Continuum of care in pediatric sepsis: a prototypical acute care delivery model
Background
Sepsis is a significant public health burden and a leading cause of morbidity and mortality among infants and children across the world (1). Over the past decade, the prevalence of pediatric severe sepsis has increased from 6.2% to 7.7% (2). While mortality from pediatric sepsis has decreased over time, it continues to be high, estimated to be 9% for all-cause mortality, and as high as 25% for those patients admitted to the intensive care unit (ICU). Among sepsis survivors, approximately one-fifth have at least moderate disability (1). Pediatric sepsis is also associated with significant resource utilization and accounts for $4.8 billion in healthcare costs in the United States (3).
Since 2002, the American College of Critical Care Medicine (ACCM) has published clinical practice parameters for hemodynamic support in pediatric and neonatal septic shock (4-6). The significant change in the 2014 update (published in 2017) is the recommendation for an institutional approach to hemodynamic support of septic shock rather than one aimed at the individual practitioner (6). It underscores the coordinated delivery of care at various levels and across settings, including the community, pre-hospital setting, emergency department (ED), hospital ward, and ICU.
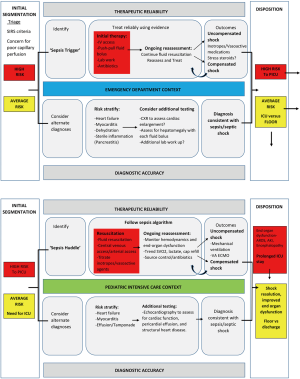
The acute care model was proposed as a construct for patients presenting to the ED with acute illness as a way to guide quality improvement of emergency care and patient outcomes (7). Four components define the acute care model: ‘segmentation’ refers to the sequential triage of patients during their evaluation and management; ‘therapeutic reliability’ refers to the standardized approach of delivering evidenced-based care that is safe, effective and timely; ‘diagnostic accuracy’ is the ability to efficiently discern the correct diagnosis for patients with some diagnostic uncertainty; and lastly, ‘disposition’ is the appropriate discharge or transfer of patients based on their initial segmentation, ongoing evaluation, and response to therapies (7).
Consistent early recognition and the timely delivery of known therapeutic interventions in pediatric sepsis remains a significant challenge (8). A subset of patients present with obvious signs of septic shock, making the initial segmentation and efficient therapeutic interventions relatively straight-forward. Others will present with more subtle signs and/or with an apparent diagnosis such as bronchiolitis or acute gastroenteritis. In patients with diagnostic uncertainty, the application of the acute care model with its iterative assessments, testing, therapies, and ongoing segmentation, may enable timely diagnosis and treatment. The continuum of care for pediatric sepsis patients often involves coordination between the ED, hospital ward and ICU. Sepsis management often continues as the patient moves from the ED to the inpatient unit. Additionally, there is a yet unquantified number of patients who develop hospital onset sepsis. Thus, management of pediatric patients with sepsis represent a prototype for the extension of the acute care model to the inpatient setting, including the ICU. In this review, we present an illustrative case highlighting opportunities to provide seamless transition of care for patients, summarize current evidence-based guidelines for the management of pediatric sepsis patients, and provide examples of the application of the acute care model.
Case presentation
A 6-year-old female with cerebral palsy and a history of recurrent urinary tract infections is brought to the ED’s shock trauma room. She was initially seen in urgent care and noted to have a fever to 39.4 °C, heart rate of 164 beats per minute, and respiratory rate of 36 breaths per minute. Her skin is cool to the touch and capillary refill is 5 seconds. Her vital signs triggered a rapid assessment for sepsis. She received rapid isotonic fluid resuscitation totaling 40 mL/kg as push-pull boluses. Point of care blood gas with lactate showed metabolic acidosis with a lactate of 4 mmol/L. Her renal profile was significant for a rise in creatinine to 0.64 mg/dL, compared to prior recorded value of 0.28 mg/dL. Blood and urine cultures were collected, and procalcitonin level was 8.4 ng/mL. The patient received ceftriaxone and vancomycin within 1 hour of arrival to the ED. Given her illness severity, the patient was admitted to the ICU for continued care.
