
Antibiotics in inflammatory bowel diseases: do we know what we’re doing?
Introduction
Inflammatory bowel disease (IBD) represents a heterogeneous group of auto-inflammatory disease primarily affecting the gastrointestinal tract (GIT). The pathogenesis of IBD is not completely understood, however it is clear that there are numerous endogenous and exogenous factors involved in its development. While it is well established that there is a genetic predisposition to IBD, with at least 200 known risk loci identified so far (1,2), the tremendous increase incidence of IBD in recent decades, particularly in the developing world (3), clearly points to other factors involved in IBD pathogenesis.
There are multiple theories as to which environmental factors are associated with this phenomenon, ranging from the “hygiene hypothesis”, dietary factors, antibiotic exposure and industrial pollutants, among others, each backed by variable levels of evidence. The role of gut flora in IBD pathogenesis is becoming increasingly evident. While it was previously thought that the microbiome is relatively stable from late childhood through to late adulthood, recent data demonstrate that the microbiome is exquisitely sensitive to dietary and other environmental changes (4,5). External environmental elements may, at least in part, exert their influence on the microbiome, which may more directly contribute to IBD development. Furthermore, recent data demonstrate that single host-genes influence the microbiome (5), hence it is possible that recognized IBD risk loci may impart their influence via changes in the gut flora.
By way of logical extension, if microbiome is so intimately involved in IBD pathogenesis, manipulation of the gut flora should be an integral component of IBD management. However existent data is inconsistent and the role of antibiotics has yet to be firmly established.
With several decades of diverse trials, and waxing and waning debate, we aim to provide a fresh perspective on the position of antibiotics in IBD management. This review will commence with an overview of the mounting evidence indicating the microbiome in the pathogenesis of IBD. Following this, the current evidence and experience relating to antibiotics in IBD management will be discussed, focusing on specific clinical scenarios. To conclude we aim to suggest a potential role of antibiotics in the management of IBD in today’s clinical practice.
The role of the microbiome in IBD
Gut flora comprises an enormously complex microenvironment, with the microbial genome outnumbering the human genome by at least an order of magnitude (6). The majority of gut microbiota has never been cultured, and the advancement in our understanding of its complex role has only begun to flourish with the development of newer culture-independent technologies. Adding to the complexity of this microenvironment is the uncertain role of other members of this microbial community including fungi and bacteriophages.
Compounding evidence exists as to the role gut microflora plays in IBD. It has long been recognized that inflamed mucosa has reduced diversity of mucosa-associated bacteria, with a noted reduction in Bacteroides and Firmicutes, thought to have anti-inflammatory properties, and a relative increase in Enterobacteriaceae, including Escherichia coli and Fusobacterium (7-11). This change has been termed “dybiosis”. Further evidence to the role of gut bacteria is noted particularly in CD whereby there is an elevated adaptive immune response to gut microbiota. This is most clearly demonstrated by the frequent presence of various circulating antibodies to E. coli (OmpC), Pseudomonas flourescens, bacterial flaggellin and ASCA mannan, among others in patients with CD (12). Mucolytic bacteria penetrating intestinal mucosa has been identified in IBD patients and mucosa-associated bacteria are found in the normally sterile submucus layer (13,14). Beyond bacteria, bacteriophages have also been shown to undergo dynamic shifts in the presence of bowel inflammation in IBD, suggesting that they also play an as yet unidentified role in IBD (15).
Indirect support for the role of gut flora in IBD pathogenesis is provided by fecal stream diversion improving disease activity in CD, with restoration of fecal stream inducing recurrence. This does not occur, however, when ileostomy effluent is filtered to remove bacteria prior to reintroduction (16,17). Ileal microflora undergoes significant expansion on restoration of contiguity with colonic remnant, however the microbial pattern in patients with CD varies significantly from that of patients without CD upon restoration of fecal flow, with significantly higher counts of E coli and bacteroides in patients with CD, as compared to higher counts of bifidobacteria and ruminococci amongst controls (18).
Further support for the critical role of the microbiome in IBD is that all genetically engineered mouse models of IBD require commensal enteric bacteria for the development of intestinal inflammation, and do not progress to an inflammatory state in germ-free conditions (12). Moreover, amongst the known IBD susceptibility genes, the majority are associated with host mucosal barrier function (19), for example depletion of NOD2 signaling pathway impairs the ability to reduce intracellular bacterial burden (20).
Current theories of IBD pathogenesis hypothesize that pathologic alterations of intestinal microbiome trigger an aberrant mucosal immune response in genetically predisposed individuals (21). Alternative theories, based on circumstantial evidence, postulate specific pathogens as the primary driver of inflammation. One suggested pathogen is Mycobacterium avium subspecies paratuberculosis (MAP), based on its frequent isolation in CD and the similarity between CD and Johne’s disease, a chronic and sometimes fatal infection in ruminants such as cattle and goats (22,23). Adherent-invasive E. coli (AIEC) has also been suggested as a major player, having been isolated in the normally sterile submucus layer and its ability to invade epithelial cells and replicate within macrophages (24). Another suggested candidate pathogen is Fusobacterium varium in UC, based on its isolation on colonic biopsies and frequent seropositivity, in addition to in vitro evidence of F. varium causing UC-like lesions in experimental models (25,26).
Specifically how dysbiosis influences the inflammatory cascade is not clear. One theory, applicable more in UC, is that dysbiosis results in excessive anaerobic nitrate respiration with resultant nitric oxide and sulfide which in turn impairs β-oxidation, lipid and protein synthesis, which may trigger changes in tight junctions and ion channels (27). While the pathophysiological mechanism of dysbiosis is far from certain, it has been observed that the extent or pattern of dysbiosis can be associated with disease activity, predict disease course and predict response to therapy. The pediatric RISK cohort, a large pediatric CD inception cohort of over 1,200 children, detected that a depletion of specific Firmicutes and Bacteroidetes taxa and expansion of Proteobacteria were associated with clinical severity (28). A regression model that included gene expression and microbial abundance more accurately predicted 6 month steroid-free remission than a model using clinical factors alone (28). In a separate study, pediatric patients with UC were shown to have significantly lower bacterial diversity than controls, and those who did not respond to corticosteroid therapy harbored a microbiome that was even less rich than steroid responders (29).
Beyond the microbial environment locally, the presence of bacterial DNA in the systemic circulation, a marker of gut translocation, is strongly associated with disease activity, cytokine levels and response to biological therapy (30). Additionally, circulating bacterial DNA serves as a predictor for disease relapse at 6 months (31,32).
While the presence of dysbiosis in IBD is indisputable, a protracted debate has long been waged as to whether it is a trigger and driver of inflammation, or whether it arises as a result of inflammation. While not resolving this controversy definitively, biopsies from uninflamed regions of ileum in the RISK cohort demonstrated that ileal dysbiosis occurs even in the absence of overt inflammation (28). Even more remotely, it has been found that subgingival oral microbiota differs in active CD than in healthy subjects, normalizing after successful induction of remission (33). These data support the assertion that the dysbiosis is independent of local inflammatory processes, hence targeting the gut flora may have a role in IBD management.
