
Single high-dose oral vitamin D3 treatment in New Zealand children with inflammatory bowel disease
Introduction
The inflammatory bowel diseases (IBD) are chronic and incurable inflammatory diseases of the gastrointestinal tract characterized by relapsing and remitting inflammation (1). IBD includes the conditions known as Crohn’s disease (CD), ulcerative colitis (UC) and IBD unclassified (IBD-U). The Canterbury region in New Zealand (NZ) has one of the highest incidence rates worldwide of IBD (2,3).
Vitamin D is a fat soluble vitamin, which is now known to have immunomodulatory properties in addition to its role in calcium and phosphorous metabolism (4,5), following the discovery of vitamin D receptors on many immunological cells in the 1980s (5-7). There is now considerable evidence that vitamin D is involved in B and T cell-mediated, and innate immunity (4,8-10).
Vitamin D deficiency may also contribute to IBD pathogenesis (4,10). A geographical gradient in IBD incidence has been previously demonstrated, with higher incidence reported at higher latitudes where there is less sunlight exposure (4,11). A recent epidemiological study reported higher rates of pediatric IBD in the South Island than in northern regions of NZ (3). IBD pathogenesis involves dysregulation of cell-mediated immunity, therefore considering the role of vitamin D in the immune system and geographical variation in epidemiology, studies have investigated an association between low vitamin D levels and pathogenesis (4,10). Vitamin D deficiency may occur in individuals with IBD secondary to malabsorption, dietary changes and less outdoor physical activity (4). Furthermore, low vitamin D levels have been associated with increased disease activity and clinical relapse of IBD (12-18). Studies identifying seasonal variation in IBD, with flares of disease and symptoms most common at the end of winter, provide further evidence for vitamin D status influencing clinical outcomes (19,20). Vitamin D supplementation was recently associated with reduced markers of interstitial inflammation in patients with active UC and vitamin D deficiency (21), and changes in the intestinal bacterial composition in CD (22).
The safety of high-dose oral vitamin D3 therapy, referred to as stoss therapy, has been demonstrated in children with vitamin D deficiency (23,24) and chronic disease (25-27). Stoss therapy has recently been described in IBD, with one previous report demonstrating the efficacy and safety of stoss therapy in children with IBD in a single center in Australia (28). The aim of this study was to retrospectively assess the efficacy, safety and impact on disease activity of single oral high-dose vitamin D3 therapy in NZ children with IBD and vitamin D deficiency.
Methods
Patient selection
A retrospective chart review was performed of children with IBD seen in the pediatric IBD clinic in Christchurch, New Zealand from 2011 to 2015. Inclusion criteria were diagnosis with vitamin D deficiency [serum 25-OH vitamin D level (25-OHD) <50 nmol/L] and management with a single oral high-dose of vitamin D3 (stoss therapy). Exclusion criteria were known disorders of calcium, parathyroid or bone health. Demographic data and disease characteristics were obtained from patient notes.
Stoss dosing and monitoring protocol
The stoss dosing regimen involved a single dose of oral cholecalciferol 100,000 to 800,000 IU provided as 50,000 IU capsules, using an age-based dosing schedule (28). The routine monitoring protocol following stoss specified measurement of serum calcium 1 to 2 weeks after stoss, with vitamin D measurement 1 month post-stoss, and again 3 months post-stoss along with standard blood monitoring tests.
The results of serum 25-OHD levels prior to and following stoss were retrieved from hospital records. Post-stoss 25-OHD levels were grouped at two time points: 1–2 months and between 3–6 months. Calcium levels after stoss therapy were obtained when available. The results of routine monitoring blood tests, including erythrocyte sedimentation rate (ESR), C reactive protein (CRP), albumin, hematocrit and platelets, were also obtained prior to and three months after stoss therapy when available. Disease activity was assessed at the time of diagnosis of vitamin D deficiency and at 3 months following stoss therapy, using the Pediatric Crohn’s Disease Activity Index (PCDAI) (29).
