Making care better in the pediatric intensive care unit
Introduction
The relatively young field of pediatric critical care has seen a shift from an approach with little consideration for the complications and adverse effects resulting from the procedures and medications to a more cautious approach with careful concern for the associated risks. Many senior pediatric intensivists recall a time when nearly every patient had a central venous line and arterial line; and hospital acquired infections, pressure injuries, unplanned extubations, and venous thromboemboli were expected costs of aggressive care. In addition to the morbidity and mortality associated with many of the HACs in children, the attributable cost due to these HACs contributes to the unsustainable health care financial crisis. The Centers for Medicare and Medicaid Services (CMS) often penalize hospitals for health care-acquired conditions (HACs), and also are beginning to reimburse in a bundled fashion such that complications become the institution’s burden. In children, payors and patients’ families are often saddling this burden of costs attributable to HACs. The direct attributable costs per event are staggering (Table 1). Payors, families, patients, and health care teams now demand a circumspect approach to care: do no harm, but how?
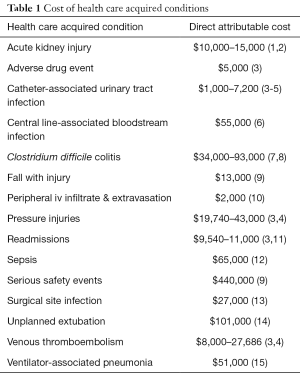
Full table
Outside of the wide range of HACs, pediatric intensive care units (PICUs) across the country work every day to improve the quality and efficiency of care delivered to children. In order to care for the growing number of children requiring critical care services, units must examine all aspects of patient care and focus on timely admissions, transfers and discharges. Just as HACs are no longer considered acceptable in health care, harm from routine care in terms of intubation or delivery of poor quality cardiopulmonary resuscitation is no longer tolerated amongst critical care professionals. Here we examine foundational strategies for building a robust culture of safety (COS) and review available quality improvement collaboratives that facilitate shared learning involving pediatric critical care patients. As a detailed description of all ongoing national improvement efforts is beyond the scope of this paper, we will highlight prescriptive bundles aimed at prevention of specific HACs as well as bundles of care and interventions to improve the quality resuscitation care delivered in the PICU.
Preparing for improvement: COS
COS can be described in a variety of ways. When a team is considering the differential diagnosis for a failed effort at harm prevention, one essential element to consider is the need for improved COS. Often COS is the “secret sauce” that leads to a team’s success. Culture may be described as how we do things when no one is looking, or the unwritten rules of a micro- or macro-environment.
How can we improve COS? First, COS should be measured regularly. AHRQ offers a tool that may be used to measure COS (16). Identifying specific domains within an environment will inform improvement strategies. Strategies for improvement of COS often involve intentional campaigns around event reporting with feedback to the reporters and assurance that retaliation will not occur as a result, leader rounding to influence, safety rounding, infusing a just culture, creating a highly reliable environment, daily operational briefings, beginning every meeting with a safety story, and verbalizing and demonstrating that safety is the top priority.
Prior to implementation of harm prevention effort teams, and especially leadership, must understand the concept that apparent harm will increase as it comes into focus with increased detection, awareness, and measurement. Otherwise, without appropriate expectations as a team embarks upon a harm prevention effort, the team will be discouraged, confused, and resources may be withdrawn. New efforts additionally require dedicated champions, political will, compelling reason to change behavior that is apparent to those involved, data, resources and infrastructure, and an appropriate change management strategy. A patient or family champion, possibly one who has experienced the harm being considered, can be an effective addition to the team as they may compel providers to eliminate the harm as well as identify strategies that can empower families to aid in prevention of the harm.
Weick and Sutcliffe describe five principles of high reliability in “Managing the Unexpected” which many hospitals have embraced as a mechanism of improving their culture of safety and eliminating preventable harm (17). These principles include a preoccupation with failure, a reluctance to simplify, sensitivity to operations, a commitment to resiliency, and deference to expertise. This approach is particularly relevant in the ever-changing health care environment.
