The concept and practice of Fanconi Anemia: from the clinical bedside to the laboratory bench
The concepts of Fanconi Anemia (FA) and the relationships with other syndromes
FA is an autosomal recessive disorder (with an exception of the complementation B, being X-linked) presenting with congenital anomalies, very high frequency of bone marrow failure, hematologic malignancies, commonly acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), and non-hematologic malignancies, commonly squamous cell carcinoma (1,2).
FA was first described and named as a disease in 1927 by the Swiss pediatrician-Guido Fanconi, 3 brothers with a specific combination of bone marrow failure and various physical abnormalities, short stature, hypo-gonadism and hyper-pigmentation. Subsequently, numbers of FA cases have been described without congenital malformations but with only progressive bone marrow failure (3). A laboratory finding of increased spontaneous chromosomal fragility of FA was reported in 1964 (4). FA was first recognised as a cause of Juvenile leukemia in 1967 (5).
So FA is classified into the chromosomal instability syndromes (Ataxia telangiectasia, Bloom syndrome, Nijmegen syndrome, and Werner syndrome) by its genetic pathological characteristics. Chromosomal instability syndromes are groups of disorders due to the defects in DNA repair, increased risk of cancer, and other phenotypic changes (6,7). FA is one of these syndromes most commonly referred disorders for chromosomal fragility testing.
FA is also classified into another group of syndromes, inherited bone marrow failure syndromes by its hematologic abnormalities. Bone marrow failure syndromes are defined as the failure of the hematopoietic function of the bone marrow and they are: Amegakaryocytic thrombocytopenia, Diamond-Blackfan anemia, Dyskeratosis congenita, Pearson syndrome, Severe congenital neutropenia, Shwachman-Diamond syndrome, thrombocytopenia absent Radii (8).
FA has several synonyms such as; Fanconi pancytopenia, inherited aplastic anemia, inherited pancytopenia, constitutional aplastic anemia, inherited bone marrow failure syndrome, pre-malignant disorder, chromosome break up syndrome, DNA-repair disorder. Among these synonyms, FA is the name well accepted and widely used. Care should be taken not to confuse it with Fanconi syndrome which is a kidney disease.
Incidence of FA
FA is found in all races and ethnic groups and the estimated incidence of FA is approximately 1 in 160,000-360,000 live births in the general population but it is much higher in some ethic groups due to the founder effects (9-11). The carrier frequency of FA in the general population worldwide is at 1:300 (12). But the carrier frequency is less than 1 in 100 in South African Afrikaners, sub-Saharan Blacks, and Spanish Gypsies and it is at 1:181 in the United State (13).
The ratio of male to female of FA patients is close 1to1. The median age of clinical onset of anaemia is 8 years old. The median diagnostic age is about 10 years (commonly from 2 to 15 years), life span is from 0-50 years (mean about 20 years old) and bone marrow failure is most common cause of death (14).
Clinical characteristics of FA
About 60-75% of patients present with the clinical characteristics including mental and physical abnormalities, bone marrow failure, increased risk of malignancies, endocrine dysfunctions including growth hormone deficiency, hypothyroidism, puberty delay, diabetes or osteopenia/osteoporosis (15-17). Patient with FA also can present with other abnormalities including multiple systems/organs: short stature; abnormal skin pigmentation; malformations of the thumbs, forearms, skeletal system, eyes, kidneys and urinary tract, ears, heart, gastrointestinal system, central nervous system; hypo-gonadism; and mental and physical developmental delay. About 35-40% of FA patients absent with any of clinical features so the delayed diagnosis or misdiagnosis can be the consequence in such cases (18).
Progressive anemia, pancytopenia or bone marrow failure is the most common clinical reasons to be seen by the clinicians. Data showed that the actuarial risk of bone marrow failure is 67% by the age of 30 and up to 90% by the age of 40 to 50 (19,20).
Infertility in patients with FA can be presented. About 50% of female patient with FA are infertile. It is often associated with pregnancy complications such as marrow failure, pre-eclampsia and premature labour. Genital malformations, hypoplastic gonads and low sperm counts are the common abnormalities with FA although fertility is rare in male (21).