In the ICU, the patient was no longer febrile, but she was persistently tachycardic, tachypneic, and had delayed capillary refill. She received an additional 20 mL/kg of isotonic fluids. Epinephrine was started via peripheral intravenous access. Repeat serum lactate was 6 mmol/L. She was intubated to decrease oxygen demand, and central venous access was obtained. A Foley catheter was placed to monitor urine output. Broad spectrum antibiotics were continued pending culture results. Echocardiography was performed and revealed mild systolic dysfunction.
Over the course of the following 48 hours, her serum lactate was monitored and showed improvement to 1.2 mmol/L. Vasoactive agents were discontinued and she was extubated. Blood cultures resulted positive for gram positive cocci bacteria in pairs, with growth of streptococcus pneumoniae, sensitive to ceftriaxone. The patient’s end-organ dysfunction resolved with improvement in urine output and decreased creatinine to 0.38 mg/dL. On ICU day 4 she was transferred to hospital medicine to complete antibiotic therapy. She was discharged from the hospital on day 9 of admission.
Definitions
In 2005, the international pediatric sepsis conference led to the development of the first consensus definition for pediatric sepsis to standardize enrollment into research studies (9). While adult definitions have seen multiple iterations (10), pediatric sepsis definitions remain largely unchanged and rely on systemic inflammatory responses syndrome (SIRS) criteria and clinical and laboratory markers of organ dysfunction. Sepsis is defined as SIRS due to or in the presence of suspected or known infection. Severe sepsis is defined as sepsis with cardiovascular dysfunction, or acute respiratory distress syndrome, or ≥2 other organ dysfunctions. Septic shock is defined as sepsis with cardiovascular dysfunction. The use of SIRS criteria in pediatric sepsis definitions is associated with low specificity. Recent data suggest that adaptation of the adult Sepsis-3 definitions may help better characterize pediatric patients with sepsis and identify patients at highest risk of mortality (11).
Recognition
Recognition of sepsis at the bedside requires strong clinical suspicion based on history, physical examination including vital signs, and corroborative laboratory data. The diagnosis of shock is supported by clinical and laboratory markers of inadequate oxygen delivery and/or organ dysfunction. Over the past decade, there has been significant effort through quality improvement initiatives toward early recognition of sepsis and institution of time sensitive fluid resuscitation and antimicrobial administration (6). Several studies conducted in ED settings have evaluated the use of ‘sepsis triggers’ to improve recognition of pediatric sepsis, and protocols to direct initial resuscitation, and have demonstrated success (12-15). For example, at Primary Children’s Hospital in Utah, the odds of death was lower in patients receiving bundle-compliant care (odds ratio 0.2) when compared to those who did not (15). However, considerable variation in sepsis recognition tools remains between and even within institutions, and none have sufficient evidence to suggest superiority of one specific tool. In a recent comparison between an electronic algorithmic alert and physician judgement in recognizing sepsis, Balamuth and colleagues reported the algorithmic approach had a higher sensitivity (92%) in identification of pediatric sepsis patients compared to physician judgement (72%), but a lower specificity, 83% compared to 99.5%, respectively (16). Utilization of electronic health records and machine learning processes may further enhance the ability of physicians to recognize patients with sepsis earlier and direct appropriate treatments.
Resuscitation
Fluid resuscitation remains a cornerstone for management of pediatric patients with septic shock (17). A study conducted in a community hospital demonstrated that reversal of shock with fluid resuscitation was associated with nine-fold improvement in survival, and each hour delay in resuscitation was associated with two-fold increased mortality (18). Rivers et al. first described early goal directed therapy in adults, which emphasized targeting resuscitative efforts to physiological end points such as mixed venous oxygen saturation (SvO2) and lactic acid. While the original study was associated with significant decrease in mortality in adults (19), subsequent large-scale, multi-national, randomized controlled trials have failed to demonstrate similar results (20-22). It is likely that in the decade between the original Rivers trial and subsequent trials, the standard of care for patients with sepsis improved drastically. An emphasis on early recognition and resuscitation, rather than achieving set physiological criteria, likely contributed to improvements in survival among patients in the control groups (20-22). Pediatric data on goal directed therapy is limited and poorly generalizable. de Oliveira et al. and Sankar et al. have demonstrated mortality benefit among pediatric sepsis patients receiving goal directed therapy in Brazilian (23) and Indian cohorts (24) respectively. However, both studies had high rates of mortality in the control groups, and thus severely limit comparison to populations in resource-rich settings. Maitland et al. conducted the FEAST trial in Sub-Saharan Africa and reported increased mortality after rapid fluid bolus, 10.5% compared to 7.3% for those receiving only maintenance fluids (25). A significant portion of subjects in the study were noted to have severe anemia and malaria, and patients with gastroenteritis and hypovolemia were excluded. This study, while of significant value in the context of resource-poor settings, has limited external validity. Despite limitations in data, rapid serial isotonic 20 mL/kg fluid boluses up to 40–60 mL/kg, with interval assessment of perfusion and for signs of heart failure, remains the standard of care (4-6). While a full discussion on choice of fluid in septic shock is beyond the scope of this review, it is important to consider that hyperchloremia is independently associated with increased mortality in pediatric septic shock (26), and lactated ringer’s may be used as an alternative to normal saline.