David Marx’s “Just Culture” concept endorses consoling people for human errors, counseling people regarding at-risk behavior, and punishing people for reckless behavior (18). Along the way, identifying and repairing systems failures is a key component of Just Culture. To account for the need for accountability, there are algorithms within Just Culture that address repetitive human error and repetitive at-risk behavior. Many organizations train leaders in this concept and it can involve quite an adjustment for hierarchical, rigid cultures.
The concept of safety coaches has been endorsed by organizations such as Solutions for Patient Safety (SPS) and Child Health Patient Safety Organization as a means of elevating and sustaining COS (19,20). These organizations have developed error prevention training intended to be shared face-to-face with all employees within a children’s hospital, and leadership methods training which endorses similar concepts and framework for leadership. Safety coaches are often initially trained, and then work with leadership to train the remainder of the employees. Safety coaches are frontline change agents empowered to identify problems and propose solutions, use effective communication techniques to prevent harm, and spread quality and safety initiatives in their microenvironments.
Improvement methods
Once an ICU has measured and established a culture of safety within the ICU it is critical to apply rigorous quality improvement methodologies to areas that require attention. There are many frameworks for improvement in healthcare: Lean and Six Sigma (21), Institute for Healthcare Improvement Model for Improvement (22), and Failure Mode Effects Analysis (23). Most hospitals have adopted a framework or created a hybrid framework in which to operate. It is important that all members of the ICU team have a basic understanding of the framework in which they are operating, and that leadership in these areas have deeper knowledge. Once all team members are on the same page with regards to the language of improvement, then the work toward improvement can begin.
It is key to measure process, outcome and balancing metrics within any improvement focus and properly display data back to the team at the bedside. ICU teams should move away from bar charts and confusing pie charts when displaying data. The utilization of run charts and control charts can quickly and scientifically display data to team members in meaningful ways. ICU providers should understand the concepts of common cause variation (or the random variation that occurs in any measured system) and special cause variation (the effect on a metric caused by something outside of the system) (24). By utilizing control charts teams can avoid wasting time and money chasing down common cause variation and focus on special cause variation. While the goal is for zero harm within the PICU, these data visualization tools can be critical to get to zero harm.
Once a team understands baseline data trends in their ICU, they can choose a focus for improvement. This may be decreasing rates of HAC’s or may be improving screening for delirium at the bedside. The first step in choosing the focus is ensuring there is actually a problem to address. Many times we perceive that there is a deficiency in care, but when properly measured the team is actually performing well. Taking the time to understand the current state is key to successful use of busy providers time. With a deficiency in care identified the team should then spend time understanding the current process as it is occurring at the point of care. Through the use of Plan-Do-Study-Act (PDSA) cycles often senior medical staff or nursing can lose touch with what is actually happening at the bedside and are not in touch with current workflow. Practitioners at the bedside, doing the daily work should be consulted and involved in proposed changes to practice. Once improvement has been made with a series of PDSA cycles the challenging work of maintaining that improvement begins. Without careful attention to maintenance of improvement efforts metrics often return to their previous level of functioning (25).
Quality improvement and patient safety collaboratives
There are multiple quality improvement and patient safety collaboratives available to providers within pediatric critical care (Table 2). Among these collaboratives, the patient populations, platform, purposes and goals, degree of interactivity, cost, and required resources vary widely. Participation fees may vary by the hospital size, and do not account for the local resources needed to implement the effort. Key features of some of the most successful collaboratives include the avoidance of competing on safety issues, sharing successes and failures in a seamless and transparent fashion, infrastructure including performance improvement consultants and web platform, requiring executive leadership buy-in, innovation, and focus on both process and outcomes measures.
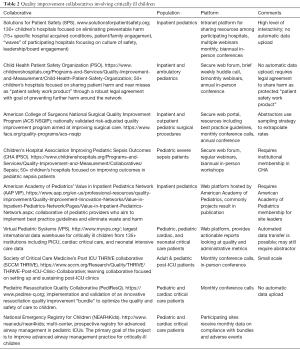
Full table
Health care acquired condition prevention
Heath care-acquired harm is no longer tolerable and is preventable in many cases. Bundles have generally been studied as a group of prevention tactics representing a comprehensive strategy. According to the IHI, a bundle is “a small set of evidence-based interventions for a defined patient segment/population and care setting that, when implemented together, will result in significantly better outcomes than when implemented individually” (26). Some adult HAC prevention bundles are more well-established and well-studied; in pediatrics, often the adult bundle isn’t adequate and a pediatric bundle must be developed and studied for a given harm which manifests or develops differently in children.
