antibody and CARD15 risk allele carriage was associated with growth failure. Russell et al. (18) reported on a possible association of OCTN1/2 variant with growth failure. Since OCTN1 variant is known to be rare in Eastern Asian populations, the prevalence of growth failure in patients with IBD might differ by ethnicity (19).
As for clinical features affecting growth failure in IBD, prepubertal onset of CD was reported to be a risk factor for reduced final height (20). Retrospective data from the United Kingdom have shown that jejunal disease was negatively associated with lower final height (21). Ley et al. (22) suggested that growth velocity was negatively correlated with elevated C-reactive protein and orosomucoid levels in patients with pediatric onset CD. However, registry data from Belgium showed no association of growth outcome with age, sex, diagnostic delay, type of treatment, disease location, or disease activity at diagnosis, but only with disease activity 3 years after disease onset (23).
A guideline from the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ECCO/ESPGHAN) suggests that growth impairment is considered rare in patients with UC who are not dependent on CS (24). Therefore, exclusion of Crohn’s colitis or primary GH deficiency should be sought when significant growth impairment is detected (24).
Epidemiology of growth failure
Growth failure can be diagnosed at any time of the disease course. Growth failure often precedes the development of abdominal symptoms. Growth retardation is mostly evaluated at the time of diagnosis of IBD. There are some population studies that compared growth data at diagnosis between CD and UC.
Data of growth failure at time of onset of IBD
Population-based study from Japan (25)
In Japan, the Ministry of Health, Labour and Welfare has created a nationwide registry of intractable diseases. We have analyzed the growth of Japanese patients with IBD by using this population-based database (unpublished data). A total of 13,916 (9,953 male and 3,963 female) and 72,394 (41,134 male and 31,260 female) new patients with CD and UC, respectively, were registered between 2004 and 2011. Among them, 2,264 (16.2%) and 5,232 (7.2%) with CD and UC, respectively, developed the disease before the age of 20 years.
Height data were available for 2,090 (1,446 male) and 4,600 (2,770 male) patients with CD and UC, respectively. The average height z-scores of registered patients below the age of 20 were −0.31 [95% confidence interval (CI): −0.37 to −0.25] for male and −0.22 (95% CI: −0.32 to −0.13) for female patients with CD. The average height z-scores were −0.09 (95% CI: −0.13 to −0.06) for male and −0.08 (95% CI: −0.14 to −0.04) for female patients with UC. The average height z-scores were significantly lower than Japanese reference values in all groups.
Male patients with CD showed lower average height z-scores when compared to male patients with UC. Pearson’s correlation analysis showed a significant correlation between the age and height z-score both in male and female patients with CD, and in male and female patients with UC. Growth failure (height z-score <−2) was detected in 96 (6.6%) male and 35 (5.4%) female patients with CD, and in 87 (3.1%) male and 60 (3.4%) female patients with UC. These percentages of growth impairment in CD were significantly higher than those detected in healthy individuals (P<0.001 for both male and female patients), whereas no significant differences were detected neither for male, nor for female patients with UC. No significant difference was observed in percentage of patients with height z-score ≤−2 between male and female patients with CD.
Data from western countries
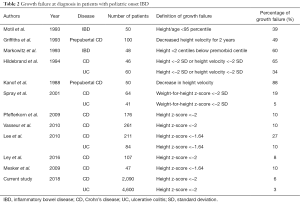
Previous reports on percentage of growth failure at diagnosis of pediatric IBD are listed in Table 2 (3,16,22,26-33). There is a trend that more recent studies detected a lower prevalence of growth failure. This might suggest that IBD is diagnosed earlier in recent years due to increased incidence of pediatric IBD worldwide.
Full table
Ghersin et al. (34) from Israel reported the outcomes of a population-based study including 2,372 patients with IBD, and showed that male and female patients with CD, but not with UC, had a significantly lower weight compared to controls. In contrast, their height at late adolescence was not statistically different from that of controls. However, patients with CD diagnosed before the age of 14 years were significantly shorter than controls in this study.