Cancer susceptibility of FA to hematologic and non-hematologic malignancies
Patients with FA have very high susceptibility to both of hematologic and non-hematologic malignancies. In haematologic malignancies, the risk to develop to AML and MDS is increased to 785 fold and the median age of patients developing to AML is 14 years (20) and AML occurs at about 9% and MDS is about 7% of all patients with FA respectively (22).
Clonal chromosomal aberrations from MDS and AML from patients with FA present and frequently they are complex in structure cytogenetically (15,23). Among the most frequent clonal abnormalities are duplications and triplications of the long arm chromosome 1, gains of portions of the long arm chromosome 3, monosomy 7 or loss of material from long arm of chromosome 7, deletions of long arm of chromosome 5, long arm of chromosome 11, rearrangement of the short arm of chromosome 6, gains of chromosome 8 and 21. The clinical significant value of those somatic aberrations is not fully understood and only the chromosome 3q gain indicates a poor prognosis (24). Interestingly, the common chromosomal rearrangements observed in non-FA patients with AML such as t[15;17], t[8;21], inv[16] or t[16;16] were rarely observed from patients with FA rarely (25).
The recent study by using high density DNA array on 57 FA patients with MDS/AML confirmed the gain of the long arm of chromosomes 1 and 3, loss of the long arm of chromosome 7 and 11. In addition in the same study, some new cryptic translocation, deletion and mutations were found including NRAS, FLT3-ITD, MLL-PTD, ERG amplification and ZFP36L2-PRDM16 translocation but no TP53, TET2, CBL, NPM11 and CEBPα mutations, revealed the association of FA and a specific pattern genomic abnormalities in FA-related leukemia and MDS (26).
FA is a cancer-prone disorder. The relative risk of patients with FA developing to non-hematologic malignancies (cancers) is increased to 700 fold by the age of 50 years commonly in squmous cell carcinomas and many other different types of solid tumors, particularly of these cancers from head and neck, skin, GI tract, and genital tract. Studies also suggested that patients with FA receiving androgen treatment for bone marrow failure are at increased risk for liver tumors (27). FA associated malignancies are very difficult to be treated (except surgically) because patients with FA are sensitive to chemotherapy and radiation.
Recently, the close links between FA genes and genes found from patients with non-FA cancers have been studied intensively. Studies demonstrated there are four FA genes identified (FA-D1, FA-J, FA-N and FA-O) found in breast cancer either in bialleleic or monoalleleic manner (28-30).
Identification of the FA genes and the elucidation of FA pathways
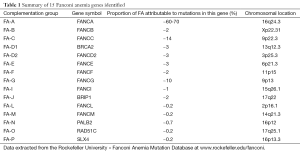
FA is caused by the mutations in FA genes coding for the production of proteins that contribute to protecting and repairing damaged DNA. FA is classified into 15 different complementation groups and these 15 different corresponding FA genes have been identified and named subsequently in an alphabetical order from A to P and they are scattered widely throughout the human genome on 11 different chromosomes as summarised in Table 1. FANCH was named before but it was found that it is the same as complementation group A (FANCA) later on (31,32). 14 of 15 FA genes are in the autosomal recessive manner but only 1 of 15 (FANCB) is in the sex chromosome recessive - X-linked (33).
The mutation of FANCA, FANCG and FANCC types consist of over 90% of the total mutated FA genes. It is still possible that other rare complementation groups remain to be identified.
Mutations of FANCA are responsible for about 60-70% of the total mutated FA genes, but it is overrepresented in some geographical regions, including Mediterranean countries such as Spain with 80% patients with belonging to this complementation group. Several types of mutations of FANCA are found including nonsense, missense, splicing mutations, micro-deletions, micro-insertions, and duplications (34).
Mutations in FANCC are responsible for about 15% of total mutated FA genes. The FANCC gene was first identified in 1992 (35) and has been most intensively studied in its expression, sub cellular localisation, protein interactions, and gain or loss of function. It is one of the large FA genes spanning more than 218 kb coding for 63 kDa protein. Studies showed that FANCC play role in the proliferation of germ cells/hematopoietic stem cells and cytokine signalling (36).
Mutations in FANCG are responsible for about 10% of the total 15 mutated FA genes. Mutations in FANCG may be associated with more severe cytopenia and a higher incidence of leukemia than other mutations; null mutations were in general more severe than mutations that produced an altered protein. The relative risk of bone marrow failure is higher in FANCG compared with FANCA and in FANCC (37).