Theoretical role of antibiotics in IBD management
Manipulation of microbiome
With mounting evidence of the critical role of the gut microflora in IBD it is reasonable to hypothesize that manipulation of this microenvironment should impact the inflammatory process. However, the overwhelming focus of research in IBD therapy, and the near exclusive focus of existent medications, has been related to modulation of an aberrant mucosal immune response (34). This limited focus on treatment ignores a significant component of potential treatment targets, possibly even a critical, primary pathogenic factor.
There are several potential mechanisms by which antibiotics may alter disease course in IBD. Antibiotics decrease luminal bacterial concentrations and may alter the composition of gut microflora, favoring “beneficial” bacteria (35-37). Antibiotics also reduce multiple bacterial enzyme activities with evidence of a direct correlation between extent of activity reduction and clinical response (36,38). Antibiotics may also decrease bacterial tissue invasion, treat microabscesses, potentially impair bacterial attachment, decrease bacterial translocation and prevent systemic dissemination (39-42). On the assumption that IBD is caused by a single pathogen, antibiotics can specifically target these organisms, at times with intracellular penetration to target embedded pathogens (43). Furthermore, antibiotics may have direct immunomodulatory (42,44-46) and anti-inflammatory properties (47-49).
Unintended consequences of microbiome manipulation
Due to the complexity and diversity of the microbiome, the effects of antibiotics are far reaching. Antibiotics are associated with increased dysbiosis with relatively higher fungal proportions in the microbiome under treatment (50). Long-term changes to microbiome continue well beyond cessation of therapy. For example, assessment of mucosal flora from IBD patients treated with a 2-week antibiotic course, demonstrate an initial suppression effect on mucosal flora followed by a massive rebound following cessation, with dramatically increased bacterial load out to 5 months (51). The long-term significance of this microbiome manipulation is not certain. There is compelling evidence that such influences on microbiome may be more pronounced, and more significant during early life when the microbiome is more malleable than in later childhood and adults (52,53).
While microbiome manipulation may positively influence mucosal inflammation in the short-term, concern has been raised of the potential for antibiotics to contribute to the inflammatory cascade. Many large cohort studies have been performed over recent decades suggesting that exposure to antibiotics, particularly in childhood, carries a higher risk of development of IBD (54-61). One particular study of over one million subjects with over six million person years follow-up demonstrated that antibiotic exposure under the age of one year of age was significantly associated with IBD development (HR, 5.51; 95% CI: 1.66–18.28) (56). An exposure-risk profile correlates a higher hazard ratio with greater exposure, up to HR 7.3 (95% CI: 2.14–24.99) with over seven antibiotic courses (59). While compelling, this association does not necessarily suggest causation. Antibiotics may be a surrogate marker for infectious processes, for example Campylobacter and Salmonella which may themselves predispose to IBD (62-67). Alternatively, the association may represent a common pathway between bacterial infections and CD with a dysregulated innate and adaptive immunity predisposing both to CD and, independently, to certain infections (68-70).
Any discussion of prolonged antibiotic exposure must also include consideration of development of antibiotic resistance. Indeed there is data to suggest that the prevalence of Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus (VRE) and extended-spectrum beta-lactamases (ESBL) is significantly higher among IBD patients. Recent hospitalization and recent antibiotic use are particularly associated with the higher prevalence (71,72).
Interestingly, despite the well-recognized risk of Clostridium difficile infection (CDI) following prolonged antibiotic exposure, patients with CD on chronic antibiotics had a low incidence of CDI (2%) in one large retrospective study, significantly lower than reported 13% incidence in patients on continuous flow left ventricular assist devices, and 22% in patients with orthopedic prosthesis infections (73).
Antibiotics and IBD: the evidence
IBD is a diverse condition with response to treatment dependent on multiple interrelated variables. To assess antibiotics in IBD as a whole, or even overall in CD or UC independently, is overly simplistic and will not reflect the true effect in specific circumstances. Hence we have attempted to review the role of antibiotics in more specific scenarios.
Evidence relating to efficacy of therapeutic interventions generally depends on a number of large, high quality randomized controlled trials (RCTs). Due to the relatively low number of high quality studies in antibiotics, and the high variability in antibiotics trialed, treatment course and outcome measures, drawing firm conclusions remains difficult.
Further complicating attempts at analysis of existent data is the potential diverse conclusions drawn from individual studies. For example, in the seminal Australian antibiotic RCT of anti-MAP combination therapy including over 200 patients followed for 3 years (74), the primary endpoint of prolonged relapse-free course out to 3 years was not met, however the antibiotic group had a higher 16-week remission rate (66% vs. 50%, P=0.02), and a clear trend to lower 12 months (39% vs. 56%, P=0.054) and 24 months (26% vs. 43%, P=0.14) relapse rate (75). However, considering that the antibiotic course was specifically tailored to target MAP, concerns were raised regarding possible underdosing of antibiotics, lack of pre-treatment testing for MAP, and levels of antibiotic resistance, further casting a doubt on the applicability of the evidence (76-80).
Due to the lack of standardized comparable high quality studies, in order to better formulate a viewpoint on the role of antibiotics in today’s IBD practice, the following analysis will also cautiously consider higher quality non-randomized and uncontrolled studies.
Evidence for antibiotics in Crohn’s disease (CD)
Induction of remission in active CD
Diverse RCTs have been performed utilizing different antibiotic combinations, varying treatment periods and with differing end-points. Studies have primarily assessed either specific anti-MAP therapy or non-specific broad spectrum antibiotics or antibiotic combinations. Overall the strongest signal by antibiotic type appears to be with clofazimine (RR, 0.70; 95% CI: 0.53–0.94) and rifamycins (RR, 0.78; 95% CI: 0.67–0.91) with macrolides failing to achieve convincing induction of remission (RR, 0.76; 95% CI: 0.42–1.36) (34).
A review of the RCTs performed for induction of remission in CD reveals a significant confounding factor. All of these trials utilized clinical indices with clinical remission and relapse being the primary outcome measures (74,81-91). It is well established that a large component of clinical activity in CD is not necessarily related to inflammation, with irritable bowel syndrome (IBS) (92), bacterial overgrowth (93) and chronic pain syndromes (94) all occurring with greater frequency than the general population. The role of antibiotics in treating IBS and bacterial overgrowth is also well supported (95). Hence it is unclear whether clinical outcomes reflects a direct effect on mucosal inflammation and disease progress, or treatment of secondary clinical manifestations of arguable importance to true mucosal response.