Some of the most established pediatric HAC prevention bundles include health care-acquired infections. Various organizations have published slight variations in the catheter-associated urinary tract infection (27-29), ventilator-associated pneumonia (27,30-32), and central line-associated bloodstream infection bundles (27,33-36). SPS also has established published prevention bundles for the following HACs: falls, pressure injuries, readmissions, surgical site infections, and venous thromboembolism (27). Many institutions within the SPS network are collaborating to test and define prevention bundles for adverse drug events, CLABSI caused by mucosal barrier injury in children with oncologic diagnoses, nephrotoxic acute kidney injury, patient behavioral events causing harm to staff, employee overexertion injuries, employee slips, trips, falls, and other HACs. Many other examples of multidisciplinary collaboration to reliably implement bundle in an effort to reduce HACs can be seen throughout pediatric critical care.
Handoffs
Handoffs have been studied extensively as a risky transition point for patients due to the potential for loss of information in transfer (37,38). Along the continuum of healthcare, often the only constants are the patient and their caregivers. In pediatric critical care, our patients experience a number of handoffs, including to/from operating room (OR), emergency room, home, long-term care facilities, rehabilitation facilities, acute care units, procedural areas, radiology, primary care providers and subspecialists, transport teams, and other hospitals. In many settings the caregivers as the constant do not accompany our patients to some of these locations; thus, it is imperative that we adequately communicate issues and concerns in a standardized format.
The majority of research and quality improvement efforts around handoffs in the PICU have focused on the OR to PICU transition. Breuer et al. (39) studied OR to PICU transfers before and after a standardized handoff protocol was implemented. They found that there were reduced antibiotic delays and improved time to analgesia administration with lower pain scores in the post-implementation group. Other reports have described improved team satisfaction with the handoff process (40) after implementation of standardized handoffs as well as improved knowledge of the surgical procedure and reduced communication errors and omissions after pediatric cardiac surgery (41). Standardized handoffs can improve communication in ICU’s and more quality improvement efforts are needed to focus on transitions outside of the OR to ICU time.
Improving the quality of cardiopulmonary resuscitation
Pediatric in hospital cardiac arrest is a low-volume, high stakes event that most often occurs in the PICU (42). Resuscitation events are chaotic and stressful to many participants leading to multiple opportunities for errors. Errors during cardiac arrest management are common, with pediatric patients particularly susceptible due to the relative infrequency of pediatric cardiac arrests and wide-ranging patient weights and sizes necessitating more complex life support algorithms (defibrillation doses, medication doses, range of CPR quality goals) (43-48). Clear evidence exists that the delivery of high quality chest compressions and rapid medication administration improves short- and long-term outcomes after cardiac arrest (49,50), however repeated studies show that the CPR delivered at the bedside does not meet guideline recommendations (51,52). Implementation of a bundle of practices has been implemented to attempt to improve the quality of care delivered to children that experience cardiac arrest, and is the focus of the Pedi-ResQ Quality Collaborative. The current bundle as of publication includes (I) identification of patients at high risk for experiencing cardiac arrest, (II) deliberate practice to improve provider chest compression skills, (III) hot debriefing immediately after cardiac arrest, (IV) attention to post-cardiac arrest care in patients that have survived the cardiac arrest event and (V) cold debriefing distant to the cardiac arrest.
