Psychosocial aspects of diabetes management: dilemma of diabetes distress
Background
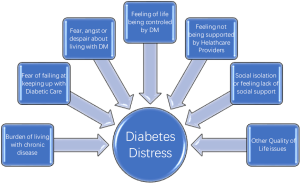
Diabetes mellitus (DM) is a debilitating chronic illness affecting 25.8 million people which accounts for 8.3% of the population in the United States as of 2011 (1). The impact of DM reaches far beyond the physical symptoms of the disease, often the emotional distress and psychosocial impact on the quality of life (QoL) of these patients complicates the effective management of their disease. A wide range of behavioral and psychiatric concerns may present in patients suffering from DM especially in patients diagnosed as adolescents (2). At times, the associated symptoms are dramatic and severe enough to draw the attention of providers, but more often than not these influences linger ominously beneath the surface for years before clinicians recognize the extent to which the psychosocial and behavioral components of DM are impacting both the course and prognosis of the disease (Figure 1).
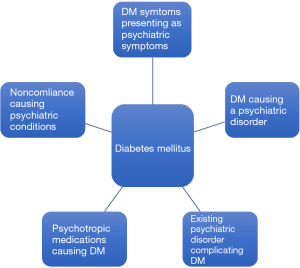
The psychosomatic manifestations in patients with diabetes arise in a variety of clinical presentations. Symptoms of diabetes can mirror psychiatric disorders and may make it difficult to distinguish between the two pathologies. For instance, a common presentation encountered in the emergency department is that of a patient endorsing the physical manifestations of an acute panic attack, diaphoresis, confusion, inattentiveness, nausea, but in reality these symptoms represent an acute episode of hypoglycemia. Although in such acute presentations, the masking of medicinal pathology by psychiatric diagnoses is rather quickly unveiled, the same cannot be said for the subtle long-term psychiatric sequelae of a chronic disease like DM. The traditional practice of medicine in which physical and mental health are catalogued as separated entities, hinders a clinician’s ability to identify the long-term psychiatric complications of chronic disease such as diabetes. Particularly, an early diagnosis of diabetes has been noted to make a profound impact on children and adolescents who struggle to accept the realities of this lifelong diagnosis. Many of them may start to experience significant behavioral rebellion when faced with the strict regimen of diet and medication management. If not dealt with properly, this may give rise to more deep seated and difficult to treat psychiatric diseases including personality disorders, depression, anxiety, as well as impulse control disorders that can further complicate the chronic treatment of DM (3,4). Furthermore, patients often face growing frustrations in light of the common experience of a cycle of adherence followed by noncompliance to the strict diet and medication regimens required to optimally treat diabetes. As diabetes education currently stands, patients receive excellent training as to the do’s and don’ts of a diabetic diet, the rules and regulations of medication administration, and a detailed account of the enumerate consequences of poor diabetes management. Despite this detailed curriculum, patients completely lack the emotional insight into the impact of this diagnosis on their psyche and QoL. The absence of this clinical guidance, leads patients to encounter difficulties as they embark upon the adapting to living with diabetes.
Epidemiology
The prevalence of DM is rising exponentially not only in United States, but globally as well. According to the 2010 International Diabetes Federation (IDF), 285 million people worldwide have diabetes (5). This reflects an increase of 39 million new cases of DM since last reported in 2007. This rate of increase in prevalence suggests that in 2030 approximately 439 million worldwide would be living with DM. By the year 2025, a vast majority of patients with DM will be concentrated in the most populous countries, of the world namely the United States, India, and China (6). Although projections of prevalence rate of DM appears to be similar among both developed and underdeveloped countries it is interesting to note that there is a very clear difference in age group of people affected among these countries. Those diagnosed in developing countries are on average between 45–64 years old, whereas people in developed countries are increasingly older than 64 years of age (6). This poses a major socioeconomic burden to young families in developing countries. Furthermore, it is estimated that DM contributes to 23 million years of life lost due to disability and reduced work-life (5). An equally staggering number of people suffer from depression worldwide, prevalence rate range from 14–16%, with women affected more than man (5,6). Like DM, depression also targets a younger population making a major economic impact. According to World Health Organization depression is the 4th and 7th leading cause of global disability, mortality, and morbidity in women and men respectively. This is likely due to work place absenteeism, reduced work productivity, and an increased rate of healthcare resource utilization (5). Further, DM is anticipated to become the 2nd leading cause of morbidity and mortality worldwide (6).