To address this confounder several studies assessed objective markers as secondary outcomes. Prantera et al. (83) assessed for endoscopic healing and Goodgame et al. (91) measured intestinal permeability as secondary outcome measures, both of which did not show any response to antibiotics. Selby et al. demonstrated a non-significant trend to improved endoscopic score and inflammatory markers out to 2 years (74). In the study by Sutherland et al. (88), CRP dropped significantly amongst patients taking metronidazole compared to placebo (0.8 vs. −0.9 mg/dL, P<0.05). In a different study by Prantera et al., overall there was a non-significant trend to higher remission (52% vs. 33%, P=NS) and response rates (67% vs. 41%, P=NS) amongst rifaximin users, however when including only those patients with elevated CRP at enrolment the differences became significant with 63% vs. 21% (P=0.03) and 75% vs. 21% (P=0.01) remission and response respectively, and treatment failure rates of 0% vs. 50% (P=0.002) (82). This suggests that the effect of Rifaximin may more specifically target inflammation rather than just associated clinical manifestation of CD, which are not known to routinely raise inflammatory markers.
There are several uncontrolled studies and case series presenting convincing data of clinical response and induction of remission in chronically active CD with the sole intervention of antibiotics (96-104). Specifically in uncontrolled trials objective markers of inflammation are critical and some of these trials demonstrated significant improvement or normalization of CRP, ESR and albumin (96,101,103), and two reports demonstrated mucosal healing in patients with previously severe endoscopic disease (97,98). Three of these studies utilized anti-MAP treatment (97,99,104), and the rest used a range of broad spectrum antibiotics.
Collaborating both the RCTs and uncontrolled trials for specific antibiotics, out of seven studies with metronidazole, four showed convincing response, two no effect and one with partial response (Table 1). Amongst the five trials of ciprofloxacin, three showed promising effect and two with no response. Three trials each of rifaximin and clarithromycin were positive in two for each with one in each of questionable response. Nine anti-MAP therapy trials were positive in five, negative in three and unclear in one. Despite the obvious risk of publication bias, uncontrolled trials with objective outcome measures, seen in the context of the RCT’s suggests that antibiotics, in various combinations and course lengths, have a signal of clinical benefit and probably an anti-inflammatory effect in active CD. The question of which patient, which antibiotic, and for how long to treat, remain largely unanswered.
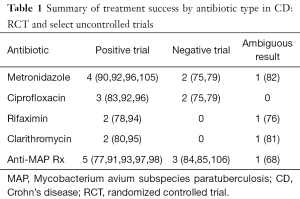
Full table
Maintenance of remission
Three trials addressed prevention of relapse in quiescent CD (74,83,90) all of whom utilized anti-MAP therapy. Overall there was a combined statistically significant preventive effect compared to placebo (RR, 0.62; 95% CI: 0.46–0.84; NNT =4) (34). One novel database derived case crossover study found that antibiotic use was associated with a reduced risk of flare during the following 60 days (OR, 0.78; 95% CI: 0.64–0.96; P=0.019) (107). While this data is provocative, it is far from conclusive, as it is possible that antibiotics may modulate, or otherwise mask flare symptoms rather than protect against a true flare.
Post-operative prophylaxis
Five RCTs investigated the effect of prolonged courses of antibiotics in prevention of post-operative recurrence of CD (106,108-111). Nitroimidazoles have been shown to be effective in reducing clinical (RR, 0.23; 95% CI: 0.09–0.57, NNT =4) and endoscopic (RR, 0.44; 95% CI: 0.26–0.74; NNT =4) recurrence (112). One small study demonstrated reduced endoscopic recurrence at 3 months with rifaximin over placebo (10% vs. 40%, P=0.03) (111). Other studies using different antibiotics were less positive, including a placebo controlled trial with ciprofloxacin which showed a non-significant trend to reduced recurrence (110), and an uncontrolled trial of rifabutin and ethambutol combination (113).
Overall there is strong evidence that antibiotics have a role in prevention of recurrence and possibly in prophylaxis, however frequent side-effects with resultant high cessation rates, in addition to the concern of developing antibiotic resistance must be considered prior to commencement (114,115). Furthermore the long-term effect beyond antibiotic cessation is questionable.
Evidence for antibiotics in perianal CD
Antibiotics have long been used in clinical practice for active perianal CD however the evidence remains relatively limited. Three randomized placebo controlled trial have been performed, two of which assessed antibiotics in combination with biological therapy (116-118). One small RCT comparing metronidazole, ciprofloxacin and placebo was too underpowered to detect any statistically significant effect (116). Ciprofloxacin as an adjunct to infliximab had a higher response than infliximab alone (73% vs. 39%, P=0.12) (117), and was also found to be significantly better as adjunct to adalimumab (71% vs. 47%, P=0.047) (118).
A large open label trial assessed response of perianal disease to ciprofloxacin or metronidazole in addition to azathioprine at 8 weeks, prior to expected azathioprine efficacy. Half of patients had a significant improvement in perianal disease scores (PDAI 8.4±2.9 to 6±4; P<0.0001) (119).
Topical metronidazole ointment was assessed in an RCT and while both groups had the same mean reduction in perianal CD activity index scores, more patients applying metronidazole ointment had at least 5 point reduction in the score (42% vs. 12%, P=0.03), significantly reduced perianal discharge (P=0.01) and a trend to less perianal pain (P=0.059) (120).
Pooled analysis of ciprofloxacin in perianal CD demonstrates efficacy in reducing fistula drainage but not for fistula healing (RR, 1.64; 95% CI: 1.16–2.32; P=0.005) (121). These results suggest that antibiotics should be used as adjunctive therapy for perianal CD, but not as sole therapy (122).
Evidence for antibiotics in ulcerative colitis
Induction of remission in active ulcerative colitis
A number or RCT (123-132) and uncontrolled studies (133-138) have been reported on antibiotics for induction of remission in chronically active and acute severe colitis (ASC). Multiple different antibiotic combinations were trialed with very different outcomes reported, ranging from dramatic induction of complete remission, through to no response.
Two meta-analyses demonstrate overall higher remission rates with antibiotics compared to placebo [RR, 1.56; 95% CI: 1.04–2.33 (34); and RR, 2.14; 95% CI: 1.48–3.09 (139)]. Considering the diverse range of antibiotics trialed, different methods of administration and course length, and the vastly divergent responses, interpretation of the data is limited, and recommendations for clinical practice is difficult to formulate. Indeed it is unclear whether it is appropriate to pool studies with a diverse assortment of antibiotics.
Interestingly, upon independent analysis of the trials a striking contrast is revealed. Those trials in which antibiotics were administered orally seemed to have a greater response than those in which they were administered intravenously. It may be that the effect of the antibiotics is by way of a local luminal effect, possibly effecting beneficial change on the microbiome, or as has been suggested, by direct effect on a particular pathogen (25,26,130,131).