The 15 FA gene protein products make up several FA pathways. Protein products from FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL and FANCM consist of FA nuclear core protein complex and this pathway can be activated by another FA pathway consisting of FANCD2 and FANCI in response to DNA damage during replication (23). Among these proteins, ubiquitin ligase, monoubiquininated proteins and helicase are the important proteins in the maintenance of genomic stability and DNA repair. Any mutation of the 15 genes can cause defects in the response to DNA damage and wrongly repaired and results in disease of FA.
Clinical course and severity of FA can vary between and even within families. It is unclear about the degree of correlation between genotype and phenotype of the disease due to presence of so many different factors affecting the gene and environment interactions.
Full table
The diagnostic guidelines for FA
A diagnosis for FA is usually made by the specialised institutes/hospitals by experienced and certified laboratories. The FA diagnostic age can start from prenatal, childhood and later life by confirming/establishing the diagnosis in a proband.
Physical abnormalities, progressive bone marrow failure, adult-onset aplastic anemia, MDS or AML and some particular types of solid tumors at an atypical young age are the main clinical clues and criteria for the suspected cases of FA.
A guideline of the indications for testing for FA is provided by the International FA Research Fund as follows (38):
Definite
- Sibling with FA;
- Aplastic anemia;
- Characteristic birth defects, particularly one or more of abnormal radii and/or thumbs; renal structural anomalies; microophthalmia; microcephaly; café au lait spots; features of VACTERL-H such as tracheo-esophageal fistula, imperforate anus, and others (see earlier listing);
- Spontaneous chromosome breaks;
- Primary MDS (at a young age);
- Primary AML (at a young age);
- Unusual sensitivity to chemo-or radiotherapy;
- Cancer typical of FA at an atypical age;
- Family history consistent with FA or with cancer (e.g., breast cancer).
Consider
- Single cytopenias;
- Macrocytosis unexplained by B12 or folate deficiency;
- Liver tumors without alcohol or hepatitis;
- Premature ovarian failure <30 years old;
- Diminished ovarian reserve <30 years old;
- Brain tumor <5 years old;
- Wilms tumor <4 years old;
- Increased Hb F not otherwise explained;
- Male (or female) infertility;
- Liver adenomas or hepatomas without alcohol or hepatitis.
Laboratory testing strategies for FA
Chromosomal fragility testing in T-lymphocytes
In the early time, it was suggested to use of spontaneous chromosomal breakage as a marker for FA (4) but it was found the testing result was inconsistent. The unique method called “chromosomal fragility testing” using clastogenic agents, mitomycin C (MMC) and diepoxybutane (DEB) were described by Cervenka et al. in 1981 (39) and Auerbach in 1993 (40) respectively. The principle of this method is to challenge the hyposensitive FA cells in the cell culture (most commonly T-lymphocytes from peripheral blood) exposed to DEB and MMC and then to analyse the chromosomal aberration, breaks, rearrangements (radials exchanges). A total 50 cells in metaphase are scored and analysed for chromosomal breakages compared to controls in the same conditions including age and sex. It is positive if the total chromosome breakage is great than 10 fold comparing to control.
This method can differentiate between FA and non-FA cell usually. It is described as the “Gold standard” with the features of simple, reliable, reproducible and sensitive comparing with other testing methods in FA diagnosis such as Immunoblot assay of FANCD2 protein monoubiquitination with a disadvantage which the rare type of FA can be missed (41) and cell cycle arrest assay using skin fibroblast exposed to MMC and then detected by flow cytometry (42). In the last two decades, the method of chromosome fragility testing has been the most widely using as the first line laboratory screen for FA although it is laborious and requires specialised personnel. It has been proposed that chromosomal fragility testing needs to be applied more widely in patients with congenital malformations even without anemia.
There are some limitations of this method: (I) it cannot detect the carriers and (II) it is often inconclusive in somatic mosaic cases of FA. In addition, false positive results from this test can be seen under the condition that tested individual referred for excluding of FA is under treatment with radiotherapy or chemotherapy in certain period of time.