Identification of patients at risk for cardiac arrest in the PICU is in its infancy and often difficult to implement at the bedside. Deliberate practice in the form of ‘rolling refreshers’ (52) encourage frequent skill refreshers and has been shown to increase skill retention in bedside care providers (53). Debriefing is a practice of reflection after an event in order to better understand actions taken during the event, and when combined with performance data can be compared to audit and feedback. Hot debriefing, or immediate review and reflection, allows teams to emotionally process events and immediately identify any systems issues that were identified during the resuscitation. Cold debriefing or post-event review, is a multidisciplinary session that reviews event data—such as monitor data, labs, radiology data and chest compression quality metrics if available. It has been associated with identification of needs for practice improvement (54) as well as improved chest compression quality leading to improved survival outcomes after cardiac arrest (55). Focus on post cardiac arrest care with attention to avoidance of fever and hypotension is recommended based on current literature (56,57). A bundle consisting of deliberate practice and cold debriefing is currently being studied in a multicenter quality improvement investigation (58) and a multitude of quality improvement investigations is ongoing via the PediResQ network to determine which of these interventions, or combinations of interventions best help this vulnerable patient population.
Conclusions
Complications and adverse events from procedures and treatments in the PICU are no longer acceptable as the price of admission for critical care. The development and maintenance of a robust culture of safety is a vital first step in ensuring that these complications are eradicated. The application of quality improvement methodologies and visualization of data utilizing process control charts can help teams during the improvement process. Robust networks are available with increasing frequency focusing on reduction of harm for patients cared for in the PICU. Participation in these collaboratives allows institutions to learn and share best practices and more rapidly advance care in these niches.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Subramanian S, Tumlin J, Bapat B, et al. Economic burden of contrast-induced nephropathy: implications for prevention strategies. J Med Econ 2007;10:119-34. [Crossref] [PubMed]
- Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg 2015;261:1207-14. [Crossref] [PubMed]
- Agency for Healthcare Research. 2013 Annual Hospital-Acquired Condition Rate and Estimates of Cost Savings and Deaths Averted From 2010 to 2013. Available online: http://www.ahrq.gov/professionals/quality-patient-safety/pfp/index.html
- Goudie A, Dynan L, Brady PW, et al. Costs of Venous Thromboembolism, Catheter-Associated Urinary Tract Infection, and Pressure Ulcer. Pediatrics 2015;136:432-9. [Crossref] [PubMed]
- Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 2011;32:101-14. [Crossref] [PubMed]
- Goudie A, Dynan L, Brady PW, et al. Attributable cost and length of stay for central line-associated bloodstream infections. Pediatrics 2014;133:e1525-32. [Crossref] [PubMed]
- Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis 2016;16:447. [Crossref] [PubMed]
- Sammons JS, Localio R, Xiao R, et al. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clin Infect Dis 2013;57:1-8. [Crossref] [PubMed]
- Brilli RJ, McClead RE Jr, Crandall WV, et al. A comprehensive patient safety program can significantly reduce preventable harm, associated costs, and hospital mortality. J Pediatr 2013;163:1638-45. [Crossref] [PubMed]
- Hanrahan K. Hyaluronidase for treatment of intravenous extravasations: implementation of an evidence-based guideline in a pediatric population. J Spec Pediatr Nurs 2013;18:253-62. [Crossref] [PubMed]
- Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics 2014;134:100-9. [Crossref] [PubMed]
- Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children's hospitals. Pediatr Crit Care Med 2014;15:798-805. [Crossref] [PubMed]
- Sparling KW, Ryckman FC, Schoettker PJ, et al. Financial impact of failing to prevent surgical site infections. Qual Manag Health Care 2007;16:219-25. [Crossref] [PubMed]
- Roddy DJ, Spaeder MC, Pastor W, et al. Unplanned Extubations in Children: Impact on Hospital Cost and Length of Stay. Pediatr Crit Care Med 2015;16:572-5. [Crossref] [PubMed]
- Brilli RJ, Sparling KW, Lake MR, et al. The business case for preventing ventilator-associated pneumonia in pediatric intensive care unit patients. Jt Comm J Qual Patient Saf 2008;34:629-38. [Crossref] [PubMed]
- AHRQ. Hospital survey on patient safety culture. Available online: https://www.ahrq.gov/sops/quality-patient-safety/patientsafetyculture/hospital/index.html
- Weick KE, Sutcliffe KM. Managing the Unexpected: What Business Can Learn from High Reliability Organizations. In: Managing the Unexpected: Resilient Performance in an Age of Uncertianty. 2nd Edition. San Francisco: Jossey-Bass, 2007.