Time between recognition of sepsis and antibiotic administration is a critical aspect of patient care and is often in the ED setting (27). In a recent meta-analysis which included 11 adult studies, Jones et al. reported no increase in mortality in patients receiving antibiotics ≥3 vs. <3 hours from ED triage, and no increase in mortality for those receiving antibiotics ≥1 vs. <1 hour from severe sepsis/shock recognition (28). Weiss et al. in a single center pediatric study in the PICU, reported an adjusted mortality odds ratio of 4.84 and increased risk of organ dysfunction associated with a more than 3-hour delay in administration of antibiotics (29).
Stabilization
Intensive care is focused on ongoing resuscitation and stabilization of hemodynamic parameters, with the ultimate goal of shock reversal. Proposed goals include achieving and maintaining a mean arterial pressure (MAP) >50th percentile for age, capillary refill time less than 2 seconds, and when central access is available, mixed venous saturation (SvO2) >70% and/or cardiac index (CI) of 3.3–6.0 L/min/m2 (6). Han et al. showed an increase in mortality for every hour hemodynamic goals were not met (18), and Ninis et al. had similar findings with increased mortality associated with delayed inotrope initiation (30). Based on these studies, peripheral inotropes should be initiated in fluid refractory shock until central access can be established. Both central access and an arterial line for invasive blood pressure monitoring should be placed as soon as possible (6). An internal jugular or subclavian central venous catheter is preferred as it allows for accurate measurement of SvO2 (6). In addition to fluid resuscitation and vasoactive agents, patients in septic shock with a SvO2 that remains below 70% and hemoglobin <10 g/dL may benefit from a blood transfusion to improve oxygen delivery (6).
The choice of vasoactive agent for pediatric septic shock is often driven by the patient’s initial clinical presentation. Patients with warm shock present with bounding pulses, warm extremities and often normal or flash capillary refill. These patients are in a low systemic vascular resistance (SVR) state and benefit from vasoconstrictors, such as norepinephrine (NE) or dopamine, with NE being the first line vasoactive of choice in warm shock (31). While there is no clear evidence regarding the use of vasopressin in pediatric septic shock, it may be a beneficial secondary agent in warm shock unresponsive to NE (6). In contrast, patients with cold shock present with poor distal pulses, cool extremities, and mottled skin. These patients are peripherally vasoconstricted with high SVR and high afterload. In these cases, epinephrine is usually the first choice given its inotropic effects at low doses. When cardiac output and perfusion remain poor despite inotropes, milrinone, a phosphodiesterase inhibitor, can be used to improve cardiac contractility and decrease SVR (31). Special consideration should be given to those patients who are refractory to both fluid and vasoactive agents. These patients may have adrenal insufficiency and stress dose steroids should be considered (31,32). Imaging modalities such as echocardiography (echo) may be useful in the management of septic shock. Ranjit et al. reported that incorporating an echo in the evaluation of patients with septic shock, in addition to invasive blood pressure monitoring, altered the fluid resuscitation and vasoactive therapy in 88% of patients (33).
Both clinical and laboratory parameters should be utilized as measures of adequate delivery of oxygen. A patient’s perfusion, mental status, and urine output should also be used as clinical markers to assess adequate resuscitation (6). de Oliveira et al. showed that maintaining a SvO2 >70% reduced mortality in septic shock when compared with only maintaining appropriate blood pressure and capillary refill (23). However, SvO2 measurement is invasive and requires central access in the internal jugular or subclavian vein. Serum lactate is used as a measure of resolving shock in adult patients. Extant pediatric data show utility of lactate as a marker of shock resolution, however, the data are limited by small sample sizes (34-38). In addition, false positives occur given elevated lactate is not solely found due to cellular hypoxia (39). While trending serum lactates may be helpful, the ACCM stresses early recognition of septic shock based on clinical presentation and not lactate measurement (6).