Final adult height of pediatric onset IBD
Population-based study from Japan (25)
In the Japanese registry, datasets of the year 2011 were reviewed and the data of patients with pediatric onset IBD (age of onset <20 years) and currently aged ≥20 years were selected for analysis. Final adult height data of 2,678 and 3,775 patients with CD and UC, respectively, were available for analysis. Patients with CD or UC showed lower average adult height when compared with healthy individuals. The average height z-scores were −0.33 (95% CI: −0.39 to −0.28) for male and −0.29 (95% CI: −0.37 to −0.21) for female patients with CD. The average height z-scores were −0.35 (95% CI: −0.41 to −0.30) for male and −0.27 (95% CI: −0.32 to −0.21) for female patients with UC. The average final adult heights in pediatric onset CD were 169.8 cm (95% CI: 169.5 to 170.1) for male and 157.2 cm (95% CI: 156.9 to 157.6) for female patients, while those in pediatric onset UC were 170.3 cm (95% CI: 170.0 to 170.6) for male and 157.7 cm (95% CI: 157.4 to 157.9) for female patients. Pearson’s correlation analysis showed a significant correlation between age and height standard deviation (SD) in male and female patients with CD and in male patients with UC, whereas female patients with UC did not show a significant correlation.
Growth failure (height z-score <−2) was detected in 120 (6.8%) male and 43 (4.7%) female patients with CD, and in 101 (5.2%) male and 85 (4.8%) female patients with UC. These percentages of growth impairment were significantly higher than those detected in healthy individuals (P<0.001 for both CD and UC, male and female patients). When comparing patients with CD and UC, male patients with CD developed growth failure more frequently than those with UC (P=0.044, chi squared test), but no significant difference was observed for female patients. Comparing male and female patients, male patients with CD developed growth failure more frequently than female patients, but no significant difference was detected between males and female patients with UC.
Data from western countries
Previous reports on adult final height in pediatric onset IBD are listed in Table 3 (3,16,21,22,27,28,32). Similar to the prevalence of growth failure at diagnosis, a trend toward lower prevalence of adult growth failure can be noted in recent studies. This fact may be associated with recent advances in treatment of IBD, which will be described below.

Full table
Effect of IBD treatment on growth of patients with pediatric onset IBD
A guideline for the management of growth, published in 2008, suggested to use CS-sparing agents and initially treat with enteral nutrition, possible in combination with azathioprine/6-mercaptopurine (6-MP) (2). Surgery can be also considered in steroid-dependent patients (2).
Newby et al. (35) reviewed the effect of IBD treatment on growth failure in childhood CD, and found three randomized controlled trials. Markowitz et al. (28) reported that there were no significant difference in linear growth between a group treated with 6-MP and a placebo group, although the total CS dose received over 18 months was significantly lower in the 6-MP group. In two randomized studies, Sanderson et al. (36) and Thomas et al. (37) reported improved growth status in patients with CD who received exclusive enteral nutrition (EEN) when compared with children treated with CS (35-38). Recently, Connors et al. (39) suggested that patients treated with EEN experienced significantly greater linear growth recovery than patients that received CS 1 year after diagnosis. Furthermore, Cohen-Dolev et al. (40) reported in a prospective study on newly diagnosed pediatric CD that patients treated with EEN had better remission rates and growth outcomes at 78 weeks when compared with patients treated with CS.
Anti-TNF agents seem to improve patients’ growth status. Borrelli et al. (41) treated 16 patients with infliximab and reported that their mean height z-score was significantly improved after 6 months of therapy. Bamberger et al. (42) retrospectively reviewed the data of 61 patients with CD who had received anti-TNF agents and showed improvement in their adult final height. A study from Canada suggested that a positive effect on height velocity was observed only in pre-pubertal or pubertal patients, but was absent in patients with Tanner stage IV–V (43). The IMAgINE 1 study group has suggested that adalimumab also improved and normalized the growth rate of patients with CD at 26 and 52 weeks of treatment compared to baseline (44).