While the comorbidity of depression in DM is a well-established phenomenon, the actual prevalence varies widely. The prevalence of this co-existence is dependent on the country in which the study was conducted, the level of awareness of depression in different populations, the cultural acceptance of a society for a person to acknowledge their depression, the socio-economic strata of the community which subsequently influences the ability to seek a provider for the appropriate diagnosis and treatment of depression (7). A growing body of literature suggests that patients with DM, especially females, are much more likely to also be simultaneously suffering from a form of depression. A meta-analysis from the United States concluded that having diabetes more than doubled the odds of developing depression. Interestingly, there was wide variation of the prevalence among the various states. Although, depressive symptoms were commonly noted in the diabetic population studied, only 11% were found to meet criteria for major depressive disorder (MDD). A much higher percentage (31%) suffering from diabetes were found to have depressive symptoms but not meeting the full criteria of depressive disorder. The risk of depression was found to be lower in people suffering from type 1 DM (T1DM) when compared to those with type 2 DM (T2DM) (8,9). Furthermore, the risk of developing psychiatric comorbid conditions was increased with earlier onset of DM. Another very large study which utilized a standardized phone survey to collect information from over 22,000 patients with diabetes in the United States found that age adjusted rate of comorbid depression in people with DM was 8.3% (4). Prevalence of concurrent depressive symptoms was lowest in Connecticut while it was highest in Alaska. Significant variations in rate of comorbid depression also were noted in people from different ethnicities, with highest rate of depression in Native Americans (27.8%) and lowest (1.1%) in Asian populations (4).
Pathophysiology of diabetes and comorbid depression
There exists a bidirectional relationship between diabetes and psychological distress. Depression is two to three times more common in DM patients than the general population (7). While the common belief is that depression is a consequence of dealing with a major illness like DM, emerging evidence suggests that depression can be a risk factor of developing DM (10). People with preexisting depression were found to have a 60% risk of developing DM while people with preexisting DM only had a 15% risk of developing depression (11). The pooled relative risk (RR) for the development of depression in patients with already existing DM was 1.15 while the RR for developing DM if you already had baseline depression was significantly greater at 1.60. This shows a much stronger association in direction of depression being a major risk factor for developing DM down the road (11).
Depression creates a deep sense of futility, amotivation, lack of energy, and hopelessness. It is therefore not surprising that when DM and depression coexist, there is a frequent association of poor compliance with diabetes care, poor glycemic control, and hence an increased risk of long-term complications. Anhedonia, or the inability to draw any pleasure from an activity or event is a core symptom of depression, and precludes patients from involvement in physical activity, interest in a healthy lifestyle, and motivation to comply with treatment recommendations (12). A prospective study of over 4,000 patients with DM and MDD were found to have a higher risk of developing macrovascular complications. These complications were found be present even when controlled for variables such as type of treatment and history of complications suffered prior to being enrolled in current study (13).
