Pediatric palliative care in the intensive care unit and questions of quality: a review of the determinants and mechanisms of high-quality palliative care in the pediatric intensive care unit (PICU)
Pediatric palliative and hospice care (HC)
Palliative care (PC)
The World Health Organization defines PC as “that which improves patients’ quality of life and that of their families facing consequences of life-threatening illness through the prevention and relief of suffering by early identification, impeccable assessment, and the treatment of pain and other problems, physical, psychosocial, and spiritual” (1). The American Academy of Pediatrics (AAP) and American College of Critical Care Medicine (ACCCM) recommend that pediatric PC be offered at the time of diagnosis and continue throughout illness, regardless of outcome (2-4). They recommend that optimal PC models involve collaboration of palliative and non-palliative health care providers with patients and families to address needs across domains “concurrently”. In this model, palliative efforts occur simultaneously with curative and life-prolonging care throughout disease course so long as life sustaining care remains in accordance with a family’s goals. The AAP and the Institute of Medicine (IOM) support a similar paralleled approach (3,5,6), arguing that “effective management of pain and other distressing symptoms, along with psychosocial care, spiritual care, and decision-making guidance are critically important beginning at diagnosis and continuing throughout the course of a child’s life and beyond” (7). For a portion of patients, PC includes end of life (EOL) care if or when disease-modifying efforts fail or become inappropriate. The Robert Wood Johnson EOL Peer Group has outlined seven care domains associated with complete physical, social, and spiritual support involved in PC (Table 1) (8).

Full table
Hospice care (HC)
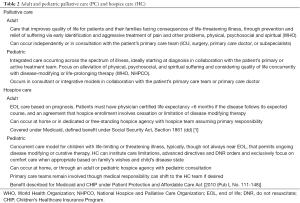
HC is a form of PC for patients at EOL (3). Licensed hospice agencies provide medical, psychosocial, and spiritual services as well as medications, durable medical equipment, and a range of diagnostic and therapeutic services (3). Generally, and particularly for adults, prognosis of 6 months or less is required for insurance approval of the hospice benefit. Pediatric HC is often home-based and coordinated by adult hospice institutions unfamiliar, uncomfortable, or unable to bear the cost of complex pediatric treatment plans on per diem reimbursement strategies (3,8). National cohort data indicate that of the 78% of adult HC agencies provide pediatric care, with average annual pediatric census of fewer than 20 children (7,9). Most providers are familiar with Medicare’s adult HC mandates (Table 2). Pediatric HC does not follow this model. Pediatric hospice patients represent a diverse cohort of diagnoses, many rare and with consequent prognostic uncertainty and unpredictable course of illness (10). Children live longer in hospice than adults and carry a diverse number of admission diagnoses and attendant morbidities (7,10,11). Recognizing that prognostics-driven HC may not be appropriate for children, the 2010 Patient Protection and Affordable Care Act [2010 (Pub L No.111-148)] specified that children can concurrently receive hospice services and curative or life-extending therapy.
Full table
Emphasis on tailored, concurrent, comfort-focused care alongside disease-modifying therapies led the AAP and Society of Critical Care Medicine (SCCM) to encourage universally available PC and HC teams whose role includes aiding providers and families navigating these systems (3,4,12). They highlight that successful PC and HC bridge the patient’s physical location, coordinates care across subspecialties, and adapts services to phase and severity of illness. Though a handful of pediatric hospitals boast pediatric home-based hospice programs, most pediatric PC teams partner with local adult hospice organizations (13).
PC statistics and populations
PC is a growing specialty. In 2016, 90% of US hospitals with more than 300 beds had PC programs. Academic and non-profit hospitals in the North East, Pacific, and Mid-Atlantic regions have a significantly higher dissemination of PC compared to for-profit and Southern institutions (14). Most recent national data estimate that 70% of children’s hospitals currently offer PC services (9,13,15).
In 2015, there were 53,000 pediatric deaths in the United States (US), reflecting a stable annual pediatric mortality (16). Congenital malformations, prematurity, cancer, and non-accidental death are the most common causes (7,9,16). The incidence of complex chronic conditions (CCCs) and associated mortality rates vary by age (Table 3). Genetic and congenital conditions rather than oncologic disorders constitute most of PC referrals and recipients of pediatric hospice (9,13). Many children receiving PC and HC remain technology-dependent with active use of medical resources including periodic in-unit care, tracheostomies with ventilators, augmented feeding, and complex medication regimens. Annual mortality of pediatric hospice patients national is 33% (Table 3) (7).

Full table
Complex chronic conditions (CCCs)
Children with CCCs are a population with significant overlap in both palliative and critical care populations. According to the US Department of Health and Human Services, the population of children with special health care needs is increasing. Fifteen percent of US children less than 18 years old (11.2 million children) and 23% of American households have at least one child with special health care needs (17); of this group, 27% have limited ability to participate in daily activities or have atypical bodily functions.
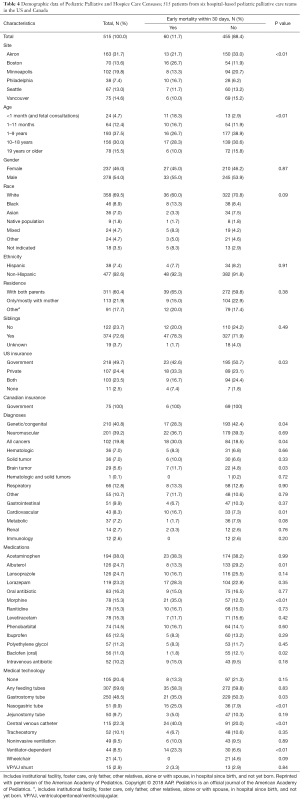
Data from 28 US hospitals demonstrate that children with CCCs account for 19% of patients and half of patient hospital days (49%) and charges (53.2%). Over the 5-year study period, the number of patients with a CCC increased by 35.6% (18). Others estimate that 24% of pediatric hospital days and 30% of hospital dollars are spent on this group (19). Further information about pediatric PC and HC cohorts was published by Feudtner and colleagues that assessed the PC census of five major children’s hospitals (Table 4) (9). They saw a distribution of ages (<1 year 17.1%, 1–18 years 67.5%, ≥19 years 15.5%) and a higher frequency of patients with primary diagnosis of congenital and inherited conditions contributing to care referral (genetic/congenital disease 40.8%, neuromuscular disease 39.2%; malignancy 19.8%). Median time from consult to death was approximately 100 days in this cohort (9).
