An overview of mechanical circulatory support in single-ventricle patients
Introduction
Individuals with single-ventricle physiology comprise a complex, and heterogenous, sub-group of patients with congenital heart disease (CHD). Palliation to single-ventricle physiology involves multiple operations eventually resulting in passive blood flow to the pulmonary circulation with the single-ventricle providing systemic blood flow. When cardiac failure develops in these patients, providing mechanical circulatory support (MCS) is an especially challenging problem due to the altered systemic venous return and arterial connections. However, MCS, in the form of extracorporeal membrane oxygenation (ECMO) or a ventricular assist device (VAD), may represent the only alternative to death. While some initial studies have been reported, the outcome of single-ventricle patients who require MCS remains unclear.
With improved operative techniques and critical care, the single-ventricle population is continually increasing. Currently, more than 1,000 new Fontan operations are performed annually in the United States and Canada alone (1). The first generation of surviving patients with Fontan physiology are now well into adulthood (2). These numbers include only patients who have reached the Fontan stage, and do not include the single-ventricle patients currently living at an earlier stage of palliation. As the single-ventricle population has increased, heart failure in patients with a single-ventricle has become the most common indication for heart transplant in children (3). Not surprisingly, in correlation, MCS for single-ventricle patients has increased, and is only likely to further increase in the future (4). Currently, nearly half of all pediatric patients who are placed on ECMO for cardiac indications have a single-ventricle (5).
ECMO has been utilized extensively in pediatric patients due to its speed of initiation, ability to provide both cardiac and pulmonary support, ability to support patients of any size (except the smallest of neonates), and the ability to seamlessly transition from cardiopulmonary bypass (CPB). However, survival for patients who require ECMO is poor for patients with CHD, and even worse for patients with a single-ventricle (5). It is employed to allow for myocardial recovery, support until a reparative operation can be performed or (more recently) as a bridge to a VAD, and potentially as a bridge to decision. ECMO, however, is hampered by a significant complication rate that increases with the duration of support (6,7). Currently, VADs have been increasingly utilized in many pediatric situations (8-13). A VAD offers the benefits of a longer duration of support, therefore, allowing more time for either recovery or to find a suitable donor (14). Therefore, ECMO and VAD support should be treated as unique therapies with different indications for use.
Importantly, while patients are often considered within a single category, “single-ventricle”, they can develop cardiac failure at any stage of palliation and at any time postoperatively. Therefore, the term “single-ventricle” encompasses a heterogenous patient population. Further, the mode of failure is often distinct, within each stage of palliation and for each patient specifically, making each patient with cardiac failure and any form of single-ventricle anatomy truly unique. Lastly, due to the small sample sizes, ECMO and VADs are often considered together as MCS, despite device selection having significant implications on the patient’s outcome.
This review focuses on the current utilization of MCS in patients with a single-ventricle. As this is an uncommon indication, and each situation is very unique, the data are limited, as is the generalizability of the published literature. We discuss the overall reported outcomes of MCS in single-ventricle patients, the implications of the varied cardiac anatomy, MCS device chosen, cannulation strategies and technical considerations. Data from the largest existing series is cited whenever possible. Case reports or small series are used as necessary, though, with appropriate consideration of a significant publication bias.
Outcomes
The current data available evaluating the survival of single-ventricle patients who require MCS are quite limited and difficult to interpret. Most consider the survival to hospital discharge of single-ventricle patients who require MCS to be 30–50% (4,15-18). When evaluating all patients with a single-ventricle who underwent MCS over a 25-year period at a single institution, of 57 patients, 18 (33%) survived to hospital discharge (16). Considering the Berlin Heart EXCORE IDE database, evaluating outcomes for single-ventricle patients, survival of 26 patients was 42% (4). Utilizing the KID (Kids Inpatient Database) to analyze survival in single-ventricle patients who require ECMO allowed for an analysis of 701 patients over 10 years; overall survival was 43%, and importantly, the outcomes did not improve over the decade evaluated (15). Though these are the most informative studies available, each evaluated the outcomes of a heterogenous patient population, with unique anatomy and indications for MCS. Definitively, the data does support that single-ventricle patients who require ECMO are likely to have poor outcomes, even worse than other CHDs (4). Additionally, patients who demonstrate any type of end-organ dysfunction prior to MCS initiation are more likely to have a poor outcome (9).
Impact of stage of palliation of single-ventricle physiology
Likely the most important factor impacting the anticipated survival for any patient requiring MCS is the stage of palliation (19). This is for many reasons, first, being that the patient’s current anatomy likely impacts their response to MCS (20). Additionally, stage of palliation correlates with many factors that likely dictate the patient’s outcome including: size, age, available devices, ability to anticoagulate and availability of donors for transplant. In addition, possibly the most important factor is indication. Clearly a patient who requires ECMO for inability to wean from CPB after Norwood is different than an adult with late Fontan failure who requires VAD as a bridge to transplant.
First stage
Patients who require MCS after the first stage of palliation are often suffering from post-cardiotomy shock. As these patients recover from CPB and adapt to their new circulation, MCS may be necessary as a bridge to recovery. In these patients, the MCS must provide cardiopulmonary support with ECMO or, if only cardiac support, provide sufficient flow through the systemic circulation and the systemic-pulmonary shunt. In the previously referenced studies evaluating the survival for single-ventricle patients who received MCS, survival of patients who required MCS after their first stage were 32% for patients supported with a VAD (16) and 11% when considering the Berlin Heart EXCOR IDE database (4).