Neuroendocrine connection between depression and diabetes
The Hypothalamic Pituitary Adrenal axis (HPA axis) is a multilevel endocrine regulatory system, the role of which is well documented in precipitating and perpetuating many chronic diseases of different body systems. The synchronized activation or down regulation of pituitary and adrenal glands is vital for the body to respond and adapt to any kind of stress (14). Corticotrophin releasing hormone (CRH) released from hypothalamus is the main switch that starts the hormonal cascade of HPA axis. Once CRH is released from hypothalamus in response to stressors, it occupies various CRH receptors in different neural circuits both in brain and in spinal cord including limbic system, and sympathetic arousal system. This is the start of a well-coordinated chain of events in response to any stress causing modulation of various body activities and defense mechanisms including physiologic response, behavioral adjustments, arousal level, regulation of sleep, appetite, sexual, and activity level. Down stream at the anterior pituitary gland level the stress response is also co-mediated by arginine vasopressin (AVP) and similar nonapeptides. CRH and AVP are secreted in near synchronized pulses, beckoning the emission of adrenocorticotropin hormone (ACTH) (15). This CRH stimulation of ACTH is diurnal with a peak between 6:00 to 8:00 AM and trough at midnight, and it is greatly affected by level of stress. The HPA axis controls the secretion of cortisol from the adrenal gland by secretion of CRF from the hypothalamus and ACTH from the pituitary gland. Glucocorticoid synthesis mainly takes place in zona fasciculata of adrenal cortex and is responsible for activating the negative feedback loop to terminate the stress response at the level of suprahypothalamic centers, hypothalamus, and pituitary gland. The main function of cortisol is to facilitate the adaptation of the organism to environmental challenges and secretion of cortisol is increased in times of stress. Melanocortin receptors 2 (MC2) are found in all three layers of adrenal cortex, and are activated under the influence of ACTH (15). MC2 exerts its action by binding with stimulating adenylyl cyclase and generating cAMP that activates enzyme pathways further down triggering steroidogenesis (15). This whole operation is self-regulatory, time sensitive and a functioning cycle has a pre-emptive safeguard against any adversarial effects. However, when stress becomes a chronic inexorable force that continue to cause relentless continual hypersecretion of CRH it causes this intricate defense system to become overburdened, causing adaptive changes like catabolism and immunosuppression to become more of a normal status (15). Continued activation of CRH in the face of persistent stress suppresses the negative feedback loop of cortisol leading to a state of hypercortisolemia. The HPA-axis stimulation also causes the stimulation of sympathetic nervous system and also activates cytokine system. This unremitting activation of HPA-axis results in a syndromal state of a chronic stress response characterized by behavioral disturbances such as depression, anxiety spectrum disorders, anorexia, central obesity, DM, metabolic syndrome, immunosuppression, and increased vulnerability to various other systemic diseases, infections, and even cancers (15).
The relationship between elevated cortisol and impairment of diurnal pulses of cortisol is well established. States of persistent hypercortisolism diagnosed by measuring 24 hours urinary free cortisol levels, and inability to suppress cortisol secretion when challenged with dexamethasone challenge (dexamethasone suppression test) This is strongly related with manifestations of depression and anxiety syndromes across the life span starting with early adolescents and continuing into adulthood . Similar association is seen in patients with DM in whom the cortisol diurnal curve is limited and does not show the typical diurnal variations and demonstrates higher bedtime cortisol level (16). It appears that hyperglycemia induced by hypercortisolism is perceived as a stress to the body, and in turn may cause further increase in secretion of ACTH and an ongoing cycle of hypercortisolism (16,17). This subclinical hypercortisolism causing depression, hyperglycemia, and possible insulin resistance can manifest as clinical hypercortisolism or Cushing syndrome. This results in central obesity with visceral adiposity, increased insulin resistance, increased hepatic catabolism of glucose, and decreased insulin release. There is high comorbidity of depression and anxiety with Cushing syndrome, with as many as 50–80% of these demonstrating depressions. Thus it is likely that a subclinical hypercortisolemic state with a flat cortisol curve and decreased variability of cortisol level can induce or perpetuate both depression and diabetes in susceptible individuals (16,17) (Figure 2).