Analysis
Statistical analysis was performed using GraphPad Prism for Windows version 7 (GraphPad Software, La Jolla, CA, USA). The paired sample t-test was used to compare continuous parametric variables, and non-parametric variables following logarithmic transformation. A P value ≤0.05 was considered significant. Children with missing data were excluded pairwise for inflammatory markers.
Results
Participant characteristics
Twenty-eight doses of stoss were given to 23 children. Five children received repeated doses of stoss. The mean age at first stoss dose was 12 years and 4 months (range, 3 years 1 month to 16 years 7 months), and 73.9% of subjects were male (17/23). CD was the most common type of IBD (82.6%, 19/23) (Table 1).
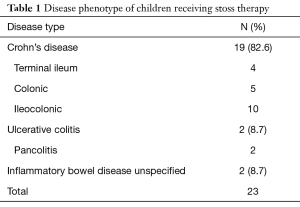
Full table
Response to high-dose oral vitamin D3 therapy
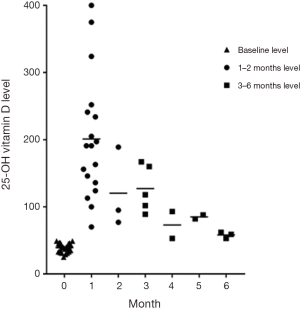
The mean 25-OHD level at baseline was 39 nmol/L (95% CI: 37–42 nmol/L, n=28). Mean 25-OHD levels increased 1–2 months after stoss to 189 nmol/L (95% CI: 148–231 nmol/L, n=21, P<0.001). All 25-OHD levels measured at one month were >75 nmol/L. The first two 25-OHD levels measured per participant at 1–2 and 3–6 months post-stoss were reviewed (Figure 1).
Serum calcium was measured after stoss therapy on 20 occasions: nineteen measurements were in the normal range. Although one child was found to have a serum calcium of 2.7 (normal range, 2.2 to 2.6 mmol/L) two weeks after treatment, this level had normalized on repeat testing ten days later. This child had a peak 25-OHD level of 77 nmol/L (2 months after stoss). No children presented with symptoms of vitamin D toxicity. The four children with 25-OHD levels >250 nmol/L had normal serum calcium levels after stoss.
Disease activity and serum inflammatory markers
There was a significant difference in mean PCDAI scores from 10.6 (95% CI: 4.9–16.3) pre-stoss to 3.8 (1.5–6.0) three months post-stoss treatment (n=28, P=0.026). Ten of the twelve children with PCDAI scores ≥10 at baseline (mild disease activity) had a reduction in disease activity scores after stoss. The mean blood platelet counts also decreased following stoss (395×109/L, 95% CI: 330–459×109/L, versus 350×109/L, 95% CI: 306–394×109/L, n=26, P=0.016). Additionally, ESR reduced three months following stoss from mean 17.6 mm/h (3.2–31.9 mm/h) to 6.7 mm/h (4.1–9.4 mm/h, n=11, P=0.017), and CRP reduced from mean 17.8 mg/L (1.5–34.1 mg/L) to 3.1 mg/L (1.1–5.1 mg/L, n=23, P=0.035). In contrast, there were no differences between baseline and follow-up hematocrit or albumin levels (data not shown).
Discussion
Single high-dose vitamin D therapy successfully increased serum 25-OHD levels at 1–2 months in these vitamin D deficient children with IBD. Furthermore, all children with serum 25-OHD measured at 1 month achieved a level greater than 75 nmol/L. Stoss therapy was well tolerated with no adverse effects seen. A reduction in markers of disease activity was observed 3 months following stoss therapy.
Stoss therapy is a novel treatment for vitamin D deficiency in children with IBD, with only one study reporting its use (28). Although mean serum 25-OHD were similar at baseline to the previous Australian study (28), the mean level achieved at 1–2 months appeared to be higher (189 nmol/L, 95% CI: 148–231) than the 1 month levels in the Australian data (146 nmol/L, 95% CI: 94–197) (28). The Australian study included a larger number of children and also demonstrated that stoss was able to maintain normal vitamin D status for 6 months in the majority of the subjects (28).