Most would expect this patient population to have a poor survival, as it is essentially selected for neonatal post-cardiotomy patients who are unable to tolerate their new circulation. Indeed, the survival for these patients in the reports referenced above is not good. Others, however, have reported over 40% survival with MCS utilization after stage I of palliation (17). Substantially different results may be in part due to device selection, surgical technique or postoperative care, however, patient selection is by far the most likely factor. Supporting this, when utilized on patients who have a repairable problem (usually shunt thrombosis), when MCS support is required between stages I and II, survival greater than 80% is reported (18). Further, when VAD implantation is utilized after every Norwood, even in patients who are stable, survival approaches 90% (21). Though some might state this as evidence for the routine use of MCS after Norwood, much more likely, it demonstrates the impact of patient selection. Whether indiscriminate use of MCS would improve overall survival by providing early assistance to the most difficult patients, remains to be seen.
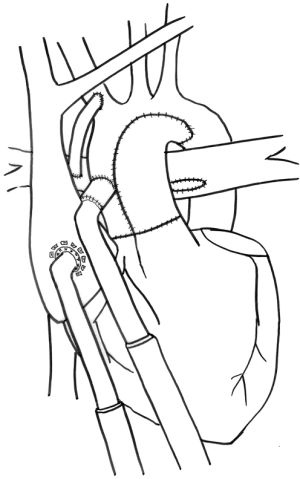
An additional consideration for post-Norwood patients is the type of systemic-pulmonary shunt, Blalock-Taussig (BT) or Sano. Though it has never been studied, the type of systemic-pulmonary shunt will influence outcomes of patients after stage I who require MCS. With a Sano shunt, flow through the pulmonary circulation is dependent on blood within the failing ventricle, which is diminished in a ventricle that is decompressed. We have typically taken down the Sano shunt and created a BT shunt at the time of VAD implant; or, on occasion, we have disconnected the proximal aspect of the Sano and reattached it to the outflow graft of the VAD (Figure 1) (22).
Second stage
MCS after the second stage of palliation has the advantage of a more efficient anatomy than a Norwood, due to the superior cavopulmonary connection (SCPC) supplying the pulmonary vasculature in series with the systemic circulation (Figure 2). However, with only a SCPC, the decompression of the single-ventricle has competing implications. Both the VAD augmented cardiac output and ventricular decompression act to increase flow through the Glenn anastomosis and improve oxygenation. Conversely, there will also be an increased flow of deoxygenated blood through the inferior vena cava (IVC). The summation has been shown in an experimental model to lead to increased oxygen delivery (23). Additionally, in case reports, it leads to improved hemodynamics (24,25). MCS use after the second stage, when considering the previously discussed manuscripts, has a survival of 36% for all VADs (16) and 58% when looking at the Berlin Heart EXCOR IDE database (4). These are both very small patient populations, 11 and 26, respectively.
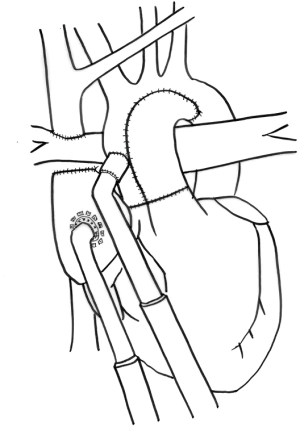
Some institutions, feel that patients who deteriorate shortly after Glenn are demonstrating their inability to tolerate the circulation and that they warrant consideration of SCPC takedown and recreation of the systemic-pulmonary shunt while implanting the VAD (16). These sentiments are based on poor outcomes with attempts at VAD support immediately post SCPC. Importantly, implantation of MCS on the systemic side would not address underlying problems with pulmonary vasculature or other limitations to pulmonary blood flow that are not related to sufficient cardiac output. These patients would subsequently require a heart transplant. As much as any, the patients who require MCS after SCPC have outcomes that remain unknown.
Third stage
For patients with a failing Fontan, when taken as a whole, the previously referenced studies noted survival to hospital discharge of 40% for any VAD (16) and 60% when considering the Berlin Heart EXCOR IDE database (4). Inflow cannulation can be performed via the ventricular apex (Figure 3A) or the atrium (Figure 3B). The outcomes for patients with a failed Fontan must be considered based on indication for VAD implant. Certainly, those with early Fontan failure are a different patient population than those with late Fontan failure. Further, in patients with late Fontan failure, the indication for VAD can be either impaired ventricular function (IVF) or preserved ventricular function (PVF) but with failing Fontan physiology.
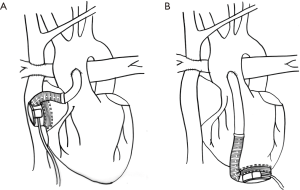
Heart failure in the acute postoperative period after Fontan has been successfully supported with MCS in multiple instances. Some are for patients in the acute postoperative Fontan period (26), others for Fontan patients who required additional cardiac operations and developed postcardiotomy shock (27,28). There have been reports of successful bridge to recovery postoperatively, after even long durations of VAD support (29).