Full table
A review of over 50,000 PICU admissions showed an association between CCCs, length of stay (LOS), morbidity, and mortality (20). This study utilized Feudtner’s definition of CCC: “one expected to last more than 12 months and involve one or more organ systems sufficiently to require pediatric subspecialty care” (21). Children with at least one CCC accounted for 53% of admissions and 77% of admissions >15 days. Cohort mortality was 2.7% and 75% of mortalities (1,078 of 1,448 deaths) were children with a CCC (20). The presence and number of CCCs increased odds ratio of mortality. UK patients with life-limiting or CCCs demonstrated higher in-unit mortality, significant decline in post-discharge functional scores, and increased mortality up to one year after PICU discharge (22).
PICU survivors have substantial medical burdens. Termed “post intensive care syndrome” or “chronically critically ill” these patients suffer significant symptom burden, poor cognitive outcomes, depression, mortality, financial, and social difficulties (23). Patients often remain dependent on intensive or near-intensive care due to continued organ failure, neuroendocrine disarray, developmental disorders, and recurrent infections (23-28). Caregivers frequently assume ongoing complex, technology-dependent homecare and continued interface with the health care system. Costs of this group are thought to exceed 20 billion dollars annually (29).
Sudden unexpected death
Sudden, unexpected death (SUD) from intentional injuries, acute illness, and unintentional trauma is a prevalent cause of death in the PICU (20,30). Traditionally, PC is targeted to deaths classified as not preventable, such as treatment failure of life-threatening or chronic conditions (13,18,23,31). Yet, PC teams can provide short-term services, decision-making support, as well as attend to complex emotion, grief, and bereavement for families affected by trauma or sudden illness. One PICU’s experience showed that 22% of mortality was a result of SUD and only 4.2% of these families received PC services compared with 28.6% of patients who died from complications of CCCs (25). Families of children with SUD may represent a vulnerable population currently underserved by PC teams (32).
Pediatric locational death in the US
PICU mortality ranges from 1% to 5% with an average of 2.7% nationally. In the US, the most common location of pediatric death is the PICU (26,33-35) versus at home or other hospital locations. Thirty to sixty percent of PICU deaths involve withdrawal of one or more forms of life sustaining medical treatment (LSMT) including mechanical support (30,33,36). Of children at EOL in the PICU, less than 1% receive HC (37,38). Some tertiary centers offer critical care transport for home withdrawal of LSMT with hospice, but the frequency of this is unknown.
A nineteen-center study of tertiary PICUs showed that 50% of death occurs following withdrawal of LSMT compared to consequences of failed cardiopulmonary resuscitation (CPR) (20%) or after limitation of support (17%) (39). Thirty-seven percent of families were approached for organ donation (range, 17–88%); of those, 20% donated. Autopsy was requested in 37% of non-medical examiner cases (30). Seventy percent of families received PC consultations. Sixty-seven percent of consults involved discussions of care limitations; 92% of families subsequently decided to limit LSMT (30).
ICU providers miss opportunities to provide straightforward PC (32,40,41). One tertiary center’s mortality review found that only half of patients with anticipated mortality received multidisciplinary supportive care (SW, chaplaincy, child life, care conferences) and symptom-directed care, including pain medication, during their terminal hospitalization. Only 26% received additional analgesia at time of withdrawal or during limitation of support. Less than 30% of SUD patients received comfort-based measures. No patients in either cohort were referred to hospice (40).
Demographics of home versus in-hospital death are changing as children with CCCs utilize more complicated and technologically-dependent home care (9,13,26,35). Ireland and Canada have experienced a decline in PICU deaths, with 95% incidence in 1998 versus 67% in 2012. PC involvement increased from 10% to 74% over this period (26). One factor is early advanced care planning (ACP), though one US study demonstrates that only 56% of children with terminal illness had evidence of any ACP on chart review and 64% had current do-not-resuscitate (DNR) orders at time of death according (34).
Methods of providing PC in the PICU
Fifty to seventy percent of children’s hospitals offer formal PC programs (13,15). Staffing varies and can include multidisciplinary providers such as nurses, nurse practitioners, social workers, child life, chaplains, bereavement coordinators, and physicians. Not all PC programs are staffed to 24/7 availability, thus resulting in institution-specific methods for providing PC within the PICU (15).
Basic tenets of PC, such as attention to symptom burden and considerations of quality of life are within the skill set of practicing intensivists and constitutes “primary PC”. This is PC within a subspecialist’s practice scope, not requiring specific pediatric palliative expertise. However, for complex decision-making, complicated grief, care transitions, and high symptom burden, intensivists might seek PC expertise. In 2012, the SCCM recommended consideration of PC for children living with CCCs, those with high risk of mortality, significant morbidity, cases complicated by conflict around goals of care, difficult symptom control, anticipated long hospitalization, frequent readmission, and staff moral distress (37). PC also serves ICU families needing extra psychosocial support and those requiring hospice referral (37).
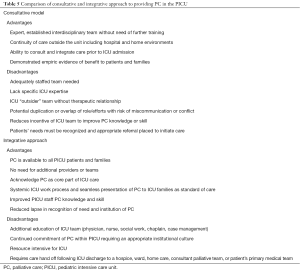
PC involvement commonly occurs in “consultation” where interdisciplinary PC certified specialists serve the “primary” ICU team. Other institutions “integrate” PC-trained providers into the PICU team to address prevalent PC needs and weave palliation into daily practice (23,42,43). Advantages exist for both models. Successful implementation likely relates to institutional-specific factors such as dedicated PC resources, census, and continuity in and out of the ICU (Table 5).
Full table
Optimal referral populations and timing continues to be studied and represents an important quality metric for PICU and PC teams. Methods for referral include “physician order” consults, parental or nurse prompted consultations, or “automatic/triggered referral” when certain criteria such as transplant, mechanical support, or LOS are met (23,32,42,44). One institution’s tool prompted consideration of PC for ICU LOS more than 2 weeks, 3 or more PICU admissions within 6 months, persistent mechanical support, organ failure, and/or disease severity criteria (32). They saw a 500% increase in PC referrals across all intensivists, illustrating that “triggers” may overcome individual provider oversight or reticence to consult PC. Populations with high morbidity and mortality such as severe neurologic injury, continuous renal replacement therapy (CRRT), and extracorporeal membrane oxygenation (ECMO) still experienced low referral (32). Seventy percent of all eligible patients based on the trigger tool were referred; 66% were new consults. Of patients who went on to die at home, 75% did so with hospice, indicating that PC consult made ICU death less likely without an increase in overall mortality rate (32). Other adults criteria trigger tools exist and are outlined in the supplemental materials (Table S1) (45).