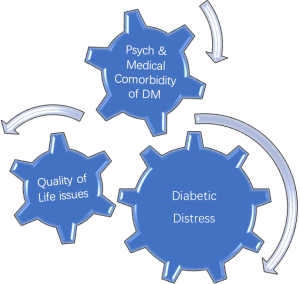
Diabetes distress
While comorbid psychiatric disorders like depressive disorders are a relatively well-known phenomenon another lesser known and rather new phenomenon is; diabetes distress or diabetes related distress. Diabetes distress is a relatively new term gaining more eminence in literature in last decade. Diabetic distress is still evolving as a phenomenon and different authors and investigators have defined it in different ways. More simplistically diabetes distress can be defined as a QoL issue due to combination of medical and psychological burden of DM as a chronic and complex malady that creates an emotional distress that often remains hidden from providers and at times from the sufferer as well. However, diabetes distress can influence diabetes management and treatment outcomes in an unfavorable way. It should be emphasized here that diabetes distress is not a psychiatric disorder but it is rather an affective state resulting from constant worry about adherence with diet, exercise, blood glucose monitoring while feeling scared, anxious, overwhelmed, at times angry and burnout. The level of diabetes distress is noted to be much higher in patients who are younger; female non-white had higher BMI, and patients who are being treated with insulin versus patients who are treated with oral hypoglycemic agents (18). Diabetes distress does not appear to be related to duration of illness. In other words, an adolescent or young adult may feel more distressed about having DM diagnosed recently versus someone who has dealt with DM for most of their lives. However, using DM management with insulin injections as proxy of severer illness (compared to DM managed by diet or medications), it can be predictive of patient with more emotional distress. On the other hand level of education, number of medications a patient was prescribed, or the level of comorbidity was not related to increased distress. When patients are dealing with greater level of diabetes related emotional distress, they are shown to have lesser compliance with anticipated treatment plan like adherence to dietary regimen, exercising on regular basis, monitoring of blood glucose levels frequently, and taking medications as prescribed. This results in patients with high score for emotional distress, not surprisingly, having higher BMI and higher HgbA1C levels (18). Another study has corroborated same findings and has also found that higher distress was correlated with having low QoL and low scores on the mental component score which may be cofactor in having low diabetes empowerment, unhealthy life style like not being physically active, or practicing unhealthy dietary practices resulting in poor glycemic control (19).
When a person is diagnosed with DM, several immediate and long term QoL issues arise. Patients are told that they have to change their life style significantly in order for them to be able to manage their DM effectively. They are also told that prognosis is dependent on their diligence and adherence to the complex regimen of dietary modifications, medications, learning to measure the blood glucose and injecting insulin as needed, making sure to include some physical activity in their routine etc. In long term, however they are facing the dilemma that they may have to continue doing whatever life style modifications they have made or are being asked to make for the rest of their life. Cognitively they may be fully aware that the prognosis of their disease is dependent on their active participation in management of DM as being proposed and any transgressions means that they are impacting the final outcome of the disease resulting in major complications. This intellectual insight is acquired from diabetes education and other informational material provided to them in a discussion with their diabetes care team and peers with DM. However, translation of this intellectual insight into emotional insight does not happen for every patient with DM. When they are unable to adhere with the ideal treatment plan being offered, they get labeled as difficult, noncompliant, or uninterested in getting better. It is seldom that most providers take in to consideration why these patients are non-compliant, and why they find it difficult to accept the most logical suggestions being made to them, or appear disinterested in their own well being. This is where assessing the QoL and may become an important screening tool for psychiatric illness (20-26) (Figure 3).
Management of diabetes distress
Provider awareness of the possibility of presence of diabetes distress is the first step in managing it. Most patients may not even be cognizant of being distressed until they are asked specific questions or they do self-assessment using a structured screening tool. Many scales are available to screen for emotional distress specifically associated with DM. These scales have been validated and are available for use in clinical practice. Providers may feel that the time constraints of a busy diabetes clinic may preclude them from utilizing long, time consuming surveys. However, the potential benefit of devoting some clinical time to reviewing these tools with patients may ultimately prove to be not only time saving in the long run but also would save the provider from frustration and angst about not been able to have good outcomes and be able to manage a patient more optimally.
Several screening scales have been used which can help delineate emotional distress related to patients with diabetes. They may aid the provider in improving DM outcomes by understanding their patient’s plight and to offer them some solutions to overcome their difficulties (Box 1).