For late failing Fontan, indications typically fall on the spectrum between IVF and PVF. Considering VAD implant for these two indications, it is unlikely they would have similar outcomes with the same support. Patients with IVF would be expected to benefit quickly from a VAD implanted to support the systemic ventricle, but those with a predominately failing physiology may take significantly more time to recover. This is what was initially seen with heart transplant for failing Fontan, the outcomes for patients with IVF were initially superior (30). Though, with improved preoperative care, operative techniques and postoperative care, it has now been shown that survival after heart transplant may now be equivalent (31,32). It has been argued that a patient with IVF would do very well with a single VAD supporting the systemic circulation, whereas a patient with predominately PVF may require support of both sides of the circulation, either with two VADs or a total artificial heart (TAH). There is insufficient data at this point to argue either against or for such approaches; further, few patients present with discrete phenotypes of one or the other, with significant overlap in most.
These patients who survive into adolescence or adulthood but subsequently develop late Fontan failure comprise a group that should be expected to have a high rate of survival. Indeed, many reports exist demonstrating survival to transplant for both PVF (33,34) and IVF (35). Considering that the vast majority are case reports, a substantial publication bias probably gives a false impression of the success rate. Likely one of the main factors leading to poor outcomes is the reluctance of the heart failure team to pursue VAD implant, either due to unfamiliarity of the anatomy or acknowledgement of the increased risk associated with the procedure (14). So when MCS is finally initiated, the patient is more debilitated than a traditional patient who requires VAD implant. Importantly, late failing Fontans are a group of patients which will only increase. Though the operations for this patient population are quite difficult, with appropriate patient selection and timing, outcomes should be expected to be quite high.
Device type
Currently, many devices are available to support patients with heart failure. The device chosen depends on many factors including the indication [anticipated recovery or bridge to transplant (BTT)], pulmonary function, anatomy and size. In addition, if utilized as a BTT, the anticipated duration on the waiting-list is an important factor and, therefore, additional factors must be considered such as antibody status.
ECMO is the first established MCS. Being comprised of both a pump and an oxygenator, it has long been utilized to treat patients with cardiorespiratory failure. It remains very useful for multiple indications, mainly for its ease and speed in initiation/cannulation and termination/decannulation. It can be initiated using the internal jugular and carotid in young children or the femoral artery and vein in larger children and adults, or centrally via median sternotomy. Additionally, transition from CPB to ECMO is quite easy. It does, however, have a significant risk profile, with a serious risk of adverse events with any significant duration. Due to these attributes it is ideally suited for emergencies or support that is anticipated to be of short duration (6,7). Limitations of ECMO stem in part from the typical required approach: peripheral cannulation generally limits flow rates (in contrast to central cannulation) due to vessel size, increased surface exposure from long segments of an intravascular cannula (and hence greater chance for activation of clotting cascades as well as the need for anticoagulation), and inherent limitations of mobility and rehabilitation potential related to concerns with cannula dislodgment. Otherwise ECMO circuitry can be quite similar to those commonly used in the pediatric setting with temporary devices (see below) with the added oxygenator.
Many pediatric VADs are simply ECMO without the oxygenator, such as a CentriMag or PediMag [St. Jude Medical Inc. (Thoratec), St. Paul, MN, USA]. Though still hampered by many of the disadvantages of ECMO, most feel they are far better tolerated with a lower risk of adverse events with a longer duration. Patients who have undergone an operation may require transition from CPB to a VAD of this type for a short period of time to allow for recovery from the stress of an operation as well as the adaptation to new anatomy (21). Additionally, for patients who initially came off bypass, but then developed progressively worsening failure, VAD support may prove necessary in the acute postoperative period, potentially urgently. ECMO or an extracorporeal VAD is ideally suited for these indications.
For longer-term support, a different set of devices has emerged as the choice whenever possible. These include the Berlin Heart EXCOR (EXCOR) (Berlin Heart, GmbH, Berlin, Germany), the HeartMate II (HMII) [St. Jude Medical Inc. (Thoratec), St. Paul, MN, USA] and the HeartWare HVAD (HVAD) (HeartWare International, Framingham, MA, USA). The EXCOR has shown significant utility in pediatric patients in nearly all settings, including patients with a single-ventricle (4,9,11,36-38). The EXCOR is an extracorporeal pneumatically driven pulsatile device with a large controller tower. The HM II and the HVAD are much smaller intracorporeal continuous-flow (CF) devices with easily transported battery packs. These CF devices, while originally designed for adults, have been used extensively in pediatrics (39,40). Their use in patients with a single-ventricle remains quite limited, though there are case reports (33-35). In pediatric patients, practice has recently shifted to CF devices in patients large enough to support one (41). The CF design allows for a smaller device, which allows for improved patient mobility and rehabilitation. Going forward, the majority of devices placed in patients of sufficient size will be a CF device.
While not as commonly utilized, some report excellent experiences with the Syncardia Total Artificial Heart (Syncardia Systems, Tucson, AZ, USA) in pediatric patients, and its use has been described in a single-ventricle patient (42,43). In a patient with a failing single-ventricle, most specifically with failing physiology or a mixed picture, it may prove to have significant utility. Theoretically, it could allow for the advantages of BiVAD support with improved rehabilitation due to the increased mobility over two devices. Indeed, its use has been described successfully in a patient with failing Fontan physiology (43).