Full table
This study and others noted delay between PC eligibility and new referral, reinforcing that PC integration at diagnosis, rather than at decline, improves timeliness to care (25,31,46). ICU days to PC consult was reduced by over a week (12 days) in one adult ICU study of integrated versus consultant models (47). They also found higher rates of PC involvement, completed ACP, hospice referrals, and lower use of medical resources at the integrated campus despite equal mortality across the two groups (47).
Barriers to PC in the PICU
PICU-related PC barriers have been reported spanning parental concerns, provider issues, knowledge gaps, and systems issues (31,48-51).
Provider barriers, staff concerns, and provider availability
Surveyed intensivists indicate avoidance of “consulting out” EOL care, worry that parents perceive PC as “giving up”, and report concerns that PC teams will provide conflicting recommendations, and at times perceive poor availability of PC providers (31,38,48,49). The most frequently cited reason is that intensivist have strong general confidence in addressing PC needs personally (38,48).
PICUs perceive poor availability of PC related to poor visibility within the ICU as well as an insufficiently staffed PC workforce outside of larger centers (14,33). Within consultative models, successful interventions include “trigger” consults for high risk patients with consequent “ripple effects” of further consults due to a reminding presence of the consultant team and observed benefits of PC for patients (33,42,44).
Emotional concerns, moral distress, and burnout represent barriers to providing and involving PC. Nurses particularly bear burdens of alleviating suffering, accommodating families’ wishes, and providing peace in an ICU environment (52). Nurses are at high risk of moral distress as they are often disenfranchised from decision-making but called upon to enact bedside care plans (53-55). PICU staff report the most common cause of moral distress is prolonged, aggressive treatment unlikely to result in a positive outcome. This frequently results from systems issues, poor communication, and lapses in unit or institutional policy and leadership (27,53,56). Moral distress is associated with feelings of frustration, guilt, self-blame and powerlessness; these feelings often persist, contributing to dissatisfaction and burn out (54,57). PC specialists may mitigate moral distress by providing additional staff and family support, advocating for staff, exploring and clarifying goals, and coordinating communication amongst the medical team and family.
Impactful builders of resilience and mitigators of burnout include one-on-one or small group discussions with colleagues and informal social interactions out of the hospital (54). Other resources such as scheduled breaks from stressful patients, relief from duty after a patient’s death, PC support for staff, Schwartz Center Rounds, and structured social interactions were helpful but underused (54,57). “Fully”-staffed PICUs with highly effective teamwork zones by the Safety Attitudes Questionnaire were associated with improved resiliency scores (55).
Symptom recognition and management
Despite reported confidence, providers demonstrate poor PC-related symptom management and under-recognition of delirium, dyspnea, nausea, secretions, agitation, and psychological symptoms (27,41,56,58). As many as 60% of bereaved parents rate ICU management of EOL symptoms, including pain, poorly (41). Intensivists’ knowledge of PC principles and perception of their role in symptom management and decision-making in the ICU has been demonstrated to be variable and at times inadequate (31). Common knowledge gaps surround legal and ethical issues of pediatric EOL care (27,55). One study found that 7% of surveyed intensivists equate a “DNR” order with “comfort care”, and 33% felt that DNR orders implied general limitation of LSMT (38). Providers also under treat EOL symptoms, citing concerns that narcotics administered during withdrawal of LSMT risks euthanasia (27,56). Most subspecialist pediatricians, including intensivists, rate themselves as knowledgeable about EOL care, though half were unfamiliar with the principle of double effect or questioned its legality (27).
PICUs poorly anticipate complicated grief, a phenomenon occurring in up to 60% of bereaved PICU parents (59-61). Routine follow-up with bereaved parents is uncommonly offered by PICUs (59,62,63). These meetings, ideally 3–6 months after death, allow parents to revisit events surrounding death and gain assurance around decision-making, especially reassurance that everything possible was done for their child. Providing feedback to the ICU team gives a degree of closure and positive feeling for bereaved parents (61,63). It is additionally possible that follow-up meetings may be used to screen for complicated grief or other morbidities. Related to appraised risk or levels of bereavement, institutions may facilitate contact with trained bereavement providers to addresses the unique needs of families grieving loss of a child (62,63).
Systems issues, the ICU environment, and PC’s “Branding”
Physicians as well as parents conflate PC with EOL (18,31,41,50,51,55,56). This impression is further solidified when referral occurs proximate to bad news or late in illness, when few or no medical interventions remain. This branding issue led some institutions to rename their PC team as “Comprehensive Care”, “Supportive Care”, “Quality of Life” or “Advanced Care” teams (23,31,64). Others use information packets explaining the role of PC (42,65), or favor the integrative model so that PC is perceived as part of “routine” intensive care (23,31).
The time-intensive nature of PC, competing demands on intensivists’ time, and poor reimbursement models represent pressures that impact PC practice (31,51,66). Weekly service changes and numerous subspecialists impair therapeutic relationships and cogent communication (29). This phenomenon multiplies across hundreds of providers and consultants over prolonged or multiple hospital stays. Short service periods skew decision-making to emphasize short-term over “big picture” goals and hinder integration of short-term choices into illness trajectory (48,49). The continuous and vigilant ICU environment negatively impacts parents’ coping (56,67). Parents often feel disenfranchised from decision-making and perceive loss of parental role particularly when experiencing language barriers, uncertain LOS, or when hospitalizations included several invasive procedures or significant changes in children’s physical appearance (29,67,68). Coping was significantly improved in parents who felt actively involved in care and well-informed (60).
Families differing needs surrounding complex decision-making are difficult to anticipate though are perhaps less time-intensive than perceived (69). Most parents forgoing LSMT made this decision after 1 or 2 meetings (70). Successful meetings involve repetition of key information within and across meetings, open parental inquiry regarding all aspects of care, consideration of spirituality and religiosity, and emotional expression (60,71). Nearly all parents desire to share fully in decision-making despite significant emotional burdens. Most parents ultimately assent to physician recommendations limiting interventions (59,61,63,70,71). Important factors in deciding to forgo LSMT included medical team recommendations, expected neurologic recovery, diagnosis, and degree of pain and suffering (60).
Parents whose children die in the PICU require and value assistance in achieving an appropriately “sacred” death that is peaceful and dignified. Parents require sufficient time and privacy with their child and calm, sensitive care that honors personhood (72). Particularly valuable persons are nurses, holistic health, music therapy, religious or spiritual providers, and other family members (63,71). Appropriate alterations to the ICU environment are imperative. Loud noises, laughing, or insensitive remarks proximate to the child’s room are vividly recalled and regretted by parents. Maintaining parental identity throughout this process is achieved through proximity to their children, staff’s respect of their decision-making, engaging in caregiving, and using cultural and religious symbols including mementos (71). At the time of death, the opportunity to say goodbye is critical, and if missed or not utilized, was a source of parental distress and deep regret, often maintained for years (50,61).