- Marx D. Patient Safety and the “ Just Culture”: A Primer For Health Care Executives; Medical Event Reporting System – Transfusion Medicine (MERS-TM). Available online: http://www.chpso.org/sites/main/files/file-attachments/marx_primer.pdf
- Harton BB, Ingram SW. Ready for lift off: Implementing a safety coach initiative. Nurs Manage 2013;44:40-5. [Crossref] [PubMed]
- Sick Kids. Safety coaches are helping us care safely everywhere every day. Available online: http://www.sickkids.ca/AboutSickKids/Newsroom/Past-News/2017/safety-coaches-helping-us-care-safely-everywhere-everyday.html
- de Koning H, Verver JP, van den Heuvel J, et al. Lean six sigma in healthcare. J Healthc Qual 2006;28:4-11. [Crossref] [PubMed]
- Lee CS, Larson DB. Beginner's guide to practice quality improvement using the model for improvement. J Am Coll Radiol 2014;11:1131-6. [Crossref] [PubMed]
- Shaqdan K, Aran S, Daftari Besheli L, et al. Root-cause analysis and health failure mode and effect analysis: two leading techniques in health care quality assessment. J Am Coll Radiol 2014;11:572-9. [Crossref] [PubMed]
- Neuhauser D, Provost L, Bergman B. The meaning of variation to healthcare managers, clinical and health-services researchers, and individual patients. BMJ Qual Saf 2011;20 Suppl 1:i36-40. [Crossref] [PubMed]
- Yaghmai BF, Di Gennaro JL, Irby GA, et al. A Pediatric Sedation Protocol for Mechanically Ventilated Patients Requires Sustenance Beyond Implementation. Pediatr Crit Care Med 2016;17:721-6. [Crossref] [PubMed]
- Resar R, Griffin FA, Haraden C, et al. Using Care Bundles to Improve Health Care Quality. IHI Innov Ser white Pap 2012. doi:. [Crossref]
- Children’s Hospitals’ Solutions for Patient Safety. SPS Prevention Bundles. Available online: https://www.solutionsforpatientsafety.org/wp-content/uploads/SPS-Prevention-Bundles.pdf
- Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 2010;31:319-26. [Crossref] [PubMed]
- Institute for Healthcare Improvement 2018 update. How-to Guide : Prevent Catheter-Associated Urinary Tract Infections. Available online: www.ihi.org
- IHI I for HI. How-to-guide: Prevent Ventilator-Associated Pneumonia - Pediatric supplement. Available online: http://www.ihi.org/resources/Pages/Tools/HowtoGuidePreventVAP.aspx
- Greene LR, Sposato K. Guide to the Elimination of Ventilator-Associated Pneumonia. 2009. Available online: http://www.apic.org/Resource_/EliminationGuideForm/18e326ad-b484-471c-9c35-6822a53ee4a2/File/VAP_09.pdf
- Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:915-36. [Crossref] [PubMed]
- Mack EH, Stem CT. Prevention of CAUTIs, CLABSIs, and VAPs in Children. Curr Treat Options Pediatr 2017;3:221-35. [Crossref]
- O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2002;30:476-89. [PubMed]
- Barnes S, Olmsted RN, Monsees E, et al. Guide to Preventing Central Line-Associated Bloodstream Infections. Available online: https://apic.org/Resource_/TinyMceFileManager/2015/APIC_CLABSI_WEB.pdf
- Centers for Disease Control and Prevention. Checklist for Prevention of Central Line Associated Blood Stream Infections. Available online: https://www.cdc.gov/hai/pdfs/bsi/checklist-for-CLABSI.pdf
- Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med 2014;371:1803-12. [Crossref] [PubMed]
- Chenault K, Moga MA, Shin M, et al. Sustainability of protocolized handover of pediatric cardiac surgery patients to the intensive care unit. Paediatr Anaesth 2016;26:488-94. [Crossref] [PubMed]
- Breuer RK, Taicher B, Turner DA, et al. Standardizing postoperative PICU handovers improves handover metrics and patient outcomes. Pediatr Crit Care Med 2015;16:256-63. [Crossref] [PubMed]
- Chen JG, Wright MC, Smith PB, et al. Adaptation of a postoperative handoff communication process for children with heart disease: a quantitative study. Am J Med Qual 2011;26:380-6. [Crossref] [PubMed]
- Sheth S, McCarthy E, Kipps AK, et al. Changes in Efficiency and Safety Culture After Integration of an I-PASS-Supported Handoff Process. Pediatrics 2016;137. [Crossref] [PubMed]
- Berg RA, Sutton RM, Holubkov R, et al. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med 2013;41:2292-7. [Crossref] [PubMed]
- Lipshutz AK, Morloc LL, Shore AD, et al. Medication errors associated with code situations in U.S. hospitals: direct and collateral damage. Jt Comm J Qual Patient Saf 2008;34:46-56, 1.