With the necessary caveat of publication and other potential biases there are a number of case series and uncontrolled trials demonstrating a dramatic response to antibiotics in both steroid-refractory ASC and in steroid dependent chronic colitis. Some of these reports demonstrate significant rates of endoscopic improvement (133,135,136). All of these trials were with various oral antibiotic cocktails mainly based around amoxicillin, tetracycline and metronidazole. The author’s team published a case series of 15 children with refractory ASC, several failing biologic therapy, in whom 47% rapidly entered remission and a further 20% partially responded to an oral antibiotic cocktail of metronidazole, amoxicillin, doxycycline and vancomycin (134). We subsequently published a RCT of the oral antibiotic cocktail, prescribed as an add-on therapy to IVCS in 28 children admitted with ASC. Day 5 PUCAI was significantly lower in the antibiotics + IVCS arm vs. IVCS alone (25±16.7 vs. 40.4±20.4, P=0.037) (140).
A large retrospective study reviewing UC patients revealed those who received antibiotics as an adjunct to IV steroids were less likely to receive hospital rescue therapy (OR, 0.42; 95% CI: 0.19–0.93), however there was no difference in hospital length of stay, surgery or re-hospitalization rates (138). No standardized antibiotic course was administered however over 90% were given ciprofloxacin or metronidazole either alone or in combination.
One study reported long-term outcomes from a RCT of oral tobramycin in acute UC. While the initial study demonstrated significantly improved remission rates following one week adjunct oral tobramycin (74% vs. 43%; P=0.008) (125), maintenance of remission over 1 and 2 years was not shown (40% vs. 20%; P=0.27 and 24% vs. 12%; P=0.40, respectively) (141). In contrast, two studies by Ohkusa et al. (130,131) on the oral antibiotic cocktail of amoxicillin, tetracycline and metronidazole in active UC, related to one year outcomes. Both of these studies reported significantly higher remission rates with antibiotics and improved remission rates, symptom scores and endoscopic scores were also demonstrated out to 12 months. Indeed these findings were more significant at 12 months than they were at 3 months, possibly suggesting long-term benefit following the short course antibiotic cocktail.
A subpopulation with specific promise for antibiotics is very early onset IBD (VEO-IBD) and infantile IBD (142). In some of these patients a monogenic etiology is identified and a poor response to conventional therapy has been noted (105,142). A recently published case-series of infants with VEO-IBD with poor response to previous medications showed a dramatic response to oral vancomycin and gentamicin in four of the five patients, including normalization of fecal calprotectin. Sustained immunosuppressant-free remission was maintained in 3 of the patients (143).
New targets of treatment and future directions
Overall there is a suggestion that altering gut microbial flora has a role in modulating IBD inflammatory activity. These findings open up new opportunities for IBD treatment utilizing modalities that differ vastly from what has been routinely used in the past.
However it remains unclear if the effect of antibiotics is due to actions on one particular bacterial species, altering the composition of the microbiome, or possibly treating secondary infections which exacerbate disease (34). Complicating the ability to formulate clear guidelines is that evidence is based on a diverse array of treatment options, with different targets of actions, in dissimilar clinical scenarios. Hence the variation in outcomes amongst the published data is not surprising. What is encouraging is the relatively large number of studies demonstrating significant effect, establishing a potential role for antibiotics. However many uncertainties remain to be clarified. One of these uncertainties is the potential contribution of antibiotic resistance. Indeed, resistance rates of local bacteria found in CD, such as AEIC and MAP have been demonstrated to be higher in patients with CD than in the general population, with multi-drug resistance demonstrated in a high proportion (144,145). Alternatively, pathogenicity of bacteria in IBD may be dependent on a large number of host-specific or environment-specific factors, hence the response to treatment may diverse between patients (35).
Furthermore, the effects of antibiotics on the microbiome and the metabolome need to be better defined. While antibiotics reduce total bacterial load, the pattern of influence is not uniform, with minimal changes noted at the phylum level, but striking changes at the family level (37). Additionally there are major shifts in microbial metabolism with an increase in short-chain fatty acids, propanol, decanol, nonanone and aromatic organic compounds, and a decrease in ethanol, methanol and glutamate (36). The effects of antibiotics may surpass microbiome and metabolome manipulation. For example, rifaximin has been shown to reverse and normalize a vast array of inflammatory cytokines including IL-17, IL-1β and TNF-α, and normalize gene expression of occludin, a tight junction protein (37). Which of these effects, or which combination of these effects, plays the dominant role in influencing inflammation remains unclear. Hence current usage of antibiotics in IBD remains somewhat unfocussed and enigmatic.
Without doubt, further studies are required to better elucidate the potential role of antibiotics in IBD. Culture-independent analysis of changes in intestinal microbiome is critical in future trials of antibiotic therapy to better explore the effect the intervention has on the microflora and to possibly provide mechanistic insight (34). Stratifying patients on the basis of genetics, immunological markers, microbiome signature and specific clinical phenotypes may help target specific antibiotic therapies more appropriately (146,147). Future trials should be more standardized, with objective outcome measures to adequately allow replication of trials.
Integration of antibiotics into IBD practice
Despite the lack of extensive trials and sufficient “hard data”, the signal from available studies suggests that antibiotics may have a complementary role in IBD management. Specifically, antibiotics can be considered in clinical practice in the following scenarios (Table 2).

Full table
Active fistulizing perianal CD
Active fistulizing perianal CD is likely the most frequent indication for antibiotics in IBD management. Oral ciprofloxacin with or without metronidazole can be used as an adjunct to immunosuppressant therapy, usually anti-TNF. This is usually continued for 3 weeks with extension of the antibiotic course as required.
Mild-moderate luminal CD
For mild luminal CD, both small bowel and colonic, it is reasonable to consider up to 8 weeks azithromycin and metronidazole to induce remission. In the absence of response within 2–3 weeks, or if antibiotics are poorly tolerated, the antibiotics should be ceased and replaced with more traditional therapies.
Post-operative CD
An extended course of oral metronidazole can be considered following re-anastomosis after CD resection. This should be considered on a case-by-case basis, accounting for risk stratification, potential metronidazole intolerance and the use of other prophylactic medications, with assumed lower contributive benefit of metronidazole when anti-TNF therapy is also administered.
Very-early onset IBD
In infants and very young children it is reasonable to consider, on a case-by-case basis, oral vancomycin and gentamicin as sole first line therapy. If the child responds this may be continued for 3 weeks and consider future courses if the disease flares. If remission is not maintained, or if no initial response is detected within the first week, more conventional treatments should be pursued.
ASC and chronic refractory colitis
Given the inconsistent data and the high-risk nature of ASC, antibiotics are not currently routinely recommended in UC. However it is reasonable to consider an oral antibiotic cocktail of amoxicillin, metronidazole, doxycycline and vancomycin if standard therapies have failed. In ASC this is usually considered if refractory to IV steroids, however salvage therapy or colectomy should never be delayed while trialing this approach.