Having a staff member assist in paperwork, contact the funeral home, and guide them through the next steps are important to families (61). The complex and myriad needs of parents at EOL led some institutions to create “End of Life” checklists or packets with items completed by designated family liaisons to ensure appropriate care (4,36,38,73). Additionally, USCF Benioff Children’s Hospital Oakland offers a “Reflection Room”, a private home-like suite for families to stay with their child during EOL and up to 24 hours after death (74). This has decreased PICU locational death and most surveyed staff ascribe value to this resource as promoting patient-and family-centered care.
Hospital memorial services are attended by up to 60% of bereaved parents despite emotionally difficult returns to the hospital (68). Services are particularly supportive when familiar staff are present. Lack of familiar staff is noted and regretted, as parents nearly universally wish that their child not be forgotten (68,71).
Parental barriers
Providers perceive parents’ “unrealistic expectations” as significant barriers to PC (48,51). However, bereaved parents frequently cite unmet needs in symptom management and emotional support, and often regret that PC was not involved earlier (35,41,61,75-77). Providers can misinterpret parents’ hope as hinderance to considerations of comfort or quality. Parents of dying children often indicate a simultaneous and seemingly contradictory recognition of their child’s ultimate prognosis and a persistent hope that their child will live a long life (15,51).
Other parents cite concerns that they want “everything done for their child”, against which PC represents no impediment. PC teams advocate for each family’s unique wishes and goals providing support through continuums of care with sensitivity to suffering. Literature also indicates that poor preparation for significant morbidity and mortality impacts receptiveness to PC (63). This can be mitigated by better communication about a child’s condition or orientation to PC’s role. Adult initiatives surrounding ACP use brochures to this end. ACP brochures decreased discordance amongst surrogate decision makers and the medical team, decreased depression and posttraumatic stress disorder (PTSD) in bereaved families, and improved satisfaction with care provided in the ICU (78). Other decision support tools such as videos discussing DNR orders and consequences of prolonged mechanical ventilation have been associated with improved family-physician concordance, perceived quality of communication, and lower hospital costs (79,80).
October and colleagues used the “Good Parent” tool to explore goals of care within the PICU. Themes of “focusing on quality of life”, “advocating for their child with the medical team”, and “putting the child’s needs above their own” emerged in all surveyed parents. These themes spanned race, socioeconomic status, and illness severity. When asked strategies that could fulfill these goals, the most common answer was “keeping parents well informed” (81).
Communication
High quality communication is perhaps the most important driver of high-quality PC in the PICU (51). PICU provider communication predicts parental perceptions of care quality regardless of illness severity or quantity of care received. It impacts decision-making, particularly surrounding technology use, care limitations, trust in the medical team, and bereavement outcomes (60,61). Parents who rated their ICU communication poorly were significantly more likely to regret their EOL decision-making or to feel misled by the medical team (60,71). Parents desire to receive all, including difficult, information promptly and honestly from a familiar person (50,71,78). News from unfamiliar sources resulted in disbelief and questioning, which staff can perceive negatively. Insensitive, abrupt, and cold remarks such as judgment of parents’ requests or decisions and statements of hopelessness are vividly recalled (71,78). Parents’ perceptions of compassion or insensitivity in communication was a theme of either comfort or distress throughout their grief (59-61).
Difficulties in communication center thematically around communication issues within teams, lack of consensus, prognostic uncertainty, insufficient communication training or confidence, and disagreement as to which team should provide news (82,83). Incidence of poor communication within an ICU admission is as high as 70% and highly predictive of conflict (55,71,84,85). PICU admissions greater than 8 days experienced a 50% incidence of conflict with consultants, parents, or within the medical team as seen in one tertiary institution’s prospective study; poor communication was causative in 48% of cases (84). Proper timing of autopsy, organ donation, and DNR requests are vital and particularly sensitive. Poorly worded or timed requests cause distress and negatively affect perceptions of care quality (63). Communication techniques more often associated with family satisfaction are those that address emotion and express empathy assuring support of the family no matter the outcome or decision (35,69,81).
Qualitative studies exploring PICU communication show differences between PC and ICU provider speech (75,85). PICU physicians speak 50% more than PC providers on average. Pediatric intensivists often poorly balance speech dedicated to benefits versus risks of interventions, offer prognostication, mention hopelessness, or speak insensitively (85). Fifty four percent of PC’s speech provides emotional support, gives plain-language summary, discusses quality of life, elicits the family’s input, embraces uncertainty, provides praise, and normalizes emotions (75,85). Average number of attendees to family meetings numbered 19, pointing to the frequently overwhelming environment and content of PICU communications (85).
Pediatric PC quality measures in the PICU
In the PICU, PC remains an effective but underutilized quality-based intervention for children to achieve higher quality care (15). Some studies find decreased costs when PC is involved, particularly early in the illness trajectory. The UCLA Center for Health Policy showed an 11% ($1,677) reduction in monthly cost per patient, a 32% reduction in average LOS, reduced stress, sleep disturbance, and increased confidence amongst primary caregivers compared to patients who did not receive PC services (86).
A single-center study evaluated the top decile of pediatric patients by cost and divided the cohort into mortalities versus survivors and PC exposure or none. Within this cohort, only 10% were referred to PC. PC patients who died had significantly lower inpatient costs. Amongst survivors receiving PC, their costs were more, but significant difference disappeared when cost was adjusted for medical complexity. This phenomenon perhaps represents the population of “chronically critically ill”, patients whom are life-long high utilizers (87). Survivors receiving PC, though cost-equivalent to non-PC survivors, were half as likely to be readmitted to the hospital in the study period (87). Adult administrative data has compared approximately 9,000 live versus deceased “usual care” and “PC” discharges across eight hospitals. Live PC discharges had net savings of $1,696 per admission and $279 per diem savings, mostly laboratory and ICU costs. Deceased PC patients had net cost savings of $4,908 in direct cost and $374 per diem. These cost savings were statistically significant compared to live and deceased patients not receiving PC (88).
High value PC in the PICU is not completely encompassed by cost savings or measures. Hospital and PICU LOS, hospital readmission rates, and Emergency Department (ED) visits are frequently considered and easily measured sources of data and are comparable across cohorts (19). Often cited process measures are PC and HC referral rates. This infers intrinsic value to PC and HC and thus is threatened by circular logic, though exploring reasons for non-referral within this measure may be enlightening for systems or provider-related barriers to PC.