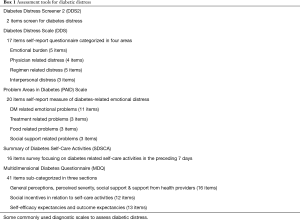
Full table
First and foremost is the acknowledgment in a provider’s mind that DM is not just a physical ailment completely isolated from a patient’s psyche. Addressing QoL issues will improve the outcome measures for DM thus improving the risk of long term complications. A study of 20 patients with T2DM showed improved fasting blood glucose and two-hour postprandial blood glucose levels in patients treated with progressive relaxation techniques and biofeedback treatment suggesting that a less anxious state may improve diabetes care (27). Treating patients in silos of specialized medicine is not conducive of good outcome in any disease and especially a complex disease such as DM. It is of utmost importance that healthcare providers know that patients with DM are likely to have some level of emotional distress, detachment from their social support system, as well as possible distrust of health care professionals affecting their psychological state. A multidisciplinary approach is key in ensuring better outcomes in DM patients. A multidisciplinary team including a clinical social worker and psychologist can address the complex concerns and difficulties a DM patient may encounter in daily routine of not only managing the DM but to also be successful in their respective lives and be able to manage their roles as a parent, spouse, student, or worker. Starting a discussion with your patient about their distress may open a door where the provider can have an opportunity to make behavioral as well as non-behavioral interventions to improve the DM outcomes (Box 2).

Full table
Psychiatric comorbidities of DM
Depression is not the only psychiatric comorbidity associated with DM. Patients with DM have a 20% lifetime risk of developing an anxiety disorder (4,9). Another rather common and potentially dangerous comorbid psychiatric disorder patient is eating disorders. These have been reported in association with diabetes, especially affecting older adolescents and young women with T1DM
Depressive spectrum disorders
Depression may be the underlying cause of non-compliance with diabetes care. Depression creates a sense of apathy towards illness and life in general in these patients that can lead to chronic non-compliance and poor diabetes control. Depressive disorder can be of different nature and vary in severity on a spectrum. They may have an adjustment disorder, a depressive state resulting from adjustment to a big stressor like being diagnosed with DM or finding out that one has developed a major complication of DM or more indolent and persistent depressive state on a chronic basis that does not reach the full syndromal state of a major depressive episode. Many DM patients suffer from a depressive disorder due to direct results of having a major medical illness like DM. We have discussed the physiologic interconnection of psyche and endocrine in pathophysiology section above to help understand this type of depression (Table 1).
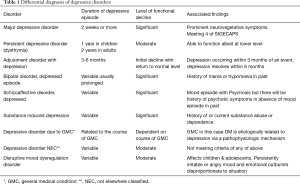
Full table
Adjustment disorder
This is a psychological response to a clearly identifiable psychosocial stressor within last 3–6 months of presentation. The prevalence rate is around 2–8% in the community but it is much higher (12%) in patients who are hospitalized due to a medical condition (28). The depressive symptoms appear within 3 months of a stressful event and usually resolve within 6 months of resolution of the index event. The symptoms are usually not severe enough to impact functioning and quickly resolve on its own or with short term supportive therapy, interpersonal therapy (IPT), or cognitive behavioral therapy (CBT). In some cases symptoms continue to persist after 6 months, at which point adjustment disorder is labeled as chronic and may require more long-term psychotherapy (28).
Persistent depressive disorder (PDD)
This was previously known as dysthymic disorder. PDD is a simmering low intensity depression of chronic nature lasting for at least 2 years. PDD usually starts early in life following a major adverse event. A person suffering from PDD commonly describes it as “I am suffering from depression for as long as I can remember.” Most patients suffer from this illness for 5 to 10 years (28). Longstanding depression gives rise to a characteristic personality with self-doubt, low self-esteem, tendency to depend on others, interpersonal sensitivity, demanding nature, passivity, and a pessimistic outlook about life (28).
MDD
A MDD episode is described as a pervasive sense of depression on most days than not, lasting for at least 2 weeks. This is accompanied by neurovegetative symptoms like disturbance of sleep, loss of interest in pleasurable activities (also called anhedonia), lack of concentration and difficulty with memory, decline in appetite or overeating, low energy, tendency to blame self or ruminate, change in psychomotor activity level, feelings of worthlessness and hopelessness with suicidal ideation. In order to make a diagnosis of MDD, other psychiatric disorders such as substance-induced mood disorder and mood disorder caused by a medical condition need to be excluded. Depressive symptoms as part of bereavement and in adjustment reaction in response to a major life event stressor should also be excluded (28) (Box 3).