We advocate for the use of an intracorporeal VAD whenever possible, for multiple reasons including, the demonstrated improved outcomes in other populations, the improved ability for rehabilitation and the improved quality of life (8,9,11,16,44). The Berlin Heart EXCOR may be necessary if the patient is small, but if the patient is large enough [body surface area (BSA) is >1 m2], a CF VAD should be used. We prefer the HeartWare HVAD due to its size. In the patient who is acutely postoperative, either unable to wean from bypass or deteriorates in the acute postoperative period, we recommend transition from CPB to ECMO or VAD such as the PediMag (depending on pulmonary function). If postoperative failure develops suddenly and requires urgent MCS, ECMO should be initiated peripherally. However, once it is determined that there is no pulmonary pathology, the patient should be converted to a VAD to allow for recovery or BTT. Specifically, we attempt to avoid the use of an oxygenator whenever possible (45).
Configuration
When utilizing MCS in a patient with a single-ventricle, the patient’s anatomy and pathology must both be considered so that the MCS is used optimally. Patients with a single-ventricle fail due to failure of the ventricle to support the systemic circulation (or systemic and pulmonary in a shunted patient), or due to their inability to tolerate their passive pulmonary circulation. Therefore, the selection of the device configuration is likely the most important controllable factor, with the exception of whether or not to utilize MCS at all. Multiple configurations are possible, systemic VAD placement, VAD support for only the pulmonary circulation and BiVAD or TAH support, all of which have been described.
When supporting patients after stage 1 of palliation, the device must provide flow to support both the systemic and pulmonary circulation. We have recently come to prefer a cannulation technique which utilizes the common atrium for inflow and the neoaorta as outflow (22). In our experience, we have found cannula selection to be very important in limiting thrombus formation. For the inflow, we prefer the arterial Berlin Heart cannula due to its smaller surface within the atrium and lower likelihood of creating stagnant flow. The outflow cannula is the Berlin Heart aortic cannula with either a homograft or Gore-Tex (W.L. Gore and Associates, Elkton, MD, USA) extension. If the patient has a Sano shunt, this is taken down. To provide pulmonary flow, either a BT shunt is created or the proximal end of the Sano shunt is anastomosed end-to-side to the outflow cannula via the homograft or Gore-Tex extension (45).
For patients after Fontan, the vast majority of patients who receive VAD support, receive support of the systemic ventricle. In a patient with IVF without other signs of failure, this is the ideal treatment option. However, for patients with failing physiology, or a more mixed picture, the decision is more difficult. Our bias is that a single VAD supporting the systemic ventricle will allow for improved physiology as we believe all of these patients suffer from a substantial, and under-appreciated, collateral burden that “steals” 20–50% of cardiac output (and in turn an increased volume load that burdens the single ventricle), in addition to addressing any other underlying pulmonary circulation issues, despite absence of hemodynamic significance (i.e., if there is no gradient by angiography demonstrates suboptimal anatomy). We do not believe that pulmonary blood flow can be augmented by “sucking” it across the pulmonary vascular bed. A decrease in central venous pressure upon initiation of inotropic support demonstrates the potential benefit of a VAD for these patients. Some have argued that support of both the systemic ventricle and the right side may be superior, especially if the elevated pulmonary pressures are thought unlikely to be “reversible”. It is hard to believe that these same patients, not too long ago, were deemed to have low enough pulmonary pressures to undergo Fontan palliation. Nevertheless, it has been shown that a single right sided VAD may be sufficient for some patients (46). However, placement of a right sided VAD may be technically quite challenging.
BiVAD support has been described in the failing Fontan patient (47). Though some feel that the addition of a second VAD increases the risk of complications, it has been shown that BiVAD support can be done without increased risk (9,11,48). Implantation of a second pump does, however, increase the surgical complexity of an already difficult operation. Further, the disadvantage of impaired rehabilitation due to a second device may have significant implications. Though, if necessary, not providing support for the pulmonary circulation would leave the patient debilitated, clearly a worse alternative.
Technical difficulties
Utilization of MCS in patients with a single-ventricle is a surgical challenge. These patients require a redo-sternotomy, often times multiple, with what can be a difficult dissection. Patients often have foreign material present, either a Fontan conduit or a systemic-pulmonary shunt. Injury to any native or foreign structures may have catastrophic implications. Further, if a cardiac chamber is inadvertently entered during dissection prior to cross-clamp application, an air embolism is possible. In addition, these patients commonly have substantial collateral flow, which can complicate the dissection prior to CPB and impair visualization once on bypass. Regardless of the configuration of VAD support chosen, sternal entry and the dissection process may be difficult in these patients. For that reason, in our population of multiple redo-sternotomies a femoral arterial and venous line are placed prior to incision. These lines are large enough to accept a wire for percutaneous CPB initiation if it becomes emergently necessary.
For patients who are going to have a VAD implanted, inflow cannula placement is more complex than in a normal ventricle. The inflow can be placed in either the apex of the ventricle or the atrium. Placement within the apex is more familiar to most surgeons but may require significant trabecular resection. Atrial placement avoids the difficulties with trabeculations, but the inherent lack of strength in the atrial wall does create concerns for suck down events. Single-ventricle patients may have a single right ventricle, which will undoubtedly have more-dense trabeculations than a single left ventricle. Further, as the ventricle dilates, the apex can be displaced, making it difficult to identify. As the geometry of the single-ventricle dilates and changes, the angle of the inflow cannula may be difficult to predict. There have been multiple reports of VAD implants into the systemic right ventricle of patients with dextro-transposition of the great arteries (D-TGA) after atrial switch (49-51) or in congenitally corrected TGA (CC-TGA) (52-54). In many, apical inflow cannula placement is aided by use of a needle with transesophageal echocardiographic guidance (55-57). This will allow the cannula to be far enough from the septum and atrioventricular valve to avoid suck down events. In pediatric patients, especially if the ventricular muscle is thinned, the depth of the inflow cannula can be decreased by placing multiple felt rings on the epicardium (35). This latter approach has also been taken with cannulation of the common atrium using the HVAD, successfully bridging Fontan patients to transplant (58).