Skin fibroblast testing for the suspected cases in somatic mosaicism
Study showed that about 15-20% patients with FA tested using lymphocytes show low response to DEB and MMC due to mosaicism (43,44). Mosaic is defined as the presence of genetically distinct two populations of lymphocytes in a given organism. It is relatively common in patients with FA: one showing increased sensitivity to DEB/MMC and the other showing normal levels of chromosomal breakage in response to DEB/MMC. In such suspected FA cases with somatic mosaicism, cultured skin fibroblasts are needed for further testing either with DEB and MMC or other methods when there is strong FA features clinically but chromosomal fragility testing is under detection level.
Determination of FA complementation groups
After the confirmation of FA from chromosomal fragility testing is obtained, the determination of which FA complementation group is the next step. Complementation group testing is used to classify patients with FA according to the specific gene defects that cause FA. The principle of the complementation group testing is to infect FA cells tested with retrovirus to contain a cDNA from a FA gene to identify the particular FA complementation group for the subsequent DNA sequencing of gene mutations (45). This testing requires living cells from patients with FA requested (either lymphocyte for non-mosaic or skin fibroblast for mosaic).
Mutation analysis
It is important to identify the specific gene mutations in each case, as the severity of the disease and the risk of developing aplastic anaemia or malignancies related to the complementation groups. Mutation analysis is to identify the specific gene mutations from the proband after confirmed by the primary complementation group result.
In the past, the conventional molecular genetic testing is complicated and time consuming due to the presence of at least 15 mutated genes involving in and also it requires many steps including DNA amplification, sequencing and detection of large deletions. Such testing usually needs to be done in laboratories with specific expertise.
International FA Research Fund provides the instructions and guidelines for mutation analysis (38).
Targeted mutation detection clues
Targeted mutation analysis is used clues obtained for the common mutations detection. These clues include Ashkenazi Jewish FANCC IVS4+4 A>T or FANCD1/BRCA2 6174delT; non-Ashkenazi Jewish Moroccan FANCA 2172-2173insG or FANCA 4275delT; Tunisian FANCA 890-893del; Indian FANCA 2574C>G (S858R); Israeli Arabs FANCA del ex 6-31, FANCA IVS 42-2A>C, and FANCG IVS4+3A>G; Japanese FANCC IVS4+4 A>T; Afrikaner FANCA del ex 12-31 and FANCA del ex 11-17; Brazil FANCA 3788-3790del; Spanish Gypsy FANCA 295C>T; and Sub-Saharan African Black FANCG 637-643delTACCGCC (39).
Sequence analysis for mutations
Sequence analysis is used for all the known genes associated with FA. Sequence analysis is complicated by the number of genes to be analysed, the large number of possible mutations in each gene, the presence of large insertions or deletions in some genes, and the large size of many of the FA-related genes. If the complementation group has been established, the responsible mutation can be determined by sequencing the corresponding gene. The majority of patients with FA worldwide are the complementation A with several hundred mutations.
Deletion/duplication analysis
Deletion/duplication analysis is also used to detect deletions of one or more exons or of an entire gene of any suspected case of FA.
The next generation sequencing technology has enabled an effective and faster molecular diagnostics approach for FA gene studies which it is able to perform the mutation analysis for FA genes without the requirement of complementation group testing step which the living cells are required. Recently Ameziane et al. applied the next generation sequencing approach to identify BRCA2, FANCD2, FANCI and FANCL mutations in novel unclassified FA patients (46).
Conclusions
Studying and testing on FA can offer potential opportunities in the understanding of the mechanisms and treatment for FA and other human genetic diseases, aging and cancer because FA possess the unique features in many aspects. The first successful application of human cord blood transplantation was in a FA patient in 1988 and the patient is still living and well.
Diagnosis on patients with FA also is challenging, particularly in its early phase. Mismanagement from the misdiagnosis of FA was not uncommon in some regions and countries because FA is a genetically and phenotypically heterogeneous disease and also because FA shares many clinical features with several group diseases/syndromes. In research, the precise biological activities and the roles of the FA proteins remain still undetermined because most FA proteins in the core complex have no enzymatic motif which is an obstacle to understand their molecular functions.