Other quality measures include discussions and documentation of ACPs. Only 20% of children with life-limiting conditions have a documented form of ACP, often related to poor recognition of patient trajectory (58,89). In a cross-sectional study of bereaved parents of childhood cancer, advanced planning for location of death increased home-based death and decreased hospital admissions in the last month of life. This is also important because the ability to plan the location of death, and to see that plan through, predicts better parental bereavement outcomes (90). Parents with ACPs had increased comfort with the setting and manner of their child’s death, and were less likely to have preferred a different location (34,58). Instances of early ACP reduced negative consequences for staff and families in terms of perceived suffering, moral and emotional distress, and poor family satisfaction (58). Certain locational death, specifically, out-of-PICU death, has been proposed to imply less resource-intensive care and instances where parental advocacy and decision-making have resulted in effective choice and medical stewardship.
Other tools assess parental PC needs such as the Parent and Children Palliative Care Needs Assessment, a 22-item survey measuring quality of life and care concerns to inform clinical practice (91). Surveyed parents of children with malignancy report unmet needs around sibling impact, symptom management, financial issues, and family cohesion. Measures evaluating coping indicate effects of critical illness on the family. Coping Resources and the Texas Revised Inventory of Grief were used to assess a group of bereaved parents. Those whose children died of acute illness had greater intensity of acute grief by inventory than those succumbing to CCCs (61,71). Parents’ physical coping resources and the perceived empathy of PICU staff were the most significant predictors of acute grief severity. Cognitive coping resources, emotional attitudes of staff, and adequacy of information predicted the intensity of long-term grief (59). Grief screening and in-unit mechanisms to provide anticipatory bereavement care may be appropriate for these high-risk groups. ICU mortality follow-up visits is another mechanism through which such tools could evaluate the coping reserves and grief of families.
Like care processes for the prevention of serious harm, such as central line associated blood stream infections (CLABSI) and catheter associated urinary tract infections (CAUTI), multiple researchers have assessed “Palliative Care-ICU Care” bundles (42,65,92). One adult effort involved a PC quality bundle with nine domains to improve the quality and quantity of PC communications (42). Domains included identification of medical decision makers, addressing advanced directive and resuscitation status, provision of an informational leaflet, systematic pain assessments and management, social work and spiritual support, and routine interdisciplinary family meeting. When applied to patients with ICU LOS greater than 5 days, compliance with the entire 9-domain bundle was 38–87% across 19 ICUs (44). The authors recognized the limitation in using process measures for compliance rather than outcome measures, but cited empiric data and society consensus statements supporting positive effects of these PC processes on outcomes (44). Other adult PC bundles utilized in high risk morbidity and mortality patients with predicted admissions of more than five days found that bundle compliance reduced LOS by one day and significantly reduced time to care plan consensus (92,93). These efforts showed variable and rather poor adherence to individual process measures at often less than 20%, particularly for care conferences (65,92,93).
Other measures considered in the literature include parental perceptions of decision-making, quality of life scores, parental stress, depression, utilization of bereavement and support groups, as well as medical decision-making support tools prior to family conferences (Question Prompt Lists), staff burn out, and resilience scores (29).
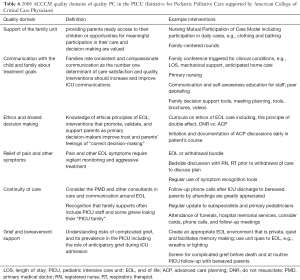
In considering measures of PC quality in the PICU, one must consider what constitutes high-quality PC. Adult ICU survivors, bereaved families, ICU, and PC providers in association with the Robert Wood Johnson Foundation’s “Promoting Excellence in EOL Care Project” elucidated several domains of quality PC for adults in the ICU (42). In a parallel effort, Pediatric Intensivists, PC experts, and parents in conjunction with the Initiative for Pediatric Palliative Care put forth 6 domains of high-quality PC that were adopted by the ACCCM in 2008 (4). Table 6 lists those domains, provides examples, and possible process measures (Table 6).
Full table
PC resources for PICUs
Palliative and intensive care experts call for still more data surrounding EOL and PC in children to further understand issues in this population (4,94) Without thorough understanding of root problems, interventional studies will be premature and not attuned to real population needs. Further, many issues are likely institution-dependent, based on population demographics, resources, and relative frequencies of conditions requiring ICU and PC. Overall, PC research is not well funded, limiting advances, and prompting concerns of early publication of insignificant findings. PC research comprises 0.2% of all NIH-funded research and from 2006–2010 only 20% of published adult or pediatric PC literature was supported by federal funding (31). Additionally, the majority of PC research funding is focused on adult and geriatric care rather than pediatrics. Potentially low cost, institution-responsive, and rapid cycle quality improvement efforts may allow teams to more efficiently address the unique issues facing PC in the PICU.
Lack of systematic PC education for trainees and providers plays a role in quality PC. Curricula within the subspecialty fellowship structure that includes PC and EOL expertise is advisable; indeed, the American College of Graduate Medical Education (ACGME) deems one of the major educational endpoints of PICU fellowship as “Care for children at EOL” (95). Perhaps, clinical exposure to home-based and ambulatory PC, including management of home ventilation, symptom management skills, and communication skills training, should be considered (29).
Training opportunities for other staff include communication skills training initiatives, such as, IntensiveTalk of the Vital Talk group, and the initiative for Pediatric Palliative Care’s Critical Care Communications skills program “C3”. Cellular phone applications such as Vital Tips has free family meeting planning and debriefing tools. For nurses, the End of Life Nursing Consortium (ELNEC) is widespread, and local hospices may offer outreach and education for nurses or other staff. Online educational modules for physicians are available from Education in Palliative and End of Life care (EPEC-Peds), and OPEN pediatrics (see Table S2)
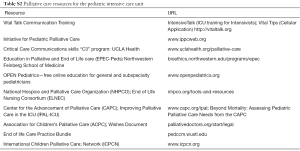
Full table
Other resources for inpatient units include a concurrent care implementation toolkit from the NHPCO as a resource for hospice organizations and individuals caring for children who would benefit from PC and HC services. The IPAL-ICU project funded by the NIH, National Institutes of Aging, and the Center to Advance Palliative Care has several resources to use in the unit and a library of growing research on PC quality in the ICU. For patients exploring PC and EOL care, the Five Wishes document supported by the Association for Children’s Palliative Care serves to alleviate difficulty “raising the issue” of the dying process. It is a conversational guide for discussions around hopes for the present, plans for when the disease progresses, goals of care during life threating events, and wishes for remembrance after death (89).