For patients who do require right sided support, usually, significant surgical reconstruction is necessary; a capacitance chamber may need to be constructed to accommodate the inflow cannula. This chamber must be created from the already surgically manipulated superior vena cava (SVC) and IVC. Outflow cannula placement is into the pulmonary arteries, which also require reconstruction. This significantly increases the operative and bypass time and the surgical risk. As an alternative to BiVAD support, a TAH can be utilized. In this instance, the operative difficulty revolves around returning the systemic venous return to the right atrium, which may need reconstruction, again a major operation (43).
Future directions
ECMO utilization has been shown to be increasing as a percentage of hospitalizations for patients with a single-ventricle (4). In addition, the population of patients who exist with a single-ventricle is continuing to increase. Knowing that a certain percentage of these Fontan patients will eventual fail means that, going forward, an increasing number of VADs will be implanted in patients with a single-ventricle. As many of these will be placed as a BTT, improved timing and patient selection of VAD implantation will become crucial. To accomplish this, risk factors for a poor outcome need to be more clearly delineated. To that end, a registry was created to identify the risk factors and evaluate the outcomes of patients with a single-ventricle who require MCS (59).
For these complicated patients, going forward, computer models have been developed (60). Using computer models, a simulator has been created to allow for the simulation of a surgical procedure to evaluate the impact of operations at all three stages of palliation (61). These, and other, computer models can be used demonstrate the impact of MCS utilization in patients with different anatomy (20). As the number of patients treated increases, these models could potentially become more accurate and robust. Theoretically, these could be used to simulate different devices placed in different configurations. This could have a significant impact on preoperative planning.
In patients with single-ventricle and other complex CHDs, three-dimensional (3D) printing has been utilized in some of the most difficult scenarios (62,63). It provides the spatial orientation to potentially improve preoperative planning. Further, it is a useful tool to aid communication with the patient and family, cardiologists and critical care team (64). It has shown utility in allowing for preoperative planning of inflow cannula placement when faced with the challenges of implanting a VAD into a systemic right ventricle (65). Going forward, it may be utilized with more frequency with these complex cases.
For Fontan patients, a unique device has been proposed, and tested in an animal model, a viscous impeller pump (VIP) (66). This device is inserted transvenous and is placed at the junction of the SVC/IVC and PAs (67). As the device rotates, the right sided pressure is augmented and cardiac output is improved (68). It could be used in the failing Fontan, specifically for patients with failing physiology. This could provide temporary support to improve the end-organ function in a patient prior to transplant (69). Further, it could improve patient selection by identifying those who recover end-organ function, presumably a marker for a good outcome post-heart transplant. Currently the use of this device is preclinical.
There are new devices on the horizon in the adult population that could be brought into the pediatric realm. Recently the relatively new HeartMate 3 device [St. Jude Medical Inc. (Thoratec)] has shown promise, with improved 6-month outcomes compared to the HM II, and has a size more comparable to the HVAD (70). The HeartWare MVAD is another device that initially showed promise, especially with its very small size, however, it is not yet available. Finally, the PumpKIN trial continues testing of the Jarvik 2015, a CF device which could support even the smallest of patients (71).
Certainly, in all centers where MCS is utilized in patients with a single-ventricle (either pediatric or adult), experts in every field must be readily available. Others have noted the benefits of a multidisciplinary approach consisting of surgeons and heart failure specialists, intensivists, hematologists, pharmacists, nurses and potentially psychiatrists and infectious disease physicians (72). We feel this is absolutely necessary to optimize the outcomes of these very difficult patients.
Conclusions
Patients with a single-ventricle comprise a heterogenous and inherently difficult patient population. When their condition necessitates the initiation of MCS, currently, their outcome is difficult to predict. Further studies are needed evaluating unique cannulation strategies, outcomes based on stage of palliation, patient selection and timing of MCS. A registry has been created to aid in answering these questions in the future (59).
The number of single-ventricle patients who require MCS is going to increase as the overall single-ventricle population increases. As patients with a failing single-ventricle age and an inevitable portion develops heart failure, some have dubbed a heart transplant the “fourth stage” (73). Likely, many of these patients will be considered at some point for possible VAD support. Though currently the outcomes are poor, there is promise, that with improved devices, increased experience and the development of risk factors to improve patient selection and operative timing, that outcomes will improve significantly.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jacobs JP, Maruszewski B. Functionally univentricular heart and the fontan operation: lessons learned about patterns of practice and outcomes from the congenital heart surgery databases of the European association for cardio-thoracic surgery and the society of thoracic surgeons. World J Pediatr Congenit Heart Surg 2013;4:349-55. [Crossref] [PubMed]
- Schilling C, Dalziel K, Nunn R, et al. The Fontan epidemic: Population projections from the Australia and New Zealand Fontan Registry. Int J Cardiol 2016;219:14-9. [Crossref] [PubMed]
- Voeller RK, Epstein DJ, Guthrie TJ, et al. Trends in the indications and survival in pediatric heart transplants: a 24-year single-center experience in 307 patients. Ann Thorac Surg 2012;94:807-15; discussion 815-6. [Crossref] [PubMed]
- Weinstein S, Bello R, Pizarro C, et al. The use of the Berlin Heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg 2014;147:697-704; discussion 704-5. [Crossref] [PubMed]
- Ford MA, Gauvreau K, McMullan DM, et al. Factors Associated With Mortality in Neonates Requiring Extracorporeal Membrane Oxygenation for Cardiac Indications: Analysis of the Extracorporeal Life Support Organization Registry Data. Pediatr Crit Care Med 2016;17:860-70. [Crossref] [PubMed]
- Imamura M, Dossey AM, Prodhan P, et al. Bridge to cardiac transplant in children: Berlin Heart versus extracorporeal membrane oxygenation. Ann Thorac Surg 2009;87:1894-901; discussion 1901.