High-dose oral vitamin D has been shown to be safe and effective at rapidly increasing serum 25-OHD levels in vitamin D deficiency (23,30,31). Stoss therapy has been used successfully in children with cystic fibrosis (25), chronic kidney disease (26) and prior to hematopoietic stem cell transplant (27). A recent study found that in children with chronic liver disease and vitamin D deficiency, weekly vitamin D supplementation was more effective than a stoss regimen (32). Providing single dose vitamin D therapy may improve compliance (31) and emerging data also suggest that high doses may have benefits beyond bone health (24,33). Despite a recent increase in publications describing the use of stoss, the majority of studies remain limited by sample size and larger studies are still required needed to further assess the efficacy of stoss therapy in children (24,34).
Vitamin D toxicity can manifest as hypercalcemia and hypercalciuria, which can lead to nephrocalcinosis (5,34). Toxicity is rarely reported in children with cases described in children taking doses within a range of 40,000 to 560,000 IU/kg (35). In the present study, one child had a single marginally raised serum calcium level 2 weeks following therapy, however the follow-up level 10 days later was normal, the child remained well and the 2-month 25-OHD level was not substantially raised. All four of the children who had 25-OHD levels >250 nmol/L at 1 month, which is a level previously suggested as a safe limit (35,36), had normal serum calcium levels. None of the children in the current study reported symptoms of toxicity. Similarly, stoss was safely administered in the Australian study, with no record of adverse effects (28).
The finding of reduced PCDAI scores and serum inflammatory markers following stoss therapy suggests further potential beneficial effects of vitamin D beyond bone health in IBD (13,14,16,17,37). A recent UK study reported reduced platelet counts, increased albumin and reduced fecal calprotectin levels in eight adults with UC following weekly cholecalciferol therapy (21). Studies in adults have also shown associations between low vitamin D levels and increased disease activity in IBD (13-15,18,38). One report indicated a relationship between low vitamin D and higher CRP in CD (13). Lower quality of life scores in adults with IBD have also been linked vitamin D deficiency (37). There are fewer studies including children. However, Samson et al. (39) previously reported an association between increased frequency of inactive disease and both higher 25-OHD levels and vitamin D supplementation, and Pappa et al. (40) found an association between low serum 25-OHD and higher ESR in children and young people with IBD. Syed et al. (41) also recently reported reduced serum hemoglobin and increased hepcidin in vitamin D insufficient children with IBD. The Australian study of stoss therapy in children found a significant increase in serum hemoglobin and albumin levels 6 months following stoss, but in contrast to the present data serum CRP or platelet levels did not change (28).
Vitamin D deficiency is prevalent in NZ children (42,43). Seventy-eight percent of 55 children aged 12–22 months had serum 25-OHD ≤50 nmol/L during winter months in one study in the South Island of NZ (42). Another report demonstrated that 31% of 1,585 children aged 5–14 years had serum 25-OHD <37.5 nmol/L (43). Rates of vitamin D deficiency in NZ children with IBD have not yet been defined, although NZ guidelines recommend yearly screening for vitamin D insufficiency (44). Recently published European guidelines also recommend routine surveillance of vitamin D status in children with IBD (45). In an Australian study, 38% of 78 of children with IBD had 25-OHD levels ≤50 nmol/L (46). Globally, a recent systematic review summarized 21 studies assessing vitamin D status in pediatric IBD (47). Although 25-OHD laboratory cut-off values varied widely between studies, vitamin D deficiency was found commonly, but appeared to occur more frequently in countries further from the equator, and in African-American compared with Caucasian children (47). A recent Canadian study found that 33% (48) of children with IBD had 25OH-D <50 nmol/L, whilst a USA study reported 62% of children with IBD had 25-OHD <30 nmol/L, although notably 75% of controls also had 25-OHD <30 nmol/L (49).