- Kozer E, Scolnik D, Macpherson A, et al. Variables associated with medication errors in pediatric emergency medicine. Pediatrics 2002;110:737-42. [Crossref] [PubMed]
- Kozer E, Seto W, Verjee Z, et al. Prospective observational study on the incidence of medication errors during simulated resuscitation in a paediatric emergency department. BMJ 2004;329:1321. [Crossref] [PubMed]
- Hansen M, Meckler G, Lambert W, et al. Patient safety events in out-of-hospital paediatric airway management: a medical record review by the CSI-EMS. BMJ Open 2016;6. [Crossref] [PubMed]
- Hansen M, Eriksson C, Skarica B, et al. Safety events in pediatric out-of-hospital cardiac arrest. Am J Emerg Med 2018;36:380-3. [Crossref] [PubMed]
- Flannery AH, Parli SE. Medication Errors in Cardiopulmonary Arrest and Code-Related Situations. Am J Crit Care 2016;25:12-20. [Crossref] [PubMed]
- Sutton RM, French B, Niles DE, et al. 2010 American Heart Association recommended compression depths during pediatric in-hospital resuscitations are associated with survival. Resuscitation 2014;85:1179-84. [Crossref] [PubMed]
- Andersen LW, Berg KM, Saindon BZ, et al. Time to Epinephrine and Survival After Pediatric In-Hospital Cardiac Arrest. JAMA 2015;314:802-10. [Crossref] [PubMed]
- Sutton RM, Wolfe H, Nishisaki A, et al. Pushing harder, pushing faster, minimizing interruptions… but falling short of 2010 cardiopulmonary resuscitation targets during in-hospital pediatric and adolescent resuscitation. Resuscitation 2013;84:1680-4. [Crossref] [PubMed]
- Niles DE, Duval-Arnould J, Skellett S, et al. Characterization of Pediatric In-Hospital Cardiopulmonary Resuscitation Quality Metrics Across an International Resuscitation Collaborative. Pediatr Crit Care Med 2018;19:421-32. [Crossref] [PubMed]
- Sutton RM, Niles D, Meaney PA, et al. "Booster" training: evaluation of instructor-led bedside cardiopulmonary resuscitation skill training and automated corrective feedback to improve cardiopulmonary resuscitation compliance of Pediatric Basic Life Support providers during simulated cardiac arrest. Pediatr Crit Care Med 2011;12:e116-21. [Crossref] [PubMed]
- Blankenship AC, Fernandez RP, Joy BF, et al. Multidisciplinary Review of Code Events in a Heart Center. Am J Crit Care 2016;25:e90-7. [Crossref] [PubMed]
- Wolfe H, Zebuhr C, Topjian AA, et al. Interdisciplinary ICU cardiac arrest debriefing improves survival outcomes. Crit Care Med 2014;42:1688-95. [Crossref] [PubMed]
- Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med 2014;42:1518-23. [Crossref] [PubMed]
- de Caen AR, Berg MD, Chameides L, et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (Reprint). Pediatrics 2015;136 Suppl 2:S176-95. [Crossref] [PubMed]
- Reeder RW, Girling A, Wolfe H, et al. Improving outcomes after pediatric cardiac arrest - the ICU-Resuscitation Project: study protocol for a randomized controlled trial. Trials 2018;19:213. [Crossref] [PubMed]