- Almond CS, Singh TP, Gauvreau K, et al. Extracorporeal membrane oxygenation for bridge to heart transplantation among children in the United States: analysis of data from the Organ Procurement and Transplant Network and Extracorporeal Life Support Organization Registry. Circulation 2011;123:2975-84. [Crossref] [PubMed]
- Fraser CD Jr, Jaquiss RD, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med 2012;367:532-41. [Crossref] [PubMed]
- Almond CS, Morales DL, Blackstone EH, et al. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation 2013;127:1702-11. [Crossref] [PubMed]
- Eghtesady P, Almond CS, Tjossem C, et al. Post-transplant outcomes of children bridged to transplant with the Berlin Heart EXCOR Pediatric ventricular assist device. Circulation 2013;128:S24-31. [Crossref] [PubMed]
- Morales DL, Almond CS, Jaquiss RD, et al. Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin Heart EXCOR ventricular assist device. J Heart Lung Transplant 2011;30:1-8. [Crossref] [PubMed]
- Morales DL, Zafar F, Rossano JW, et al. Use of ventricular assist devices in children across the United States: analysis of 7.5 million pediatric hospitalizations. Ann Thorac Surg 2010;90:1313-8; discussion 1318-9. [Crossref] [PubMed]
- Fraser CD Jr, Jaquiss RD. The Berlin Heart EXCOR Pediatric ventricular assist device: history, North American experience, and future directions. Ann N Y Acad Sci 2013;1291:96-105. [Crossref] [PubMed]
- Everitt MD, Donaldson AE, Stehlik J, et al. Would access to device therapies improve transplant outcomes for adults with congenital heart disease? Analysis of the United Network for Organ Sharing (UNOS). J Heart Lung Transplant 2011;30:395-401. [Crossref] [PubMed]
- Misfeldt AM, Kirsch RE, Goldberg DJ, et al. Outcomes of Single-Ventricle Patients Supported With Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med 2016;17:194-202. [Crossref] [PubMed]
- Poh CL, Chiletti R, Zannino D, et al. Ventricular assist device support in patients with single ventricles: the Melbourne experience. Interact Cardiovasc Thorac Surg 2017;25:310-6. [Crossref] [PubMed]
- Hoskote A, Bohn D, Gruenwald C, et al. Extracorporeal life support after staged palliation of a functional single ventricle: subsequent morbidity and survival. J Thorac Cardiovasc Surg 2006;131:1114-21. [Crossref] [PubMed]
- Allan CK, Thiagarajan RR, del Nido PJ, et al. Indication for initiation of mechanical circulatory support impacts survival of infants with shunted single-ventricle circulation supported with extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2007;133:660-7. [Crossref] [PubMed]
- Carlo WF, Villa CR, Lal AK, et al. Ventricular assist device use in single ventricle congenital heart disease. Pediatr Transplant 2017;21. [Crossref] [PubMed]
- Di Molfetta A, Amodeo A, Gagliardi MG, et al. Hemodynamic Effects of Ventricular Assist Device Implantation on Norwood, Glenn, and Fontan Circulation: A Simulation Study. Artif Organs 2016;40:34-42. [Crossref] [PubMed]
- Ungerleider RM, Shen I, Yeh T, et al. Routine mechanical ventricular assist following the Norwood procedure--improved neurologic outcome and excellent hospital survival. Ann Thorac Surg 2004;77:18-22. [Crossref] [PubMed]
- Eghtesady P, Manning PB. Mechanical Circulatory Support Following Norwood Palliation. Oper Tech Thorac Cardiovasc Surg 2017;21:330-8. [Crossref]
- Sinha P, Deutsch N, Ratnayaka K, et al. Effect of mechanical assistance of the systemic ventricle in single ventricle circulation with cavopulmonary connection. J Thorac Cardiovasc Surg 2014;147:1271-5. [Crossref] [PubMed]
- Niebler RA, Shah TK, Mitchell ME, et al. Ventricular Assist Device in Single-Ventricle Heart Disease and a Superior Cavopulmonary Anastomosis. Artif Organs 2016;40:180-4. [Crossref] [PubMed]
- Chu MW, Sharma K, Tchervenkov CI, et al. Berlin Heart ventricular assist device in a child with hypoplastic left heart syndrome. Ann Thorac Surg 2007;83:1179-81. [Crossref] [PubMed]
- Calvaruso DF, Ocello S, Salviato N, et al. Implantation of a Berlin Heart as single ventricle by-pass on Fontan circulation in univentricular heart failure. ASAIO J 2007;53:e1-2. [Crossref] [PubMed]
- Newcomb AE, Negri JC, Brizard CP, et al. Successful left ventricular assist device bridge to transplantation after failure of a fontan revision. J Heart Lung Transplant 2006;25:365-7. [Crossref] [PubMed]
- Russo P, Wheeler A, Russo J, et al. Use of a ventricular assist device as a bridge to transplantation in a patient with single ventricle physiology and total cavopulmonary anastomosis. Paediatr Anaesth 2008;18:320-4. [Crossref] [PubMed]