In patients with persistent shock, methods of decreasing metabolic demand, such as intubation, sedation, and paralysis should be considered early in their PICU course. Mechanical ventilation is beneficial in pediatric shock via removing work of breathing and improving oxygen delivery (31). The change to positive pressure ventilation and effects of sedation administered for intubation can cause hemodynamic instability or collapse in patients who are not properly volume resuscitated (31).
The use of veno-arterial extracorporeal membrane oxygenation (VA ECMO) as a treatment for severe septic shock is a controversial treatment that is still being investigated (40). The current recommendation from the Surviving Sepsis Campaign (SSC) is that VA ECMO should be reserved only for cases of refractory severe septic shock (grade 2C) given the high risks associated with ECMO (27). One of controversies surrounding the use of VA ECMO for septic shock is the amount of blood flow that should be used. Some studies recommend flows of 150 mL/kg/min while others recommend more conventional flows of 110 mL/kg/min (5). Oberender et al. performed an international multicenter, retrospective cohort study to determine if the use of VA ECMO in severe septic shock in pediatric patients altered morbidity, mortality, or length of ICU stay and hospital stay when compared to conventional treatments (40). Results showed no significant difference in survival to discharge between the two groups. However, those patients who suffered an in-hospital cardiac arrest secondary to sepsis and were cannulated onto VA ECMO had a 24% survival advantage. This study also showed improved survival rates when ECMO flows were targeted for 150 mL/kg/min (40).
Quality improvement in pediatric sepsis care
Bundling of care in the ED has been proposed as an effective strategy to improve outcomes in pediatric sepsis (41-43). In a recent study, Evans et al. reported that completion of a sepsis bundle within 1 hour of protocol initiation-including collection of blood cultures, delivery of antibiotics and 20 mL/kg fluid bolus, was associated with decreased mortality in pediatric patients with sepsis (odds ratio 0.59), compared to those completing the bundle over more than an hour (43). Interestingly, completion of the individual elements of the bundle were not associated with a lower odds of mortality. Several limitations of the study should be considered, including limited sample size to detect the effect of individual elements of the bundle on outcomes and the effect of confounding factors such as severity of illness that affected the ability of all elements of the bundle to be completed within an hour. Of note, only one-fourth of the patients received all elements of the bundle within one hour. The SSC guidelines reinforce the use of the one-hour bundle (44).
Three bundles are recommended by the ACCM guidelines as an institutional approach to the management of septic shock. Institutions should develop or adopt a recognition bundle, a resuscitation and stabilization bundle, and a performance bundle. The recognition bundle includes implementation of a sepsis screening tool to rapidly identify patients with suspected septic shock and then prompt a rapid clinical assessment. The resuscitation and stabilization bundle standardizes care of septic shock patients, including specific goals of resuscitation as recommended by the ACCM, and drives adherence to best practices. The performance bundle monitors and measures processes and practices, and includes efforts aimed at improving and sustaining compliance with institutional best practices (6).
As in the ED, recognition of sepsis within the inpatient setting is imperative. Similar screening tools can be adapted for use on the pediatric inpatient floor and in the ICU. Inpatient tools should include a combination of diagnoses, vital signs and/or exam findings, and patient specific findings such as high-risk conditions. When these defined criteria are met, further prompt assessment should follow. For example, a trigger tool recently deployed in our PICU uses an electronic health record (EMR) generated best practice alert (BPA) which then prompts a bedside perfusion assessment. Findings of poor perfusion activates a sepsis huddle with the medical team, bedside nurse, and respiratory therapist, and a decision is made to initiate our septic shock management algorithm. The sepsis huddle allows the team to articulate a shared mental model, identify and assign tasks and interventions, and do so in a timely manner. The use of the algorithm ensures key aspects of the evaluation and management are not missed. The algorithm is posted at the bedside, which helps the multidisciplinary treatment team stay on task during resuscitation, and helps the team anticipate next steps in management. By developing, adapting, and customizing evidence-based guidelines and care pathways, institutions may help improve delivery of pediatric sepsis care.