Summary
We can expect further supportive data for the role of antibiotics in IBD in the future. As the trials and analyses improve we will hopefully gain better insight into mechanisms of action to enable better “fine-tuning” of potential treatment targets. Regardless it already seems that manipulation of gut flora with antibiotics have a complimentary and potentially symbiotic role with current treatments which target mucosal immunity. Each medication class has a potential role and the delicate symphony of IBD management requires judicious utilization of various treatment options which needs to be conducted with a blend of experience, evidence, and at times a little flare.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979-86. [Crossref] [PubMed]
- McGovern DP, Kugathasan S, Cho JH. Genetics of Inflammatory Bowel Diseases. Gastroenterology 2015;149:1163-76.e2. [Crossref] [PubMed]
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720-7. [Crossref] [PubMed]
- Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105-8. [Crossref] [PubMed]
- Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 2011;9:279-90. [Crossref] [PubMed]
- Li J, Jia H, Cai X, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 2014;32:834-41. [Crossref] [PubMed]
- Ott SJ, Musfeldt M, Wenderoth DF, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004;53:685-93. [Crossref] [PubMed]
- Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007;104:13780-5. [Crossref] [PubMed]
- Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008;105:16731-6. [Crossref] [PubMed]
- Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 2006;55:205-11. [Crossref] [PubMed]
- Lupp C, Robertson ML, Wickham ME, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007;2:204. [Crossref] [PubMed]
- Subramanian S, Campbell BJ, Rhodes JM. Bacteria in the pathogenesis of inflammatory bowel disease. Curr Opin Infect Dis 2006;19:475-84. [Crossref] [PubMed]
- Liu Y, van Kruiningen HJ, West AB, et al. Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn's disease. Gastroenterology 1995;108:1396-404. [Crossref] [PubMed]
- Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122:44-54. [Crossref] [PubMed]
- Duerkop BA, Kleiner M, Paez-Espino D, et al. Murine colitis reveals a disease-associated bacteriophage community. Nat Microbiol 2018;3:1023-31. [Crossref] [PubMed]
- Flanagan PK, Campbell BJ, Rhodes JM. Lessons from diversion studies and antibacterial interventions. Dig Dis 2012;30:347-50. [Crossref] [PubMed]
- Harper PH, Lee EC, Kettlewell MG, et al. Role of the faecal stream in the maintenance of Crohn's colitis. Gut 1985;26:279-84. [Crossref] [PubMed]
- Neut C, Bulois P, Desreumaux P, et al. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn's disease. Am J Gastroenterol 2002;97:939-46. [Crossref] [PubMed]
- Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119-24. [Crossref] [PubMed]
- Oehlers SH, Flores MV, Hall CJ, et al. The inflammatory bowel disease (IBD) susceptibility genes NOD1 and NOD2 have conserved anti-bacterial roles in zebrafish. Dis Model Mech 2011;4:832-41. [Crossref] [PubMed]
- Lane ER, Zisman TL, Suskind DL. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res 2017;10:63-73. [Crossref] [PubMed]
- Scribano ML, Prantera C. Antibiotics and inflammatory bowel diseases. Dig Dis 2013;31:379-84. [Crossref] [PubMed]
- Liverani E, Scaioli E, Cardamone C, et al. Mycobacterium avium subspecies paratuberculosis in the etiology of Crohn's disease, cause or epiphenomenon? World J Gastroenterol 2014;20:13060-70. [Crossref] [PubMed]
- Subramanian S, Roberts CL, Hart CA, et al. Replication of Colonic Crohn's Disease Mucosal Escherichia coli Isolates within Macrophages and Their Susceptibility to Antibiotics. Antimicrob Agents Chemother 2008;52:427-34. [Crossref] [PubMed]
- Ohkusa T, Sato N, Ogihara T, et al. Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species-specific antibody. J Gastroenterol Hepatol 2002;17:849-53. [Crossref] [PubMed]
- Ohkusa T, Okayasu I, Ogihara T, et al. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut 2003;52:79-83. [Crossref] [PubMed]
- Roediger WE. Review article: nitric oxide from dysbiotic bacterial respiration of nitrate in the pathogenesis and as a target for therapy of ulcerative colitis. Aliment Pharmacol Ther 2008;27:531-41. [Crossref] [PubMed]
- Haberman Y, Tickle TL, Dexheimer PJ, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest 2014;124:3617-33. [Crossref] [PubMed]
- Michail S, Durbin M, Turner D, et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis 2012;18:1799-808. [Crossref] [PubMed]
- Gutiérrez A, Holler E, Zapater P, et al. Antimicrobial peptide response to blood translocation of bacterial DNA in Crohn's disease is affected by NOD2/CARD15 genotype. Inflamm Bowel Dis 2011;17:1641-50. [Crossref] [PubMed]
- Gutiérrez A, Scharl M, Sempere L, et al. Genetic susceptibility to increased bacterial translocation influences the response to biological therapy in patients with Crohn's disease. Gut 2014;63:272-80. [Crossref] [PubMed]
- Chan SS, Watson AJ. Bacterial translocation influences the response to biological therapy in Crohn's disease. Gastroenterology 2013;145:898-901. [Crossref] [PubMed]
- Kelsen J, Bittinger K, Pauly-Hubbard H, et al. Alterations of the Subgingival Microbiota in Pediatric Crohn's Disease Studied Longitudinally in Discovery and Validation Cohorts. Inflamm Bowel Dis 2015;21:2797-805. [Crossref] [PubMed]
- Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:661-73. [Crossref] [PubMed]
- Perencevich M, Burakoff R. Use of antibiotics in the treatment of inflammatory bowel disease. Inflamm Bowel Dis 2006;12:651-64. [Crossref] [PubMed]
- Maccaferri S, Vitali B, Klinder A, et al. Rifaximin modulates the colonic microbiota of patients with Crohn's disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother 2010;65:2556-65. [Crossref] [PubMed]
- Gao J, Gillilland MG 3rd, Owyang C. Rifaximin, gut microbes and mucosal inflammation: unraveling a complex relationship. Gut Microbes 2014;5:571-5. [Crossref] [PubMed]
- Rafii F, Ruseler-Van Embden JG, van Lieshout LM. Changes in bacterial enzymes and PCR profiles of fecal bacteria from a patient with ulcerative colitis before and after antimicrobial treatments. Dig Dis Sci 1999;44:637-42. [Crossref] [PubMed]