Full table
Anxiety disorders
Though not mentioned in the literature as frequently as depression, anxiety spectrum disorders are quite common in patients with DM. Patients with DM have a 17–20% higher lifetime prevalence of developing an anxiety disorder in comparison to their age matched controls who do not have DM (9). Anxiety disorder can significantly impact a person especially in adolescents or young adults suffering from a chronic illness like DM. It has been shown that patients who have higher levels of anxiety symptoms independent of depressive symptoms have suboptimal glycemic control, as they were less likely to do frequent blood glucose monitoring (3). Patients with DM may confuse their symptoms of hypoglycemia as having an anxiety or panic attack and that may delay getting the help they need at that crucial time.
Other common psychiatric conditions comorbid with DM
Diabulimia and eating disorders
Diabulimia, also known as manipulation of insulin dosing, is characterized by either decreasing or skipping of the dose of insulin as a purging mechanism for the purpose of losing weight. Diabulimia is the second most common method of weight control in adolescent girls with T1DM. Restriction of insulin doses may or may not be associated with other eating disorders. As many as 30% of young adolescent patients with T1DM are considered to have some level of eating disorder. Jones et al. in Canada surveyed 2,000 children with a self-assessment survey tool and found that children with DM were 2.4% more likely to have an eating disorder than their peers who did not have DM (29). However combination of diabulimia and binge eating disorder (BED) is the most common way of weight control in adolescents (29,30).The patients who have eating disorders and who are also manipulating insulin doses are likely to have higher mean hemoglobin A1C levels when compared to patients with DM who did manipulate insulin without a comorbid eating disorder (30). Around 10% of adolescent girls aged 12 to 19 years with T1DM were found to meet the criteria of an eating disorder compared to 4% of age matched controls, while 15% of the girls met partial diagnostic criteria compared to 8% of the controls (29,31). Once the patients with DM develop eating disorders they are likely to continue to have an eating disorder even at 4- to 5-year follow-up. The patients with T1DM who also have comorbid eating disorders are more likely to develop microvascular complications, have more episodes of diabetic ketoacidosis and hospitalizations (32). Recurrent hypoglycemia and hyperglycemia can have profound impact on the developing brain of children and adolescents who develop DM early on. Neurocognitive difficulties like deficient memory, learning difficulties, and performance intelligence quotient are some of the common neurocognitive deficits seen in early onset T1DM (33). Some of the neurocognitive deficits are mild and may go undetected.
The most common eating disorder seen in patients with DM is BED. BED is characterized by consumption of unusually large amount of food in a short period of time, usually within 2 hours with a feeling that they have lost control on their eating behavior. They may eat alone to avoid embarrassment, eat at a faster pace, eat when not hungry, and continue eating even when feeling uncomfortably full. The diagnosis is not made unless these episodes occur at least once a week for 3 months. Depending on the number of episodes, it can be classified as mild with 1–2 episodes per week to severe with 14 or more binge eating episodes occurring in a week (28).
Bulimia nervosa (BN) is another quite common eating disorder that may be seen in up to 27% of DM patients (34). Although BN is usually a disease of late adolescence to young adulthood, it may continue to develop as a chronic problem of late adulthood. BN is characterized by recurrent episodes of binge eating with a sense of lack of control within a discrete period of time. These symptoms are accompanied with inappropriate compensatory behaviors such as induced vomiting, misuse of laxatives, diuretics, or thyroid hormone or insulin, fasting, and excessive exercise. Despite frantic efforts to lose weight, most patients with BN have normal weight or remain overweight. BN is highly associated with body image issues and low self-esteem along with comorbid psychiatric disorders including depression, bipolar disorder, anxiety disorders, social anxiety, borderline personality disorder, and substance abuse disorders. BN patients have 2% per year risk of dying due to medical complications associated with binging and purging and from suicide (35).