Optimal serum 25-OHD levels are not well defined. The Australian and New Zealand Bone and Mineral Society and Osteoporosis Australia position statement recommend levels in children should be maintained at ≥50 nmol/L (50), although definitions of vitamin D deficiency and insufficiency remain controversial (36). Vieth et al. (51) have shown that levels of 75 nmol/L are associated with maximal enteral calcium absorption, suggesting optimal bone health with levels at or greater than this. There is increasing evidence of effects beyond bone health, with levels ≥75 nmol/L associated with reduced disease activity and better health outcomes in the setting of IBD (38). In this present study, all children with 25-OHD measured at 1 month achieved a level >75 nmol/L, comparable with the Australian study which reported that following 98% of doses levels >75 were achieved at 1 month (28).
Studies have also evaluated other oral vitamin D regimens in IBD. A USA study found that daily vitamin D3 2,000 IU or weekly vitamin D2 50,000 IU resulted in higher serum 25-OHD levels at 6 weeks compared with daily vitamin D2 2,000 IU (52). A further study by the same group compared low dose (400 IU daily) to higher dose (1,000 IU daily summer/autumn, 2,000 IU daily winter/spring) vitamin D2 in children with IBD and reported that neither regimen successfully maintained serum 25-OHD levels of ≥32 ng/mL (80 nmol/L) over 12 months (53). Comparative studies between stoss and other regimens have not yet been undertaken.
The current study was limited by the number of subjects included and the retrospective design, with consequent incomplete availability of monitoring tests which had been completed at variable times. However, all children in this study received consistent care from one gastroenterology clinic and were managed with the same stoss protocol, and these are some of the only data available to assess stoss in children with IBD.
Single high-dose oral vitamin D therapy was used successfully to manage vitamin D insufficiency in these children with IBD, with children achieving serum 25-OHD levels of >75 nmol/L at 1 month post-therapy. An improvement in inflammatory markers and disease activity scores was also apparent in this group of children. These data confirm and support the conclusions drawn from the earlier report of stoss therapy in children with IBD (28). Further prospective studies are now required to assess the impact of vitamin D therapy as an adjunctive therapy in children with IBD and to clearly establish an optimal dosing regimen.
Acknowledgements
The support of Cure Kids, New Zealand is acknowledged.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the University of Otago Human Ethics Committee (Health), number HD16/078, as a minimal risk project. The Ethics Committee approval permitted access to past health records without individual patient consent required.
References
- Griffiths AM, Buller HB. Inflammatory Bowel Diseases. In: Walker WA, Durie PR, Hamilton JH, editors. Pediatric gastrointestinal disease. Pathophysiology, diagnosis, management. 3rd edition. Hamilton Ontario: BC Decker, 2000:613-52.