- Farrell RJ, LaMont JT. Microbial factors in inflammatory bowel disease. Gastroenterol Clin North Am 2002;31:41-62. [Crossref] [PubMed]
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004;126:1620-33. [Crossref] [PubMed]
- Isaacs KL, Sartor RB. Treatment of inflammatory bowel disease with antibiotics. Gastroenterol Clin North Am 2004;33:335-45. x. [Crossref] [PubMed]
- Sartor RB. Review article: the potential mechanisms of action of rifaximin in the management of inflammatory bowel diseases. Aliment Pharmacol Ther 2016;43 Suppl 1:27-36. [Crossref] [PubMed]
- Brown CL, Smith K, Wall DM, et al. Activity of Species-specific Antibiotics Against Crohn's Disease-Associated Adherent-invasive Escherichia coli. Inflamm Bowel Dis 2015;21:2372-82. [PubMed]
- Di Marco R, Mangano K, Quattrocchi C, et al. Curative effects of sodium fusidate on the development of dinitrobenzenesulfonic acid-induced colitis in rats. Clin Immunol 2003;109:266-71. [Crossref] [PubMed]
- Wan YC, Li T, Han YD, et al. Effect of Pregnane Xenobiotic Receptor Activation on Inflammatory Bowel Disease Treated with Rifaximin. J Biol Regul Homeost Agents 2015;29:401-10. [PubMed]
- Kolios G, Manousou P, Bourikas L, et al. Ciprofloxacin inhibits cytokine-induced nitric oxide production in human colonic epithelium. Eur J Clin Invest 2006;36:720-9. [Crossref] [PubMed]
- Labro MT. Antibiotics as anti-inflammatory agents. Curr Opin Investig Drugs 2002;3:61-8. [PubMed]
- Huckle AW, Fairclough LC, Todd I. Prophylactic Antibiotic Use in COPD and the Potential Anti-Inflammatory Activities of Antibiotics. Respir Care 2018;63:609-19. [Crossref] [PubMed]
- Garrido-Mesa N, Camuesco D, Arribas B, et al. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol Res 2011;63:308-19. [Crossref] [PubMed]
- Lewis JD, Chen EZ, Baldassano RN, et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn's Disease. Cell Host Microbe 2015;18:489-500. [Crossref] [PubMed]
- Swidsinski A, Loening-Baucke V, Bengmark S, et al. Bacterial biofilm suppression with antibiotics for ulcerative and indeterminate colitis: consequences of aggressive treatment. Arch Med Res 2008;39:198-204. [Crossref] [PubMed]
- Tamburini S, Shen N, Wu HC, et al. The microbiome in early life: implications for health outcomes. Nat Med 2016;22:713-22. [Crossref] [PubMed]
- Yassour M, Vatanen T, Siljander H, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 2016;8:343ra81. [Crossref] [PubMed]
- Li WQ, Cho E, Khalili H, et al. Rosacea, Use of Tetracycline, and Risk of Incident Inflammatory Bowel Disease in Women. Clin Gastroenterol Hepatol 2016;14:220-5.e1-3.
- Ungaro R, Bernstein CN, Gearry R, et al. Antibiotics associated with increased risk of new-onset Crohn's disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol 2014;109:1728-38. [Crossref] [PubMed]
- Kronman MP, Zaoutis TE, Haynes K, et al. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012;130:e794-803. [Crossref] [PubMed]
- Virta L, Auvinen A, Helenius H, et al. Association of repeated exposure to antibiotics with the development of pediatric Crohn's disease--a nationwide, register-based finnish case-control study. Am J Epidemiol 2012;175:775-84. [Crossref] [PubMed]
- Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn's disease and ulcerative colitis. Am J Gastroenterol 2011;106:2133-42. [Crossref] [PubMed]
- Hviid A, Svanstrom H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011;60:49-54. [Crossref] [PubMed]
- Card T, Logan RF, Rodrigues LC, et al. Antibiotic use and the development of Crohn's disease. Gut 2004;53:246-50. [Crossref] [PubMed]
- Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol 2010;105:2687-92. [Crossref] [PubMed]
- De Vroey B, De Cassan C, Gower-Rousseau C, et al. Editorial: Antibiotics earlier, IBD later? Am J Gastroenterol 2010;105:2693-6. [Crossref] [PubMed]
- García Rodríguez LA, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 2006;130:1588-94. [Crossref] [PubMed]
- Porter CK, Tribble DR, Aliaga PA, et al. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology 2008;135:781-6. [Crossref] [PubMed]
- Gradel KO, Nielsen HL, Schonheyder HC, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 2009;137:495-501. [Crossref] [PubMed]
- Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog 2009;1:2. [Crossref] [PubMed]
- Kalischuk LD, Buret AG. A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease? Am J Physiol Gastrointest Liver Physiol 2010;298:G1-9. [Crossref] [PubMed]
- Nguyen GC. Editorial: bugs and drugs: insights into the pathogenesis of inflammatory bowel disease. Am J Gastroenterol 2011;106:2143-5. [Crossref] [PubMed]
- Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 2011;140:1729-37. [Crossref] [PubMed]
- Henckaerts L, Nielsen KR, Steffensen R, et al. Polymorphisms in innate immunity genes predispose to bacteremia and death in the medical intensive care unit. Crit Care Med 2009;37:192-201, e1-3.
- Nguyen GC. Tip of the iceberg? The emergence of antibiotic-resistant organisms in the IBD population. Gut Microbes 2012;3:434-6. [Crossref] [PubMed]
- Leung W, Malhi G, Willey BM, et al. Prevalence and predictors of MRSA, ESBL, and VRE colonization in the ambulatory IBD population. J Crohns Colitis 2012;6:743-9. [Crossref] [PubMed]
- Roy A, Lichtiger S. Clostridium difficile Infection: A Rarity in Patients Receiving Chronic Antibiotic Treatment for Crohn's Disease. Inflamm Bowel Dis 2016;22:648-53. [Crossref] [PubMed]
- Selby W, Pavli P, Crotty B, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn's disease. Gastroenterology 2007;132:2313-9. [Crossref] [PubMed]
- Chamberlin W. Importance of the Australian Crohn's disease antibiotic study. Gastroenterology 2007;133:1744-5; author reply 1745-6.
- Gitlin L, Biesecker J. Australian Crohn's antibiotic study opens new horizons. Gastroenterology 2007;133:1743-4; author reply 1745-6.
- Lipton JE, Barash DP. Flawed Australian CD study does not end MAP controversy. Gastroenterology 2007;133:1742; author reply 1745-6.
- Kuenstner JT. The Australian antibiotic trial in Crohn's disease: alternative conclusions from the same study. Gastroenterology 2007;133:1742-3; author reply 1745-6.