Duncan et al. (45) retrospectively reviewed the data of 16 patients with CD who received percutaneous endoscopic gastrostomy placement for the administration of supplementary nutrition and showed that their height SD improved significantly from −1.85 at the time of treatment to −1.03 at 2 years after treatment. However, five of them also received GH therapy after gastrostomy placement.
Wong et al. (46) reported the findings of a randomized control trial on recombinant human GH treatment for patients with pediatric onset CD and compared growth velocity before and after 6 months of GH administration. The median growth velocity was significantly improved in the GH group, whereas no significant change was observed in the control group. They suggested that the long-term efficacy of GH should be evaluated and that the therapy has only limited benefit for reducing clinical disease activity. The ECCO/ESPGHAN guideline suggests that GH treatment should be only considered in a limited number of cases due to limited evidence of efficacy (47).
As for effect of treatment on the growth of patients with UC, the ECCO/ESPGHAN guideline suggests that children with UC may have more CS-related complications, and even low CS doses (0.1–0.4 mg/kg/day) can suppress their growth (24,48). Although cohort data are lacking, since growth failure is rarely detected at UC onset, avoiding CS and choosing immunomodulators, anti-TNF agents, or surgical resection in refractory cases seems to be beneficial in achieving optimal growth in patients with pediatric onset UC.
Conclusions
Growth impairment is still a major complication of pediatric onset IBD, especially in CD. Avoiding CS and achieving deep remission using enteral nutrition or anti-TNF agents seems to be important to maintain optimal growth in patients with pediatric IBD.
Acknowledgements
This registry data was supported by a grant from ‘‘Intractable diseases, the Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare.’’ I appreciate Dr. Thomas Walters at The Hospital for Sick Children, Toronto, Canada, for his precious advice.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol 2004;18:509-23. [Crossref] [PubMed]
- Heuschkel R, Salvestrini C, Beattie RM, et al. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis 2008;14:839-49. [Crossref] [PubMed]
- Hildebrand H, Karlberg J, Kristiansson B. Longitudinal growth in children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 1994;18:165-73. [Crossref] [PubMed]
- Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314-21. [Crossref] [PubMed]
- Shamir R, Phillip M, Levine A. Growth retardation in pediatric Crohn's disease: pathogenesis and interventions. Inflamm Bowel Dis 2007;13:620-8. [Crossref] [PubMed]
- Ballinger A, El-Haj T, Perrett D, et al. The role of medial hypothalamic serotonin in the suppression of feeding in a rat model of colitis. Gastroenterology 2000;118:544-53. [Crossref] [PubMed]
- Molfino A, Iannace A, Colaiacomo MC, et al. Cancer anorexia: hypothalamic activity and its association with inflammation and appetite-regulating peptides in lung cancer. J Cachexia Sarcopenia Muscle 2017;8:40-47. [Crossref] [PubMed]
- Shamir R. Nutritional aspects in inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2009;48 Suppl 2:S86-8. [Crossref] [PubMed]