Clinicians with a broad clinical knowledge about FA from genetics, hematology and oncology fields being able to referring of such patients for testing and the experienced laboratory being able to perform the particular testing are the prerequisiting conditions for FA diagnosis. The diagnosis of FA is a team effort from the clinic to the laboratory with the proper strategies. Commonly paediatric hematology is the frontline in the clinic; and cytogenetics is the frontline in the laboratory. The differential diagnosis of FA from many other different diseases, particularly from both chromosomal instability syndromes and bone marrow failure syndromes can be difficult.
Acknowledgements
Author wish to thank the supporting from Dr Greg Peters and Mr Dale Wright, The Department Head of The Western Sydney Genomics, Western Sydney Genetic Program, The Children’s Hospital at Westmead and also wish to thank the International FA Research Fund (IFAR) for their friendly encouraging and support.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Young NS, Alter BP. eds. Aplastic Anemia: Acquired and Inherited. Philadelphia PA: WB Saunders, 1994;17:P100-32.
- Alter BP. Inherited bone marrow failure syndromes. Nathan DG, Orkin SH, Look AT, et al. eds. Nathan and Oski’s Hematology of Infancy and Childhood 2003: 6th ed. Philadelphia, PA: WB Saunders, 2003:280-365.
- Fanconi G. Familiäre infantile perniziosaartige Anämie (pernizioses Blutbild und Konstitution). Jahrb Kinderh 1927;117:257-80.
- Schmid W, Scharer K, Baumann T, et al. Chromosomal fragility in familial panmyelopathy (Fanconi type). Schweiz Med Wochenschr 1965;95:1461-4. [PubMed]
- Fanconi G. Familial constitutional panmyelocytopathy, Fanconi’s anemia (F.A.). I. Clinical aspects. Semin Hematol 1967;4:233-40. [PubMed]
- Taylor AM. Chromosome instability syndromes. Best Pract Res Clin Haematol 2001;14:631-44. [PubMed]
- Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res 2009;668:4-10. [PubMed]
- Dokal I. Fanconi’s anaemia and related bone marrow failure syndromes. Br Med Bull 2006;77-78:37-53. [PubMed]
- Moustacci E. Fanconi anemia. Orphanet 2002;1-5.
- Auerbach AD, Rogatko A, Schroeder-Kurth TM. International Fanconi Anemia Registry: relation of clinical symptoms to diepoxybutane sensitivity. Blood 1989;73:391-6. [PubMed]
- Tipping AJ, Pearson T, Morgan NV, et al. Molecular and genealogical evidence for a founder effect in Fanconi anemia families of the Afrikaner population of South Africa. Proc Natl Acad Sci U S A 2001;98:5734-9. [PubMed]
- Tischkowitz MD, Hodgson SV. Fanconi anaemia. J Med Genet 2003;40:1-10. [PubMed]
- Tipping AJ, Pearson T, Morgan NV, et al. Molecular and genealogical evidence for a founder effect in Fanconi anemia families of the Afrikaner population of South Africa. Proc Natl Acad Sci U S A 2001;98:5734-9. [PubMed]
- Rosenberg PS, Tamary H, Alter BP. How high are carrier frequencies of rare recessive syndromes? Contemporary estimates for Fanconi Anemia in the United States and Israel. Am J Med Genet A 2011;155A:1877-83. [PubMed]
- Saxon B. Nathan and Oski’hematology of infancy and children. J Paediatr Child Helath 2004;40:244.
- Butturini A, Gale RP, Verlander PC, et al. Hematologic abnormalities in Fanconi anemia: an International Fanconi Anemia Registry study. Blood 1994;84:1650-5. [PubMed]
- Wajnrajch MP, Gertner JM, Huma Z, et al. Evaluation of growth and hormonal status in patients referred to the International Fanconi Anemia Registry. Pediatrics 2001;107:744-54. [PubMed]
- Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev 2010;24:101-22. [PubMed]
- Giampietro PF, Adler-Brecher B, Verlander PC, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics 1993;91:1116-20. [PubMed]
- Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood 2003;101:822-6. [PubMed]
- Liu JM, Auerbach AD, Young NS. Fanconi anemia presenting unexpectedly in an adult kindred with no dysmorphic features. Am J Med 1991;91:555-7. [PubMed]
- Alter BP. Cancer in Fanconi anemia, 1927-2001. Cancer 2003;97:425-40. [PubMed]
- Hodson C, Walden H. Towards a molecular understanding of the fanconi anemia core complex. Anemia 2012;2012:926787.
- Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol 2010;150:179-88. [PubMed]
- Tönnies H, Huber S, Kuhl JS, et al. Clonal chromosomal aberrations in bone marrow cells of Fanconi anemia patients: gains of the chromosomal segment 3q26q29 as an adverse risk factor. Blood 2003;101:3872-4. [PubMed]
- Quentin S, Cuccuini W, Ceccaldi R, et al. Myelodysplasia and leukemia of Fanconi anemia are associated with a specific pattern of genomic abnormalities that includes cryptic RUNX1/AML1 lesions. Blood 2011;117:e161-70. [PubMed]
- Auerbach AD, Allen RG. Leukemia and preleukemia in Fanconi anemia patients. A review of the literature and report of the International Fanconi Anemia Registry. Cancer Genet Cytogenet 1991;51:1-12. [PubMed]
- Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica 2008;93:511-7. [PubMed]
- Howlett NG, Taniguchi T, Olson S, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 2002;297:606-9. [PubMed]
- Zhang F, Ma J, Wu J, et al. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol 2009;19:524-9. [PubMed]
- Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci U S A 2009;106:7155-60. [PubMed]
- Oostra AB, Nieuwint AW, Joenje H, et al. Diagnosis of fanconi anemia: chromosomal breakage analysis. Anemia 2012;2012:238731.
- Meetei AR, Levitus M, Xue Y, et al. X-linked inheritance of Fanconi anemia complementation group B. Nat Genet 2004;36:1219-24. [PubMed]
- Castella M, Pujol R, Callén E, et al. Chromosome fragility in patients with Fanconi anaemia: diagnostic implications and clinical impact. J Med Genet 2011;48:242-50. [PubMed]
- Strathdee CA, Duncan AM, Buchwald M. Evidence for at least four Fanconi anaemia genes including FACC on chromosome 9. Nat Genet 1992;1:196-8. [PubMed]
- Faivre L, Guardiola P, Lewis C, et al. Association of complementation group and mutation type with clinical outcome in fanconi anemia. European Fanconi Anemia Research Group. Blood 2000;96:4064-70. [PubMed]
- Futaki M, Yamashita T, Yagasaki H, et al. The IVS4 + 4 A to T mutation of the fanconi anemia gene FANCC is not associated with a severe phenotype in Japanese patients. Blood 2000;95:1493-8. [PubMed]
- Guidelines for Diagnosis and Management, Fanconi Anemia, Third Edition, 2008;P38-9.
- Yamashita T, Wu N, Kupfer G, et al. Clinical variability of Fanconi anemia (type C) results from expression of an amino terminal truncated Fanconi anemia complementation group C polypeptide with partial activity. Blood 1996;87:4424-32. [PubMed]
- Cervenka J, Arthur D, Yasis C. Mitomycin C test for diagnostic differentiation of idiopathic aplastic anemia and Fanconi anemia. Pediatrics 1981;67:119-27. [PubMed]
- Auerbach AD. Fanconi anemia diagnosis and the diepoxybutane (DEB) test. Exp Hematol 1993;21:731-3. [PubMed]
- Pulsipher M, Kupfer GM, Naf D, et al. Subtyping analysis of Fanconi anemia by immunoblotting and retroviral gene transfer. Mol Med 1998;4:468-79. [PubMed]
- Schindler D and H. Hoehn H. eds. Flow cytometric testing for syndromes with chromosomal instability, aplastic anemia and related hematological disorders in Diagnostic Cytogenetics. Heidelberg, Germany: Springer, 1999:269-81.
- Gregory JJ Jr, Wagner JE, Verlander PC, et al. Somatic mosaicism in Fanconi anemia: evidence of genotypic reversion in lymphohematopoietic stem cells. Proc Natl Acad Sci U S A 2001;98:2532-7. [PubMed]
- Chandra S, Levran O, Jurickova I, et al. A rapid method for retrovirus-mediated identification of complementation groups in Fanconi anemia patients. Mol Ther 2005;12:976-84. [PubMed]
- Ameziane N, Sie D, Dentro S, et al. Diagnosis of fanconi anemia: mutation analysis by next-generation sequencing. Anemia 2012;2012:132856.