- Zanetti S, Molicotti P, Cannas S, et al. "In vitro" activities of antimycobacterial agents against Mycobacterium avium subsp. paratuberculosis linked to Crohn's disease and paratuberculosis. Ann Clin Microbiol Antimicrob 2006;5:27. [Crossref] [PubMed]
- Behr MA, Hanley J. Antimycobacterial therapy for Crohn's disease: a reanalysis. Lancet Infect Dis 2008;8:344. [Crossref] [PubMed]
- Prantera C, Zannoni F, Scribano ML, et al. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 1996;91:328-32. [PubMed]
- Prantera C, Lochs H, Campieri M, et al. Antibiotic treatment of Crohn's disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther 2006;23:1117-25. [Crossref] [PubMed]
- Prantera C, Kohn A, Mangiarotti R, et al. Antimycobacterial therapy in Crohn's disease: results of a controlled, double-blind trial with a multiple antibiotic regimen. Am J Gastroenterol 1994;89:513-8. [PubMed]
- Prantera C, Lochs H, Grimaldi M, et al. Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn's disease. Gastroenterology 2012;142:473-81.e4. [Crossref] [PubMed]
- Steinhart AH, Feagan BG, Wong CJ, et al. Combined budesonide and antibiotic therapy for active Crohn's disease: a randomized controlled trial. Gastroenterology 2002;123:33-40. [Crossref] [PubMed]
- Graham D. Prolonged remission in CD following treatment for Mycobacterium paratuberculosis. Gastroenterology 1995;108:A826. (AGA abstract). [Crossref]
- Leiper K, Martin K, Ellis A, et al. Clinical trial: randomized study of clarithromycin versus placebo in active Crohn's disease. Aliment Pharmacol Ther 2008;27:1233-9. [Crossref] [PubMed]
- Sutherland L, Singleton J, Sessions J, et al. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut 1991;32:1071-5. [Crossref] [PubMed]
- Arnold GL, Beaves MR, Pryjdun VO, et al. Preliminary study of ciprofloxacin in active Crohn's disease. Inflamm Bowel Dis 2002;8:10-5. [Crossref] [PubMed]
- Afdhal NH, Long A, Lennon J, et al. Controlled trial of antimycobacterial therapy in Crohn's disease. Clofazimine versus placebo. Dig Dis Sci 1991;36:449-53. [Crossref] [PubMed]
- Goodgame RW, Kimball K, Akram S, et al. Randomized controlled trial of clarithromycin and ethambutol in the treatment of Crohn's disease. Aliment Pharmacol Ther 2001;15:1861-6. [Crossref] [PubMed]
- Hoekman DR, Zeevenhooven J, D'Haens GR, et al. The prevalence of irritable bowel syndrome-type symptoms in inflammatory bowel disease patients in remission. Eur J Gastroenterol Hepatol 2017;29:1086-90. [Crossref] [PubMed]
- Ricci JEJ, Chebli LA, Ribeiro TC, et al. Small-Intestinal Bacterial Overgrowth is Associated With Concurrent Intestinal Inflammation But Not With Systemic Inflammation in Crohn's Disease Patients. J Clin Gastroenterol 2018;52:530-6. [PubMed]
- Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis 2009;15:778-88. [Crossref] [PubMed]
- Cash BD. Emerging role of probiotics and antimicrobials in the management of irritable bowel syndrome. Curr Med Res Opin 2014;30:1405-15. [Crossref] [PubMed]
- Levine A, Turner D. Combined azithromycin and metronidazole therapy is effective in inducing remission in pediatric Crohn's disease. J Crohns Colitis 2011;5:222-6. [Crossref] [PubMed]
- Chamberlin W, Ghobrial G, Chehtane M, et al. Successful treatment of a Crohn's disease patient infected with bacteremic Mycobacterium paratuberculosis. Am J Gastroenterol 2007;102:689-91. [Crossref] [PubMed]
- Elliott PR, Moore GT, Bell SJ, et al. Severe recurrent Crohn's disease of the ileocolonic anastomosis disappearing completely with antibacterial therapy. Gut 2005;54:1818-9. [Crossref] [PubMed]
- Shafran I, Kugler L, El-Zaatari FA, et al. Open clinical trial of rifabutin and clarithromycin therapy in Crohn's disease. Dig Liver Dis 2002;34:22-8. [Crossref] [PubMed]
- Shafran I, Johnson LK. An open-label evaluation of rifaximin in the treatment of active Crohn's disease. Curr Med Res Opin 2005;21:1165-9. [Crossref] [PubMed]
- Leiper K, Morris AI, Rhodes JM. Open label trial of oral clarithromycin in active Crohn's disease. Aliment Pharmacol Ther 2000;14:801-6. [Crossref] [PubMed]
- Greenbloom SL, Steinhart AH, Greenberg GR. Combination ciprofloxacin and metronidazole for active Crohn's disease. Can J Gastroenterol 1998;12:53-6. [Crossref] [PubMed]
- Gui GP, Thomas PR, Tizard ML, et al. Two-year-outcomes analysis of Crohn's disease treated with rifabutin and macrolide antibiotics. J Antimicrob Chemother 1997;39:393-400. [Crossref] [PubMed]
- Hampson SJ, Parker MC, Saverymuttu SH, et al. Quadruple antimycobacterial chemotherapy in Crohn's disease: results at 9 months of a pilot study in 20 patients. Aliment Pharmacol Ther 1989;3:343-52. [Crossref] [PubMed]
- Ledder O, Catto-Smith AG, Oliver MR, et al. Clinical patterns and outcome of early-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2014;59:562-4. [Crossref] [PubMed]
- Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology 1995;108:1617-21. [Crossref] [PubMed]
- Aberra FN, Brensinger CM, Bilker WB, et al. Antibiotic use and the risk of flare of inflammatory bowel disease. Clin Gastroenterol Hepatol 2005;3:459-65. [Crossref] [PubMed]
- Rutgeerts P, Van Assche G, Vermeire S, et al. Ornidazole for prophylaxis of postoperative Crohn's disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2005;128:856-61. [Crossref] [PubMed]
- Mañosa M, Cabre E, Bernal I, et al. Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn's disease: a randomized, double-blind, placebo-controlled trial. Inflamm Bowel Dis 2013;19:1889-95. [PubMed]
- Herfarth HH, Katz JA, Hanauer SB, et al. Ciprofloxacin for the prevention of postoperative recurrence in patients with Crohn's disease: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 2013;19:1073-9. [Crossref] [PubMed]
- Campieri M. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn’s disease: A randomized controlled study versus mesalazine. Gastroenterology 2000;118:A781. [Crossref]
- Doherty G, Bennett G, Patil S, et al. Interventions for prevention of post-operative recurrence of Crohn's disease. Cochrane Database Syst Rev 2009.CD006873. [PubMed]
- Rutgeerts P, Geboes K, Vantrappen G, et al. Rifabutin and ethambutol do not help recurrent Crohn's disease in the neoterminal ileum. J Clin Gastroenterol 1992;15:24-8. [Crossref] [PubMed]
- Sokol H. Probiotics and antibiotics in IBD. Dig Dis 2014;32 Suppl 1:10-7. [Crossref] [PubMed]