- Gearry RB, Richardson A, Frampton CM, et al. High incidence of Crohn's disease in Canterbury, New Zealand: results of an epidemiologic study. Inflamm Bowel Dis 2006;12:936-43. [Crossref] [PubMed]
- Lopez RN, Appleton L, Gearry RB, et al. Rising Incidence of Paediatric Inflammatory Bowel Disease in Canterbury, New Zealand, 1996-2015. J Pediatr Gastroenterol Nutr 2018;66:e45-50. [Crossref] [PubMed]
- Limketkai BN, Mullin GE, Limsui D, et al. Role of Vitamin D in Inflammatory Bowel Disease. Nutr Clin Pract 2017;32:337-45. [Crossref] [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [Crossref] [PubMed]
- Baeke F, Korf H, Overbergh L, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol 2010;121:221-7. [Crossref] [PubMed]
- Provvedini DM, Tsoukas CD, Deftos LJ, et al. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983;221:1181-3. [Crossref] [PubMed]
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770-3. [Crossref] [PubMed]
- Palmer MT, Lee YK, Maynard CL, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem 2011;286:997-1004. [Crossref] [PubMed]
- Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology 2012;142:482-9. [Crossref] [PubMed]
- Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013;62:630-49. [Crossref] [PubMed]
- Nielsen OH, Rejnmark L, Moss AC. Role of Vitamin D in the Natural History of Inflammatory Bowel Disease. J Crohns Colitis 2018;12:742-52. [Crossref] [PubMed]
- Jorgensen SP, Hvas CL, Agnholt J, et al. Active Crohn's disease is associated with low vitamin D levels. J Crohns Colitis 2013;7:e407-13. [Crossref] [PubMed]
- Ham M, Longhi MS, Lahiff C, et al. Vitamin D levels in adults with Crohn's disease are responsive to disease activity and treatment. Inflamm Bowel Dis 2014;20:856-60. [Crossref] [PubMed]
- Dolatshahi S, Pishgar E, Jamali R. Does serum 25 hydroxy vitamin D level predict disease activity in ulcerative colitis patients? Acta Clin Belg 2016;71:46-50. [Crossref] [PubMed]
- Gubatan J, Mitsuhashi S, Zenlea T, et al. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2017;15:240-6.e1. [Crossref] [PubMed]
- Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn's disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 2010;32:377-83. [Crossref] [PubMed]
- Blanck S, Aberra F. Vitamin d deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci 2013;58:1698-702. [Crossref] [PubMed]
- Aratari A, Papi C, Galletti B, et al. Seasonal variations in onset of symptoms in Crohn's disease. Dig Liver Dis 2006;38:319-23. [Crossref] [PubMed]
- Lewis JD, Aberra FN, Lichtenstein GR, et al. Seasonal variation in flares of inflammatory bowel disease. Gastroenterology 2004;126:665-73. [Crossref] [PubMed]
- Garg M, Hendy P, Ding JN, et al. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J Crohns Colitis 2018;12:963-72. [Crossref] [PubMed]
- Schaffler H, Herlemann DP, Klinitzke P, et al. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn's disease patients, but not in healthy controls. J Dig Dis 2018;19:225-34. [Crossref] [PubMed]
- Kocyigit C, Catli G, Ince G, et al. Can Stoss Therapy Be Used in Children with Vitamin D Deficiency or Insufficiency without Rickets? J Clin Res Pediatr Endocrinol 2017;9:150-5. [Crossref] [PubMed]
- Nama N, Menon K, Iliriani K, et al. A systematic review of pediatric clinical trials of high dose vitamin D. PeerJ 2016;4:e1701. [Crossref] [PubMed]
- Shepherd D, Belessis Y, Katz T, et al. Single high-dose oral vitamin D3 (stoss) therapy--a solution to vitamin D deficiency in children with cystic fibrosis? J Cyst Fibros 2013;12:177-82. [Crossref] [PubMed]
- Deveci M, Aytac MB, Altun G, et al. Effect of high-dose oral cholecalciferol on cardiac mechanics in children with chronic kidney disease. Cardiol Young 2017;27:1807-14. [Crossref] [PubMed]
- Wallace G, Jodele S, Myers KC, et al. Single Ultra-High-Dose Cholecalciferol to Prevent Vitamin D Deficiency in Pediatric Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2018;24:1856-60. [Crossref] [PubMed]
- Shepherd D, Day AS, Leach ST, et al. Single High-Dose Oral Vitamin D3 Therapy (Stoss): A Solution to Vitamin D Deficiency in Children With Inflammatory Bowel Disease? J Pediatr Gastroenterol Nutr 2015;61:411-4. [Crossref] [PubMed]
- Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr 1991;12:439-47. [Crossref] [PubMed]
- Cipriani C, Romagnoli E, Pepe J, et al. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: implications for treatment and prophylaxis. J Clin Endocrinol Metab 2013;98:2709-15. [Crossref] [PubMed]
- Tan JK, Kearns P, Martin AC, et al. Randomised controlled trial of daily versus stoss vitamin D therapy in Aboriginal children. J Paediatr Child Health 2015;51:626-31. [Crossref] [PubMed]
- Lal BB, Alam S, Khanna R, et al. Weekly regimen of vitamin D supplementation is more efficacious than stoss regimen for treatment of vitamin D deficiency in children with chronic liver diseases. Eur J Pediatr 2018;177:827-34. [Crossref] [PubMed]
- Abrams SA, Coss-Bu JA, Tiosano D. Vitamin D: effects on childhood health and disease. Nat Rev Endocrinol 2013;9:162-70. [Crossref] [PubMed]
- Munns CF, Shaw N, Kiely M, et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab 2016;101:394-415. [Crossref] [PubMed]
- Vogiatzi MG, Jacobson-Dickman E, DeBoer MD. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab 2014;99:1132-41. [Crossref] [PubMed]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-30. [Crossref] [PubMed]
- Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr 2011;35:308-16. [Crossref] [PubMed]
- Raftery T, O'Sullivan M. Optimal vitamin D levels in Crohn's disease: a review. Proc Nutr Soc 2015;74:56-66. [Crossref] [PubMed]
- Samson CM, Morgan P, Williams E, et al. Improved outcomes with quality improvement interventions in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2012;55:679-88. [Crossref] [PubMed]
- Pappa HM, Langereis EJ, Grand RJ, et al. Prevalence and risk factors for hypovitaminosis D in young patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2011;53:361-4. [PubMed]
- Syed S, Michalski ES, Tangpricha V, et al. Vitamin D Status Is Associated with Hepcidin and Hemoglobin Concentrations in Children with Inflammatory Bowel Disease. Inflamm Bowel Dis 2017;23:1650-8. [Crossref] [PubMed]
- Houghton LA, Szymlek-Gay EA, Gray AR, et al. Predictors of vitamin D status and its association with parathyroid hormone in young New Zealand children. Am J Clin Nutr 2010;92:69-76. [Crossref] [PubMed]
- Rockell JE, Green TJ, Skeaff CM, et al. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5-14 y. J Nutr 2005;135:2602-8. [Crossref] [PubMed]
- Day AS. Paediatric Gastroenterology Clinical Network: Management of Inflammatory Bowel Disease in Children and Adolescents in New Zealand, A Clinical Guideline. 2014. Available online: https://www.starship.org.nz/media/256562/nz_ibd_clinical_guideline_aug_2015.pdf. Accessed 29 August 2018.
- Miele E, Shamir R, Aloi M, et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;66:687-708. [Crossref] [PubMed]
- Levin AD, Wadhera V, Leach ST, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci 2011;56:830-6. [Crossref] [PubMed]
- Fritz J, Walia C, Elkadri A, et al. A Systematic Review of Micronutrient Deficiencies in Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Wingate KE, Jacobson K, Issenman R, et al. 25-Hydroxyvitamin D concentrations in children with Crohn's disease supplemented with either 2000 or 400 IU daily for 6 months: a randomized controlled study. J Pediatr 2014;164:860-5. [Crossref] [PubMed]
- Alkhouri RH, Hashmi H, Baker RD, et al. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2013;56:89-92. [Crossref] [PubMed]
- Paxton GA, Teale GR, Nowson CA, et al. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: a position statement. Med J Aust 2013;198:142-3. [Crossref] [PubMed]
- Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007;85:649-50. [Crossref] [PubMed]
- Pappa HM, Mitchell PD, Jiang H, et al. Treatment of vitamin D insufficiency in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing three regimens. J Clin Endocrinol Metab 2012;97:2134-42. [Crossref] [PubMed]
- Pappa HM, Mitchell PD, Jiang H, et al. Maintenance of optimal vitamin D status in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing two regimens. J Clin Endocrinol Metab 2014;99:3408-17. [Crossref] [PubMed]