- Cardarelli MG, Salim M, Love J, et al. Berlin heart as a bridge to recovery for a failing Fontan. Ann Thorac Surg 2009;87:943-6. [Crossref] [PubMed]
- Griffiths ER, Kaza AK, Wyler von Ballmoos MC, et al. Evaluating failing Fontans for heart transplantation: predictors of death. Ann Thorac Surg 2009;88:558-63; discussion 563-4. [Crossref] [PubMed]
- Kanter KR, Mahle WT, Vincent RN, et al. Heart transplantation in children with a Fontan procedure. Ann Thorac Surg 2011;91:823-9; discussion 829-30. [Crossref] [PubMed]
- Miller JR, Simpson KE, Epstein DJ, et al. Improved survival after heart transplant for failed Fontan patients with preserved ventricular function. J Heart Lung Transplant 2016;35:877-83. [Crossref] [PubMed]
- Morales DL, Adachi I, Heinle JS, et al. A new era: use of an intracorporeal systemic ventricular assist device to support a patient with a failing Fontan circulation. J Thorac Cardiovasc Surg 2011;142:e138-40. [Crossref] [PubMed]
- Niebler RA, Ghanayem NS, Shah TK, et al. Use of a HeartWare ventricular assist device in a patient with failed Fontan circulation. Ann Thorac Surg 2014;97:e115-6. [Crossref] [PubMed]
- Imielski BR, Niebler RA, Kindel SJ, et al. HeartWare Ventricular Assist Device Implantation in Patients With Fontan Physiology. Artif Organs 2017;41:40-6. [Crossref] [PubMed]
- Shah NR, Lam WW, Rodriguez FH 3rd, et al. Clinical outcomes after ventricular assist device implantation in adults with complex congenital heart disease. J Heart Lung Transplant 2013;32:615-20. [Crossref] [PubMed]
- Irving CA, Cassidy JV, Kirk RC, et al. Successful bridge to transplant with the Berlin Heart after cavopulmonary shunt. J Heart Lung Transplant 2009;28:399-401. [Crossref] [PubMed]
- Pearce FB, Kirklin JK, Holman WL, et al. Successful cardiac transplant after Berlin Heart bridge in a single ventricle heart: use of aortopulmonary shunt as a supplementary source of pulmonary blood flow. J Thorac Cardiovasc Surg 2009;137:e40-2. [Crossref] [PubMed]
- Cabrera AG, Sundareswaran KS, Samayoa AX, et al. Outcomes of pediatric patients supported by the HeartMate II left ventricular assist device in the United States. J Heart Lung Transplant 2013;32:1107-13. [Crossref] [PubMed]
- Miera O, Potapov EV, Redlin M, et al. First experiences with the HeartWare ventricular assist system in children. Ann Thorac Surg 2011;91:1256-60. [Crossref] [PubMed]
- Miller JR, Lancaster TS, Epstein DJ, et al. Outcomes and Trends of Ventricular Assist Device Selection in Children with End-Stage Heart Failure. ASAIO J 2017;63:464-9. [Crossref] [PubMed]
- Copeland JG, Copeland H, Gustafson M, et al. Experience with more than 100 total artificial heart implants. J Thorac Cardiovasc Surg 2012;143:727-34. [Crossref] [PubMed]
- Rossano JW, Goldberg DJ, Fuller S, et al. Successful use of the total artificial heart in the failing Fontan circulation. Ann Thorac Surg 2014;97:1438-40. [Crossref] [PubMed]
- Miller JR, Boston US, Epstein DJ, et al. Pediatric Quality of Life while Supported with a Ventricular Assist Device. Congenit Heart Dis 2015;10:E189-96. [Crossref] [PubMed]
- Gazit AZ, Petrucci O, Manning P, et al. A Novel Surgical Approach to Mechanical Circulatory Support in Univentricular Infants. Ann Thorac Surg 2017;104:1630-6. [Crossref] [PubMed]
- Prêtre R, Haussler A, Bettex D, et al. Right-sided univentricular cardiac assistance in a failing Fontan circulation. Ann Thorac Surg 2008;86:1018-20. [Crossref] [PubMed]
- Nathan M, Baird C, Fynn-Thompson F, et al. Successful implantation of a Berlin heart biventricular assist device in a failing single ventricle. J Thorac Cardiovasc Surg 2006;131:1407-8. [Crossref] [PubMed]
- Miller JR, Epstein DJ, Henn MC, et al. Early Biventricular Assist Device Use In Children: A Single Center Review Of 31 Patients. ASAIO J 2015;61:688-94. [Crossref] [PubMed]
- Agusala K, Bogaev R, Frazier OH, et al. Ventricular assist device placement in an adult with D-transposition of the great arteries with prior Mustard operation. Congenit Heart Dis 2010;5:635-7. [Crossref] [PubMed]
- Akay MH, Cooley DA, Frazier OH. Implantation of the heartmate II in a patient of 34 years after a Mustard procedure. J Card Surg 2012;27:769-70. [Crossref] [PubMed]
- Arendt K, Doll S, Mohr FW. Failing Mustard circulation with secondary pulmonary hypertension: mechanical assist device to achieve reverse pulmonary vascular remodelling for subsequent heart transplantation. Heart 2010;96:1164. [Crossref] [PubMed]