- El-Haj T, Poole S, Farthing MJ, et al. Anorexia in a rat model of colitis: interaction of interleukin-1 and hypothalamic serotonin. Brain Res 2002;927:1-7. [Crossref] [PubMed]
- Gryboski JD, Burger J, McCallum R, et al. Gastric emptying in childhood inflammatory bowel disease: nutritional and pathologic correlates. Am J Gastroenterol 1992;87:1148-53. [PubMed]
- Seidman EG. Nutritional management of inflammatory bowel disease. Gastroenterol Clin North Am 1989;18:129-55. [PubMed]
- Sanderson IR. Growth problems in children with IBD. Nat Rev Gastroenterol Hepatol 2014;11:601-10. [Crossref] [PubMed]
- Soendergaard C, Young JA, Kopchick JJ. Growth Hormone Resistance-Special Focus on Inflammatory Bowel Disease. Int J Mol Sci 2017.18. [PubMed]
- Vortia E, Kay M, Wyllie R. The role of growth hormone and insulin-like growth factor-1 in Crohn's disease: implications for therapeutic use of human growth hormone in pediatric patients. Curr Opin Pediatr 2011;23:545-51. [Crossref] [PubMed]
- Akobeng AI, Clayton PE, Miller V, et al. Low serum concentrations of insulin-like growth factor-I in children with active Crohn disease: effect of enteral nutritional support and glutamine supplementation. Scand J Gastroenterol 2002;37:1422-7. [Crossref] [PubMed]
- Lee JJ, Essers JB, Kugathasan S, et al. Association of linear growth impairment in pediatric Crohn's disease and a known height locus: a pilot study. Ann Hum Genet 2010;74:489-97. [Crossref] [PubMed]
- D'Mello S, Trauernicht A, Ryan A, et al. Innate dysfunction promotes linear growth failure in pediatric Crohn's disease and growth hormone resistance in murine ileitis. Inflamm Bowel Dis 2012;18:236-45. [Crossref] [PubMed]
- Russell RK, Drummond HE, Nimmo ER, et al. Analysis of the influence of OCTN1/2 variants within the IBD5 locus on disease susceptibility and growth indices in early onset inflammatory bowel disease. Gut 2006;55:1114-23. [Crossref] [PubMed]
- Li M, Gao X, Guo CC, et al. OCTN and CARD15 gene polymorphism in Chinese patients with inflammatory bowel disease. World J Gastroenterol 2008;14:4923-7. [Crossref] [PubMed]
- Herzog D, Fournier N, Buehr P, et al. Early-onset Crohn's disease is a risk factor for smaller final height. Eur J Gastroenterol Hepatol 2014;26:1234-9. [Crossref] [PubMed]
- Sawczenko A, Ballinger AB, Savage MO, et al. Clinical features affecting final adult height in patients with pediatric-onset Crohn's disease. Pediatrics 2006;118:124-9. [Crossref] [PubMed]
- Ley D, Duhamel A, Behal H, et al. Growth Pattern in Paediatric Crohn's Disease is Related to Inflammatory Status. J Pediatr Gastroenterol Nutr 2016;63:637-43. [Crossref] [PubMed]
- De Greef E, Hoffman I, Smets F, et al. Paediatric Crohn Disease: Disease Activity and Growth in the BELCRO Cohort After 3 Years Follow-up. J Pediatr Gastroenterol Nutr 2016;63:253-8. [Crossref] [PubMed]
- Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:257-91. [Crossref] [PubMed]
- Ishige T, Tomomasa T, Hatori R, et al. Final Adult Height of Pediatric Onset Inflammatory Bowel Disease Patients in Japan: Analysis and Comparison of Nationwide Registry Between 2005 and 2011. J Pediatr Gastroenterol Nutr 2016.S373.