Application of the acute care model to pediatric sepsis
To ensure seamless delivery of care across different care contexts, an institutional approach to pediatric sepsis care in needed. Zackoff and colleagues have suggested extending the acute care model to the inpatient setting to provide a global approach to care (7,45). The four components of the acute care model, as they relate to the care of patients with pediatric sepsis are presented (Figure 1).
Segmentation
Begins with onset of care, often in the ED or on the hospital floor, and includes triage of patients with vital sign abnormalities that meet SIRS criteria, clinical assessment and laboratory evaluation, recognition of shock, and subsequent reassessment of patients after fluid resuscitation. Segmentation continues as new clinical and laboratory data emerge, and at each transition of care.
Therapeutic reliability
In a majority of patients who meet criteria for sepsis, this pathway provides a standardized approach to fluid resuscitation, collection of cultures and timely delivery of appropriate antibiotics. Patients are risk stratified based on illness severity, presence of high-risk co-morbidities, whether shock is compensated or uncompensated, and whether end-organ dysfunction is present. The process continues until disposition to the ICU versus the acute care unit is determined.
Diagnostic accuracy
In patients with diagnostic uncertainty, other causes of shock should be considered. A high degree of suspicion for cardiogenic shock is necessary. Patients should be assessed for signs of heart failure such as worsening tachycardia, hypotension, and hepatomegaly with fluid resuscitation. Patients with a more subtle presentation, or treated on another clinical pathway, such as bronchiolitis or pneumonia, need ongoing segmentation and resuscitation until the diagnosis of sepsis becomes more apparent or they are clinically improved.
Disposition
Early consultation of the ICU is encouraged in the management of pediatric patients with sepsis. Admission to the ICU is strongly recommended for all patients with signs of ongoing or refractory shock despite fluid resuscitation and those requiring inotropes or vasopressors.
Conclusions
A majority of pediatric septic shock management is extrapolated from adult data and there remains a large knowledge gap in pediatric septic shock outcomes. Nonetheless, while advances in basic and translational research help provide a better understanding of the pathophysiologic derangements in pediatric sepsis, an emphasis on optimizing delivery of known therapeutic interventions is crucial. Application of the acute care model to the recognition and management of pediatric sepsis may provide opportunities to improve care delivery at the institutional level. In addition, by providing a framework to standardize care and drive adherence to recommended guidelines, it may help reduce variability in care within and across institutions, and ultimately contribute to improvement in pediatric sepsis outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015;191:1147-57. [Crossref] [PubMed]
- Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014;15:828-38. [Crossref] [PubMed]
- Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013;14:686-93. [Crossref] [PubMed]
- Carcillo JA, Fields AI. American College of Critical Care Medicine Task Force Committee Members. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med 2002;30:1365-78. [Crossref] [PubMed]
- Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009;37:666-88. [Crossref] [PubMed]
- Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit Care Med 2017;45:1061. [Crossref] [PubMed]
- Iyer S, Reeves S, Varadarajan K, et al. The Acute Care Model: A New Framework for Quality Care in Emergency Medicine. Clin Pediatr Emerg Med 2011;12:91-101. [Crossref]
- Odetola FO, Freed G, Shevrin C, et al. In-Hospital Quality-of-Care Measures for Pediatric Sepsis Syndrome. Pediatrics 2017;140. [Crossref] [PubMed]
- Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2005;6:2-8.