PC in the PICU is further guided by consensus statements and guidelines published by the AAP, ACCCM, the IOM, and American Heart Association (3,4,6). Additionally, clinical practice guidelines for quality PC and consensus guidelines on hospital PC programs from the Center to Advance Palliative Care supply guidance on team construct, role, and availability for PC teams.
Conclusions
Children in the PICU require both primary and subspecialty forms of PC often. Both forms are part of complete and high-quality care in the PICU. PC affects traditional quality measures such as hospital resource expenditures, LOS, and return to the hospital. It augments staff and parental experience of ICU care because the support, symptom management, communication, and advocacy associated with excellent PC augments these endpoints. Further research is needed to elucidate effective means to achieve quality PC surrounding accepted and known quality metrics and further address barriers to PC in the PICU. Quality improvement research will play a part as problem-focused, institutionally-responsive measures are more likely to align the resources, culture, issue, and needs of individual units.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. World Health Organization Definition of Palliative Care. World Health Organization Website. Available online: http://www.who.int/cancer/palliative/definition/en
- American Academy of Pediatrics. Committee on Bioethics and Committee on Hospital Care. Palliative care for children. Pediatrics 2000;106:351-7. [PubMed]
- Section on Hospice and Palliative Medicine and Committee on Hospital Care. Pediatric Palliative Care and Hospice Care Commitments, Guidelines, and Recommendations. Pediatrics 2013;132:966-72. [Crossref] [PubMed]
- Truog RD, Campbell ML, Curtis JR, et al. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Crit Care Med. Crit Care Med 2008;36:953-63. [Crossref] [PubMed]
- Berlinger N, Barfield R, Fleischman AR. Facing persistent challenges in pediatric decision-making: new Hastings Center guidelines. Pediatrics 2013;132:789-91. [Crossref] [PubMed]
- Field M, Behrman R. Institute of Medicine (US). Committee on palliative and end-of-life care for children and their families. When children die: improving palliative and end-of-life care for children and their families. Washington, DC: National Academy Press, 2003.
- Friebert S. Williams C. NHPCO’s facts and figures: pediatric palliative and hospice care in America. 2015. Available online: https://www.nhpco.org/sites/default/files/public/quality/Pediatric_Facts-Figures.pdf
- Mularski RA, Curtis JR, Billings JA, et al. Proposed quality measures for palliative care in the critically ill: a consensus from the Robert Wood Johnson Foundation Critical Care Workgroup. Crit Care Med 2006;34:S404-11. [Crossref] [PubMed]
- Feudtner C, Kang TI, Hexem KR, et al. Pediatric palliative care patients: a prospective multicenter cohort study. Pediatrics 2011;127:1094-101. [Crossref] [PubMed]
- Dingfield L, Bender L, Harris P, et al. Comparison of pediatric and adult hospice patients using electronic medical record data from nine hospices in the United States, 2008-2012. J Palliat Med 2015;18:120-6. [Crossref] [PubMed]
- Thienprayoon R, Lee SC, Leonard D, et al. Hospice Care for Children With Cancer: Where Do These Children Die? J Pediatr Hematol Oncol 2015;37:373-7. [Crossref] [PubMed]
- Bioethics Co. Palliative care for children. Pediatrics 2000;106:351-7. [Crossref] [PubMed]
- Feudtner C, Womer J, Augustin R, et al. Pediatric palliative care programs in children’s hospitals: a cross-sectional national survey. Pediatrics 2013;132:1063-70. [Crossref] [PubMed]
- Dumanovsky T, Augustin R, Rogers M, et al. The growth of palliative care in US hospitals: a status report. J Palliat Med 2016;19:8-15. [Crossref] [PubMed]
- Johnston EE, Rosenberg AR, Kamal AH. Pediatric-Specific End-of-Life Care Quality Measures: An Unmet Need of a Vulnerable Population. J Oncol Pract 2017;13:e874-80. [Crossref] [PubMed]
- Kochanek K, Murphy S, Xu J, et al. Mortality in the United States, 2016. NCHS data brief, no 293. Hyattsville, MD: National Center for Health Statistics, 2017.
- van Dyck PC, Kogan MD, McPherson MG, et al. Prevalence and characteristics of children with special health care needs. Arch Pediatr Adolesc Med 2004;158:884-90. [Crossref] [PubMed]
- Berry JG, Hall M, Hall DE, et al. Inpatient growth and resource use in 28 children's hospitals: a longitudinal, multi-institutional study. JAMA Pediatr 2013;167:170-7. [Crossref] [PubMed]
- Levetown M. Increasing Access to the Benefits of Palliative Care in the PICU. Pediatr Crit Care Med 2016;17:804-5. [Crossref] [PubMed]
- Edwards JD, Houtrow AJ, Vasilevskis EE, et al. Chronic conditions among children admitted to US PICUs: Their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med 2012;40:2196. [Crossref] [PubMed]
- Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics 2000;106:205-9. [PubMed]
- Fraser LK, Parslow R. Children with life-limiting conditions in paediatric intensive care units: a national cohort, data linkage study. Arch Dis Child 2018;103:540-7. [Crossref] [PubMed]
- Aslakson RA, Curtis JR, Nelson JE. The changing role of palliative care in the ICU. Crit Care Med 2014;42:2418. [Crossref] [PubMed]
- Garros D, Rosychuk RJ, Cox PN. Circumstances surrounding end of life in a pediatric intensive care unit. Pediatrics 2003;112. [Crossref] [PubMed]
- Peeler K, Wolfe J. Pediatric Unexpected Death: Examination of a Unique Population and Its End-of-Life Care Management (S741). J Pain Symptom Manage 2018;55:679. [Crossref]
- Roth A, Rapoport A, Widger K, et al. General paediatric inpatient deaths over a 15-year period. Paediatr Child Health 2017;22:80-3. [Crossref] [PubMed]
- Solomon MZ, Sellers DE, Heller KS, et al. New and lingering controversies in pediatric end-of-life care. Pediatrics 2005;116:872-83. [Crossref] [PubMed]
- Petrinec AB, Martin BR. Post-intensive care syndrome symptoms and health-related quality of life in family decision-makers of critically ill patients. Palliat Support Care 2017.1-6. [Epub ahead of print]. [Crossref] [PubMed]
- Marcus KL, Henderson CM, Boss RD. Chronic critical illness in infants and children: a speculative synthesis on adapting ICU care to meet the needs of long-stay patients. Pediatr Crit Care Med 2016;17:743-52. [Crossref] [PubMed]
- Meert KL, Keele L, Morrison W, et al. End-of-Life Practices Among Tertiary Care PICUs in the United States: A Multicenter Study. Pediatr Crit Care Med 2015;16:e231-8. [Crossref] [PubMed]
- Aldridge MD, Hasselaar J, Garralda E, et al. Education, implementation, and policy barriers to greater integration of palliative care: a literature review. Palliat Med 2016;30:224-39. [Crossref] [PubMed]
- Lutmer JE, Humphrey L, Kempton TM, et al. Screening criteria improve access to palliative care in the PICU. Pediatr Crit Care Med 2016;17:e335-42. [Crossref] [PubMed]
- Roth A, Friedman J, Rapoport A, et al. editors. A retrospective review of general paediatric inpatient deaths over time. BMC proceedings; 2015: BioMed Central.