- Motil KJ, Grand RJ, Davis-Kraft L, et al. Growth failure in children with inflammatory bowel disease: a prospective study. Gastroenterology 1993;105:681-91. [Crossref] [PubMed]
- Griffiths AM, Nguyen P, Smith C, et al. Growth and clinical course of children with Crohn's disease. Gut 1993;34:939-43. [Crossref] [PubMed]
- Markowitz J, Grancher K, Rosa J, et al. Growth failure in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 1993;16:373-80. [Crossref] [PubMed]
- Kanof ME, Lake AM, Bayless TM. Decreased height velocity in children and adolescents before the diagnosis of Crohn's disease. Gastroenterology 1988;95:1523-7. [Crossref] [PubMed]
- Spray C, Debelle GD, Murphy MS. Current diagnosis, management and morbidity in paediatric inflammatory bowel disease. Acta Paediatr 2001;90:400-5. [Crossref] [PubMed]
- Pfefferkorn M, Burke G, Griffiths A, et al. Growth abnormalities persist in newly diagnosed children with crohn disease despite current treatment paradigms. J Pediatr Gastroenterol Nutr 2009;48:168-74. [Crossref] [PubMed]
- Vasseur F, Gower-Rousseau C, Vernier-Massouille G, et al. Nutritional status and growth in pediatric Crohn's disease: a population-based study. Am J Gastroenterol 2010;105:1893-900. [Crossref] [PubMed]
- Mesker T, van Rheenen PF, Norbruis OF, et al. Pediatric Crohn's disease activity at diagnosis, its influence on pediatrician's prescribing behavior, and clinical outcome 5 years later. Inflamm Bowel Dis 2009;15:1670-7. [Crossref] [PubMed]
- Ghersin I, Khateeb N, Katz LH, et al. Anthropometric Measures in Adolescents With Inflammatory Bowel Disease: A Population-Based Study. Inflamm Bowel Dis 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Newby EA, Sawczenko A, Thomas AG, et al. Interventions for growth failure in childhood Crohn's disease. Cochrane Database Syst Rev 2005.CD003873. [PubMed]
- Sanderson IR, Udeen S, Davies PS, et al. Remission induced by an elemental diet in small bowel Crohn's disease. Arch Dis Child 1987;62:123-7. [Crossref] [PubMed]
- Thomas AG, Taylor F, Miller V. Dietary intake and nutritional treatment in childhood Crohn's disease. J Pediatr Gastroenterol Nutr 1993;17:75-81. [Crossref] [PubMed]
- Dziechciarz P, Horvath A, Shamir R, et al. Meta-analysis: enteral nutrition in active Crohn's disease in children. Aliment Pharmacol Ther 2007;26:795-806. [Crossref] [PubMed]
- Connors J, Basseri S, Grant A, et al. Exclusive Enteral Nutrition Therapy in Paediatric Crohn's Disease Results in Long-term Avoidance of Corticosteroids: Results of a Propensity-score Matched Cohort Analysis. J Crohns Colitis 2017;11:1063-70. [Crossref] [PubMed]
- Cohen-Dolev N, Sladek M, Hussey S, et al. Differences in Outcomes Over Time With Exclusive Enteral Nutrition Compared With Steroids in Children With Mild to Moderate Crohn's Disease: Results From the GROWTH CD Study. J Crohns Colitis 2018;12:306-12. [Crossref] [PubMed]
- Borrelli O, Bascietto C, Viola F, et al. Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn's disease. Dig Liver Dis 2004;36:342-7. [Crossref] [PubMed]
- Bamberger S, Martinez Vinson C, Mohamed D, et al. Growth and Adult Height in Patients with Crohn's Disease Treated with Anti-Tumor Necrosis Factor alpha Antibodies. PLoS One 2016;11:e0163126. [Crossref] [PubMed]
- Walters TD, Gilman AR, Griffiths AM. Linear growth improves during infliximab therapy in children with chronically active severe Crohn's disease. Inflamm Bowel Dis 2007;13:424-30. [Crossref] [PubMed]
- Walters TD, Faubion WA, Griffiths AM, et al. Growth Improvement with Adalimumab Treatment in Children with Moderately to Severely Active Crohn's Disease. Inflamm Bowel Dis 2017;23:967-75. [Crossref] [PubMed]
- Duncan H, Painesi A, Buchanan E, et al. Percutaneous endoscopic gastrostomy placement in paediatric Crohn's disease patients contributes to both improved nutrition and growth. Acta Paediatr 2018;107:1094-9. [Crossref] [PubMed]
- Wong SC, Kumar P, Galloway PJ, et al. A preliminary trial of the effect of recombinant human growth hormone on short-term linear growth and glucose homeostasis in children with Crohn's disease. Clin Endocrinol (Oxf) 2011;74:599-607. [Crossref] [PubMed]
- Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014;8:1179-207. [Crossref] [PubMed]
- Sidoroff M, Kolho KL. Glucocorticoids in pediatric inflammatory bowel disease. Scand J Gastroenterol 2012;47:745-50. [Crossref] [PubMed]