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Schlapbach LJ, Straney L, Bellomo R, et al. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med 2018;44:179-88. [Crossref] [PubMed]
- Cruz AT, Perry AM, Williams EA, et al. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics 2011;127:e758-66. [Crossref] [PubMed]
- Larsen GY, Mecham N, Greenberg R. An Emergency Department Septic Shock Protocol and Care Guideline for Children Initiated at Triage. Pediatrics 2011;127:e1585-92. [Crossref] [PubMed]
- Paul R, Melendez E, Stack A, et al. Improving adherence to PALS septic shock guidelines. Pediatrics 2014;133:e1358-66. [Crossref] [PubMed]
- Lane RD, Funai T, Reeder R, et al. High Reliability Pediatric Septic Shock Quality Improvement Initiative and Decreasing Mortality. Pediatrics 2016;138. [Crossref] [PubMed]
- Balamuth F, Alpern ER, Grundmeier RW, et al. Comparison of Two Sepsis Recognition Methods in a Pediatric Emergency Department. Acad Emerg Med 2015;22:1298-306. [Crossref] [PubMed]
- Martin K, Weiss SL. Initial resuscitation and management of pediatric septic shock. Minerva Pediatr 2015;67:141-58. [PubMed]
- Han YY, Carcillo JA, Dragotta MA, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics 2003;112:793-9. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-93. [Crossref] [PubMed]
- ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. [Crossref] [PubMed]
- de Oliveira CF, de Oliveira DS, Gottschald AFC, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med 2008;34:1065-75. [Crossref] [PubMed]
- Sankar J, Sankar MJ, Suresh CP, et al. Early goal-directed therapy in pediatric septic shock: comparison of outcomes “with” and “without” intermittent superior venacaval oxygen saturation monitoring: a prospective cohort study. Pediatr Crit Care Med 2014;15:e157-67. [Crossref] [PubMed]
- Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011;364:2483-95. [Crossref] [PubMed]
- Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia Is Associated With Complicated Course and Mortality in Pediatric Patients With Septic Shock. Pediatr Crit Care Med 2018;19:155-60. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Crossref] [PubMed]
- Sterling SA, Miller WR, Pryor J, et al. The Impact of Timing of Antibiotics on Outcomes in Severe Sepsis and Septic Shock: A Systematic Review and Meta-analysis. Crit Care Med 2015;43:1907-15. [Crossref] [PubMed]
- Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014;42:2409-17. [Crossref] [PubMed]
- Ninis N, Phillips C, Bailey L, et al. The role of healthcare delivery in the outcome of meningococcal disease in children: case-control study of fatal and non-fatal cases. BMJ 2005;330:1475. [Crossref] [PubMed]
- Sinha R. Recognition and Initial Management of Shock. In: Rogers’ Textbook of Pediatric Intensive Care. 2016th ed. Wolters Kluwer, 2016:380-92.
- Pizarro CF, Troster EJ, Damiani D, et al. Absolute and relative adrenal insufficiency in children with septic shock. Crit Care Med 2005;33:855-9. [Crossref] [PubMed]
- Ranjit S, Aram G, Kissoon N, et al. Multimodal monitoring for hemodynamic categorization and management of pediatric septic shock: a pilot observational study. Pediatr Crit Care Med 2014;15:e17-26. [Crossref] [PubMed]
- Hatherill M, Waggie Z, Purves L, et al. Mortality and the nature of metabolic acidosis in children with shock. Intensive Care Med 2003;29:286-91. [Crossref] [PubMed]
- Dugas MA, Proulx F, de Jaeger A, et al. Markers of tissue hypoperfusion in pediatric septic shock. Intensive Care Med 2000;26:75-83. [Crossref] [PubMed]
- Scott HF, Donoghue AJ, Gaieski DF, et al. The utility of early lactate testing in undifferentiated pediatric systemic inflammatory response syndrome. Acad Emerg Med 2012;19:1276-80. [Crossref] [PubMed]
- Kim YA, Ha EJ, Jhang WK, et al. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med 2013;39:1818-23. [Crossref] [PubMed]
- Jat KR, Jhamb U, Gupta VK. Serum lactate levels as the predictor of outcome in pediatric septic shock. Indian J Crit Care Med 2011;15:102-7. [Crossref] [PubMed]
- James JH, Luchette FA, McCarter FD, et al. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999;354:505-8. [Crossref] [PubMed]
- Oberender F, Ganeshalingham A, Fortenberry JD, et al. Venoarterial Extracorporeal Membrane Oxygenation Versus Conventional Therapy in Severe Pediatric Septic Shock. Pediatr Crit Care Med 2018;19:965. [PubMed]
- Long E, Babl FE, Angley E, et al. A prospective quality improvement study in the emergency department targeting paediatric sepsis. Arch Dis Child 2016;101:945-50. [Crossref] [PubMed]
- Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 2017;376:2235-44. [Crossref] [PubMed]
- Evans IVR, Phillips GS, Alpern ER, et al. Association Between the New York Sepsis Care Mandate and In-Hospital Mortality for Pediatric Sepsis. JAMA 2018;320:358-67. [Crossref] [PubMed]
- Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med 2018;44:925-8. [Crossref] [PubMed]
- Zackoff MW, Iyer S, Dewan M. An overarching approach for acute care delivery: extension of the acute care model to the entire inpatient admission. Transl Pediatr 2018;7:246-52. [Crossref]