- Kelly J, Ritchie J, Donovan L, et al. A Retrospective Review of Resuscitation Planning at a Children’s Hospital. Children (Basel) 2018;5. [Crossref] [PubMed]
- Michelson K, Siegel L, Morgan C. Palliative care in the pediatric intensive care unit. Pediatr Crit Care Med: basic science and clinical evidence. 2014;1. Available online: http://learnicu.net/Communications/Critical-Connections/Archives/Pages/Palliative-Care-in-the-Pediatric-Intensive-Care-Unit.aspx
- Truog RD, Meyer EC, Burns JP. Toward interventions to improve end-of-life care in the pediatric intensive care unit. Crit Care Med 2006;34:S373-9. [Crossref] [PubMed]
- Sprung CL, Truog RD, Curtis JR, et al. Seeking worldwide professional consensus on the principles of end-of-life care for the critically ill. The Consensus for Worldwide End-of-Life Practice for Patients in Intensive Care Units (WELPICUS) study. Am J Respir Crit Care Med 2014;190:855-66. [Crossref] [PubMed]
- Suttle ML, Jenkins TL, Tamburro RF. End-of-Life and Bereavement Care in Pediatric Intensive Care Units. Pediatr Clin North Am 2017;64:1167-83. [Crossref] [PubMed]
- Burns JP, Sellers DE, Meyer EC, et al. Epidemiology of death in the pediatric intensive care unit at five US teaching hospitals. Crit Care Med 2014;42:2101. [Crossref] [PubMed]
- Carter BS, Howenstein M, Gilmer MJ, et al. Circumstances surrounding the deaths of hospitalized children: opportunities for pediatric palliative care. Pediatrics 2004;114:e361-6. [Crossref] [PubMed]
- Contro NA, Larson J, Scofield S, et al. Hospital staff and family perspectives regarding quality of pediatric palliative care. Pediatrics 2004;114:1248-52. [Crossref] [PubMed]
- Nelson JE, Bassett R, Boss RD, et al. Models for structuring a clinical initiative to enhance palliative care in the intensive care unit: a report from the IPAL-ICU Project (Improving Palliative Care in the ICU). Crit Care Med 2010;38:1765. [Crossref] [PubMed]
- Grunauer M, Mikesell C. A review of the Integrated Model of Care: An opportunity to respond to extensive palliative care needs in pediatric intensive care units in under-resourced settings. Front Pediatr 2018;6:3. [Crossref] [PubMed]
- Nelson JE, Mulkerin CM, Adams LL, et al. Improving comfort and communication in the ICU: a practical new tool for palliative care performance measurement and feedback. Qual Saf Health Care 2006;15:264-71. [Crossref] [PubMed]
- Hua MS, Li G, Blinderman CD, et al. Estimates of the need for palliative care consultation across United States intensive care units using a trigger-based model. Am J Respir Crit Care Med 2014;189:428-36. [Crossref] [PubMed]
- McKane K. Implementing Early Pediatric Palliative Care for Patients in the Pediatric Intensive Care Unit (QI837). J Pain Symptom Manage 2018;55:720. [Crossref]
- O’Mahony S, McHenry J, Blank AE, et al. Preliminary report of the integration of a palliative care team into an intensive care unit. Palliat Med 2010;24:154-65. [Crossref] [PubMed]
- Jones PM, Carter BS. Pediatric palliative care: feedback from the pediatric intensivist community. Am J Hosp Palliat Care 2010;27:450-5. [Crossref] [PubMed]
- Howes C. Caring until the end: a systematic literature review exploring Paediatric Intensive Care Unit end-of-life care. Nurs Crit Care 2015;20:41-51. [Crossref] [PubMed]
- Bartel DA, Engler AJ, Natale JE, et al. Working with families of suddenly and critically ill children: physician experiences. Arch Pediatr Adolesc Med 2000;154:1127-33. [Crossref] [PubMed]
- Davies B, Sehring SA, Partridge JC, et al. Barriers to palliative care for children: perceptions of pediatric health care providers. Pediatrics 2008;121:282-8. [Crossref] [PubMed]
- Eagle S, Creel A, Alexandrov A. The effect of facilitated peer support sessions on burnout and grief management among health care providers in pediatric intensive care units: A pilot study. J Palliat Med 2012;15:1178-80. [Crossref] [PubMed]
- Epstein EG, Hamric AB. Moral distress, moral residue, and the crescendo effect. J Clin Ethics 2009;20:330. [PubMed]
- Stayer D, Lockhart JS. Living with dying in the pediatric intensive care unit: a nursing perspective. Am J Crit Care 2016;25:350-6. [Crossref] [PubMed]
- Burns JP, Mitchell C, Griffith JL, et al. End-of-life care in the pediatric intensive care unit: attitudes and practices of pediatric critical care physicians and nurses. Crit Care Med 2001;29:658-64. [Crossref] [PubMed]
- Lee KJ, Dupree CY. Staff experiences with end-of-life care in the pediatric intensive care unit. J Palliat Med 2008;11:986-90. [Crossref] [PubMed]
- Lee KJ, Forbes ML, Lukasiewicz GJ, et al. Promoting staff resilience in the pediatric intensive care unit. Am J Crit Care 2015;24:422-30. [Crossref] [PubMed]
- Mitchell S, Dale J. Advance Care Planning in palliative care: a qualitative investigation into the perspective of Paediatric Intensive Care Unit staff. Palliat Med 2015;29:371-9. [Crossref] [PubMed]
- Meert KL, Thurston CS, Thomas R. Parental coping and bereavement outcome after the death of a child in the pediatric intensive care unit. Pediatr Crit Care Med 2001;2:324-8. [Crossref] [PubMed]
- Meert KL, Thurston CS, Sarnaik AP. End-of-life decision-making and satisfaction with care: parental perspectives. Pediatr Crit Care Med 2000;1:179-85. [Crossref] [PubMed]
- Meert KL, Briller SH, Schim SM, et al. Examining the needs of bereaved parents in the pediatric intensive care unit: a qualitative study. Death Stud 2009;33:712-40. [Crossref] [PubMed]
- Brooten D, Youngblut JM, Caicedo C, et al. Parents' Acute Illnesses, Hospitalizations, and Medication Changes During the Difficult First Year After Infant or Child NICU/PICU Death. Am J Hosp Palliat Care 2018;35:75-82. [Crossref] [PubMed]