- Vaughn BP, Moss AC. Prevention of post-operative recurrence of Crohn's disease. World J Gastroenterol 2014;20:1147-54. [Crossref] [PubMed]
- Thia KT, Mahadevan U, Feagan BG, et al. Ciprofloxacin or metronidazole for the treatment of perianal fistulas in patients with Crohn's disease: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 2009;15:17-24. [Crossref] [PubMed]
- West RL, van der Woude CJ, Hansen BE, et al. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn's disease with infliximab: a double-blind placebo-controlled study. Aliment Pharmacol Ther 2004;20:1329-36. [Crossref] [PubMed]
- Dewint P, Hansen BE, Verhey E, et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn's disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut 2014;63:292-9. [Crossref] [PubMed]
- Dejaco C, Harrer M, Waldhoer T, et al. Antibiotics and azathioprine for the treatment of perianal fistulas in Crohn's disease. Aliment Pharmacol Ther 2003;18:1113-20. [Crossref] [PubMed]
- Maeda Y, Ng SC, Durdey P, et al. Randomized clinical trial of metronidazole ointment versus placebo in perianal Crohn's disease. Br J Surg 2010;97:1340-7. [Crossref] [PubMed]
- Su JW, Ma JJ, Zhang HJ. Use of antibiotics in patients with Crohn's disease: a systematic review and meta-analysis. J Dig Dis 2015;16:58-66. [Crossref] [PubMed]
- Panés J, Rimola J. Perianal fistulizing Crohn's disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol 2017;14:652-64. [Crossref] [PubMed]
- Gionchetti P, Rizzello F, Ferrieri A, et al. Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: a double-blind, placebo-controlled trial. Dig Dis Sci 1999;44:1220-1. [Crossref] [PubMed]
- Dickinson RJ, O'Connor HJ, Pinder I, et al. Double blind controlled trial of oral vancomycin as adjunctive treatment in acute exacerbations of idiopathic colitis. Gut 1985;26:1380-4. [Crossref] [PubMed]
- Burke DA, Axon AT, Clayden SA, et al. The efficacy of tobramycin in the treatment of ulcerative colitis. Aliment Pharmacol Ther 1990;4:123-9. [Crossref] [PubMed]
- Turunen UM, Farkkila MA, Hakala K, et al. Long-term treatment of ulcerative colitis with ciprofloxacin: a prospective, double-blind, placebo-controlled study. Gastroenterology 1998;115:1072-8. [Crossref] [PubMed]
- Mantzaris GJ, Hatzis A, Kontogiannis P, et al. Intravenous tobramycin and metronidazole as an adjunct to corticosteroids in acute, severe ulcerative colitis. Am J Gastroenterol 1994;89:43-6. [PubMed]
- Mantzaris GJ, Petraki K, Archavlis E, et al. A prospective randomized controlled trial of intravenous ciprofloxacin as an adjunct to corticosteroids in acute, severe ulcerative colitis. Scand J Gastroenterol 2001;36:971-4. [Crossref] [PubMed]
- Mantzaris GJ, Archavlis E, Christoforidis P, et al. A prospective randomized controlled trial of oral ciprofloxacin in acute ulcerative colitis. Am J Gastroenterol 1997;92:454-6. [PubMed]
- Ohkusa T, Nomura T, Terai T, et al. Effectiveness of antibiotic combination therapy in patients with active ulcerative colitis: a randomized, controlled pilot trial with long-term follow-up. Scand J Gastroenterol 2005;40:1334-42. [Crossref] [PubMed]
- Ohkusa T, Kato K, Terao S, et al. Newly developed antibiotic combination therapy for ulcerative colitis: a double-blind placebo-controlled multicenter trial. Am J Gastroenterol 2010;105:1820-9. [Crossref] [PubMed]
- Petersen AM, Mirsepasi H, Halkjaer SI, et al. Ciprofloxacin and probiotic Escherichia coli Nissle add-on treatment in active ulcerative colitis: a double-blind randomized placebo controlled clinical trial. J Crohns Colitis 2014;8:1498-505. [Crossref] [PubMed]
- Terao S, Yamashiro K, Tamura I, et al. Antibiotic combination therapy for steroid withdrawal in steroid-dependent ulcerative colitis. Digestion 2011;83:198-203. [Crossref] [PubMed]
- Turner D, Levine A, Kolho KL, et al. Combination of oral antibiotics may be effective in severe pediatric ulcerative colitis: a preliminary report. J Crohns Colitis 2014;8:1464-70. [Crossref] [PubMed]
- Kato K, Ohkusa T, Terao S, et al. Adjunct antibiotic combination therapy for steroid-refractory or -dependent ulcerative colitis: an open-label multicentre study. Aliment Pharmacol Ther 2014;39:949-56. [Crossref] [PubMed]
- Uehara T, Kato K, Ohkusa T, et al. Efficacy of antibiotic combination therapy in patients with active ulcerative colitis, including refractory or steroid-dependent cases. J Gastroenterol Hepatol 2010;25 Suppl 1:S62-6. [Crossref] [PubMed]
- Peppercorn MA. Are antibiotics useful in the management of nontoxic severe ulcerative colitis? J Clin Gastroenterol 1993;17:14-7. [Crossref] [PubMed]
- Gupta V, Rodrigues R, Nguyen D, et al. Adjuvant use of antibiotics with corticosteroids in inflammatory bowel disease exacerbations requiring hospitalisation: a retrospective cohort study and meta-analysis. Aliment Pharmacol Ther 2016;43:52-60. [Crossref] [PubMed]
- Rahimi R, Nikfar S, Rezaie A, et al. A meta-analysis of antibiotic therapy for active ulcerative colitis. Dig Dis Sci 2007;52:2920-5. [Crossref] [PubMed]
- Turner D, Vlamakis H, Marcus D, et al. Manipulating the microbiome in pediatric Acute Severe Colitis with antibiotics cocktail: a pilot randomized controlled trial. ESPGHAN 51st Annual meeting 9-12 May, 2018.
- Lobo AJ, Burke DA, Sobala GM, et al. Oral tobramycin in ulcerative colitis: effect on maintenance of remission. Aliment Pharmacol Ther 1993;7:155-8. [Crossref] [PubMed]
- Uhlig HH, Schwerd T, Koletzko S, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014;147:990-1007.e3. [Crossref] [PubMed]
- Lev-Tzion R, Ledder O, Shteyer E, et al. Oral Vancomycin and Gentamicin for Treatment of Very Early Onset Inflammatory Bowel Disease. Digestion 2017;95:310-3. [Crossref] [PubMed]
- Dogan B, Scherl E, Bosworth B, et al. Multidrug resistance is common in Escherichia coli associated with ileal Crohn's disease. Inflamm Bowel Dis 2013;19:141-50. [Crossref] [PubMed]
- Beckler DR, Elwasila S, Ghobrial G, et al. Correlation between rpoB gene mutation in Mycobacterium avium subspecies paratuberculosis and clinical rifabutin and rifampicin resistance for treatment of Crohn's disease. World J Gastroenterol 2008;14:2723-30. [Crossref] [PubMed]
- Isaacs KL, Lewis JD, Sandborn WJ, et al. State of the art: IBD therapy and clinical trials in IBD. Inflamm Bowel Dis 2005;11 Suppl 1:S3-12. [Crossref] [PubMed]
- Greenberg GR. Antibiotics should be used as first-line therapy for Crohn's disease. Inflamm Bowel Dis 2004;10:318-20. [Crossref] [PubMed]