- Rajagopalan N, Booth DC, Diaz-Guzman E, et al. Successful ventricular assist device placement in transposition of the great arteries with pulmonary hypertension. Ann Thorac Surg 2013;95:e47. [Crossref] [PubMed]
- Huang J, Slaughter MS. HeartWare ventricular assist device placement in a patient with congenitally corrected transposition of the great arteries. J Thorac Cardiovasc Surg 2013;145:e23-5. [Crossref] [PubMed]
- Morgan JA, Paone G, Brewer RJ. Long-term right ventricular assist device support for congenitally corrected transposition of the great arteries. Heart Surg Forum 2013;16:E27-9. [Crossref] [PubMed]
- Woods RK, Ghanayem NS, Mitchell ME, et al. Mechanical Circulatory Support of the Fontan Patient. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2017;20:20-7. [Crossref] [PubMed]
- Joyce DL, Crow SS, John R, et al. Mechanical circulatory support in patients with heart failure secondary to transposition of the great arteries. J Heart Lung Transplant 2010;29:1302-5. [Crossref] [PubMed]
- Neely RC, Davis RP, Stephens EH, et al. Ventricular assist device for failing systemic ventricle in an adult with prior mustard procedure. Ann Thorac Surg 2013;96:691-3. [Crossref] [PubMed]
- Mascio CE, Malankar DP, Rome JJ. HeartWare Ventricular Assist Device as a Bridge-to-Transplant in a Small Boy with Complicated Kawasaki Disease. ASAIO J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Rossano JW, Woods RK, Berger S, et al. Mechanical support as failure intervention in patients with cavopulmonary shunts (MFICS): rationale and aims of a new registry of mechanical circulatory support in single ventricle patients. Congenit Heart Dis 2013;8:182-6. [Crossref] [PubMed]
- Di Molfetta A, Ferrari G, Filippelli S, et al. Use of Ventricular Assist Device in Univentricular Physiology: The Role of Lumped Parameter Models. Artif Organs 2016;40:444-53. [Crossref] [PubMed]
- Conover T, Hlavacek AM, Migliavacca F, et al. An interactive simulation tool for patient-specific clinical decision support in single-ventricle physiology. J Thorac Cardiovasc Surg 2018;155:712-21. [Crossref] [PubMed]
- Anwar S, Singh GK, Varughese J, et al. 3D Printing in Complex Congenital Heart Disease: Across a Spectrum of Age, Pathology, and Imaging Techniques. JACC Cardiovasc Imaging 2017;10:953-6. [Crossref] [PubMed]
- McGovern E, Kelleher E, Snow A, et al. Clinical application of three-dimensional printing to the management of complex univentricular hearts with abnormal systemic or pulmonary venous drainage. Cardiol Young 2017;27:1248-56. [Crossref] [PubMed]
- Meier LM, Meineri M, Qua Hiansen J, et al. Structural and congenital heart disease interventions: the role of three-dimensional printing. Neth Heart J 2017;25:65-75. [Crossref] [PubMed]
- Farooqi KM, Saeed O, Zaidi A, et al. 3D Printing to Guide Ventricular Assist Device Placement in Adults With Congenital Heart Disease and Heart Failure. JACC Heart Fail 2016;4:301-11. [Crossref] [PubMed]
- Rodefeld MD, Boyd JH, Myers CD, et al. Cavopulmonary assist in the neonate: an alternative strategy for single-ventricle palliation. J Thorac Cardiovasc Surg 2004;127:705-11. [Crossref] [PubMed]
- Myers CD, Mattix K, Presson RG Jr, et al. Twenty-four hour cardiopulmonary stability in a model of assisted newborn Fontan circulation. Ann Thorac Surg 2006;81:264-70; discussion 270-1. [Crossref] [PubMed]
- Giridharan GA, Koenig SC, Kennington J, et al. Performance evaluation of a pediatric viscous impeller pump for Fontan cavopulmonary assist. J Thorac Cardiovasc Surg 2013;145:249-57. [Crossref] [PubMed]
- Rodefeld MD, Frankel SH, Giridharan GA. Cavopulmonary assist: (em)powering the univentricular fontan circulation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2011;14:45-54. [Crossref] [PubMed]
- Mehra MR, Naka Y, Uriel N, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med 2017;376:440-50. [Crossref] [PubMed]
- Adachi I, Burki S, Horne D, et al. The miniaturized pediatric continuous-flow device: Preclinical assessment in the chronic sheep model. J Thorac Cardiovasc Surg 2017;154:291-300. [Crossref] [PubMed]
- Mackling T, Shah T, Dimas V, et al. Management of single-ventricle patients with Berlin Heart EXCOR Ventricular Assist Device: single-center experience. Artif Organs 2012;36:555-9. [Crossref] [PubMed]
- Michielon G, Carotti A, Pongiglione G, et al. Orthotopic heart transplantation in patients with univentricular physiology. Curr Cardiol Rev 2011;7:85-91. [Crossref] [PubMed]