- Brooten D, Youngblut JM, Seagrave L, et al. Parent’s perceptions of health care providers actions around child ICU death: what helped, what did not. Am J Hosp Palliat Care 2013;30:40-9. [Crossref] [PubMed]
- Nelson JE, Puntillo KA, Pronovost PJ, et al. In their own words: Patients and families define high-quality palliative care in the intensive care unit. Crit Care Med 2010;38:808. [Crossref] [PubMed]
- Black MD, Vigorito MC, Curtis JR, et al. A multifaceted intervention to improve compliance with process measures for ICU clinician communication with ICU patients and families. Crit Care Med 2013;41:2275-83. [Crossref] [PubMed]
- Kaye EC, Abramson ZR, Snaman JM, et al. Productivity in pediatric palliative care: Measuring and monitoring an elusive metric. J Pain Symptom Manage 2017;53:952-61. [Crossref] [PubMed]
- Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics 2006;118 Suppl 3:S203-18. [Crossref] [PubMed]
- Macdonald ME, Liben S, Carnevale FA, et al. Parental perspectives on hospital staff members' acts of kindness and commemoration after a child's death. Pediatrics 2005;116:884-90. [Crossref] [PubMed]
- Michelson KN, Clayman ML, Haber-Barker N, et al. The use of family conferences in the pediatric intensive care unit. J Palliat Med 2013;16:1595-601. [Crossref] [PubMed]
- de Vos MA, Bos AP, Plötz FB, et al. Talking with parents about end-of-life decisions for their children. Pediatrics 2015;135:e465-76. [Crossref] [PubMed]
- Macnab AJ, Northway T, Ryall K, et al. Death and bereavement in a paediatric intensive care unit: Parental perceptions of staff support. Paediatr Child Health 2003;8:357-62. [Crossref] [PubMed]
- Davies R. Mothers' stories of loss: their need to be with their dying child and their child's body after death. J Child Health Care 2005;9:288-300. [Crossref] [PubMed]
- Treece PD, Engelberg RA, Crowley L, et al. Evaluation of a standardized order form for the withdrawal of life support in the intensive care unit. Crit Care Med 2004;32:1141-8. [Crossref] [PubMed]
- Vesely C, Newman V, Winters Y, et al. Bringing Home to the Hospital: Development of the Reflection Room and Provider Perspectives. J Palliat Med 2017;20:120-6. [Crossref] [PubMed]
- Ciriello AG, Dizon ZB, October TW. Speaking a Different Language: A Qualitative Analysis Comparing Language of Palliative Care and Pediatric Intensive Care Unit Physicians. Am J Hosp Palliat Care 2018;35:384-9. [Crossref] [PubMed]
- García-Salido A, Santos-Herranz P, Puertas-Martín V, et al. Retrospective study of children referred from paediatric intensive care to palliative care: Why and for what. An Pediatr (Barc) 2018;88:3-11. [PubMed]
- McDonagh JR, Elliott TB, Engelberg RA, et al. Family satisfaction with family conferences about end-of-life care in the intensive care unit: increased proportion of family speech is associated with increased satisfaction. Crit Care Med 2004;32:1484-8. [Crossref] [PubMed]
- Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med 2007;356:469-78. [Crossref] [PubMed]
- Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med 2012;40:2327-34. [Crossref] [PubMed]
- Curtis JR, Nielsen EL, Treece PD, et al. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med 2011;183:348-55. [Crossref] [PubMed]
- October TW, Fisher KR, Feudtner C, et al. The parent perspective: "being a good parent" when making critical decisions in the PICU. Pediatr Crit Care Med 2014;15:291-8. [Crossref] [PubMed]
- Odeniyi F, Nathanson PG, Schall TE, et al. Communication Challenges of Oncologists and Intensivists Caring for Pediatric Oncology Patients: A Qualitative Study. J Pain Symptom Manage 2017;54:909-15. [Crossref] [PubMed]
- Guidance for clinicians involved in end-of-life care of children. Lancet 2015;385:1261. [Crossref] [PubMed]
- Studdert DM, Burns JP, Mello MM, et al. Nature of conflict in the care of pediatric intensive care patients with prolonged stay. Pediatrics 2003;112:553-8. [Crossref] [PubMed]
- Hebert LM, Watson AC, Madrigal V, et al. Discussing Benefits and Risks of Tracheostomy: What Physicians Actually Say. Pediatr Crit Care Med 2017;18:e592-7. [Crossref] [PubMed]
- Gans D, Kominski GF, Roby DH, et al. Better outcomes, lower costs: palliative care program reduces stress, costs of care for children with life-threatening conditions. Policy Brief UCLA Cent Health Policy Res 2012;(PB2012-3):1-8.
- Smith AG, Andrews S, Bratton SL, et al. Pediatric palliative care and inpatient hospital costs: a longitudinal cohort study. Pediatrics 2015;135:694-700. [Crossref] [PubMed]
- Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med 2008;168:1783-90. [Crossref] [PubMed]
- Fraser J, Harris N, Berringer A, et al. Advanced care planning in children with life-limiting conditions–the Wishes Document. BMJ Publishing Group Ltd., 2010.
- Dussel V, Kreicbergs U, Hilden JM, et al. Looking beyond where children die: determinants and effects of planning a child's location of death. J Pain Symptom Manage 2009;37:33-43. [Crossref] [PubMed]
- Donnelly JP, Downing K, Cloen J, et al. Development and Assessment of a Measure of Parent and Child Needs in Pediatric Palliative Care. J Pain Symptom Manage 2018;55:1077-84.e2. [Crossref] [PubMed]
- Lilly CM, De Meo DL, Sonna LA, et al. An intensive communication intervention for the critically ill. Am J Med 2000;109:469-75. [Crossref] [PubMed]
- Penrod JD, Pronovost PJ, Livote EE, et al. Meeting standards of high-quality intensive care unit palliative care: clinical performance and predictors. Crit Care Med 2012;40:1105. [Crossref] [PubMed]
- Treece PD, Engelberg RA, Shannon SE, et al. Integrating palliative and critical care: description of an intervention. Crit Care Med 2006;34:S380-7. [Crossref] [PubMed]
- Carraccio C, Burke AE. Beyond competencies and milestones: adding meaning through context. J Grad Med Educ 2010;2:419-22. [Crossref] [PubMed]