Differentiating depression from diabetes distress
The most important tool in this regard is to take a careful and thorough history paying special attention to the time of onset, temporal relationship to psychosocial stressors, past history of depression or other psychiatric disorders, family psychiatric history, history of alcohol and substance abuse and its relationship to the disorder, and detailed medical history with focus on onset of DM and its course, including the attitude of the person towards having the illness of DM and the perspectives about the course and prognosis of the disease. It is also important to obtain a thorough review of current or recently used medications especially if any medications are complicating DM, for example antidepressants or antipsychotics usually used as adjunct to antidepressant or as mood stabilizers which may be causing weight gain or complicating DM in any other way. It is important to look deeper into the dynamics playing a role in the overall success or failure of DM management, like difficulties in personal or family relations, current psychosocial stressors, chronic psychological trauma all of which can affect a person’s ability to deal with the added stress of a chronic disease like DM.
Management of psychiatric comorbidities
Early diagnosis and treatment of psychiatric conditions coexisting and complicating DM management have been shown to improve treatment outcomes such as hemoglobin A1C levels (36). DM, when diagnosed early in life in young children and adolescents has profound effects on psychological development leading to lifelong patterns of faulty coping mechanisms including self-destructive behaviors, aggressiveness, anxieties about their disease state, and not being able to utilize healthy coping skills leading to disengagement and poor adherence (36). The presence of these disengaging behaviors and defiant attitudes makes it difficult to have an in-depth evaluation leading to proper diagnosis.
Multidisciplinary clinic visits are extremely useful, patients and families can interact with their endocrinologist, nurse, a dietician/nutritionist, a diabetes educator along with a clinical social worker, and a psychologist/psychiatrist. It has been shown that when DM is complicated with depression, health care services utilization increases many fold reflecting a 4.5 times increased burden on the health care system, amounting to $247 million monetary burden. The DM patients who are also depressed also tend to utilize more prescriptions (43 vs. 21, P<0.0001) than controls (P<0.0001) (10). A comprehensive care approach is vital for these patients and families to optimize the success of treatment. The goal of treatment should be to establish long-term relationships between patients and their treatment team to promote open communication, common goals of care, and frequent follow-ups. Diabetes patients with comorbid psychiatric illness should be referred for psychotherapy and counseling sessions and for assessment for need of psychotropic medications. It is of utmost importance that we do prompt identification, provide comprehensive treatment, and continue to follow-up these patients regularly to avoid major complications and adverse outcomes of the DM.
Psychotherapeutic management of diabetes distress and psychiatric comorbidities of DM
Many psychological interventions have shown promise in addressing emotional distress relating to general medical conditions. Simple interventions like engaging patient in supportive psychotherapy where they can talk about their emotional distress and work with the therapist to understand their frustrations can be helpful. Behavior therapies where modifications are made by learning to improve specific behaviors that can be self-destructive such as being non-compliant with treatment protocol and adhering to the healthy life style can be beneficial. CBT which makes a person understand one’s own flawed cognitive schemas and thought patterns leading to negative emotional state that in turn leads to maladaptive behaviors has also been shown to be very effective. Some alternative therapies like neuro and biofeedback, distraction techniques, relaxation, and imagery therapy, all have shown some level of efficacy. Psychiatric screening for all DM patients is not necessary, however, identifying patients at risk and screening them for particular psychiatric conditions is prudent. Other treatment modalities like group therapy and family therapy have all been shown to be effective in mild cases of depression (27). More serious and recurrent episodes that fail to respond to psychosocial support and psychotherapy should be assessed carefully for a trial of psychotropic medications.
Supportive psychotherapy (ST)
ST is a psychotherapeutic approach that can be utilized when patient is in relative acute stages of illness and is not yet ready to engage in deep insight-oriented or other structured therapies. ST can be performed with relative ease with patients facing acute changes in their life circumstances affecting their emotional wellbeing like dealing with a major complication of DM or having a setback in the management of DM. ST strives to reinforce a patient’s ego by providing them support for their current stressors and to enable patients to adapt well to challenges they are facing. ST was conceptualized to identify and enhance the inherent abilities of the patient and to help them realize their own potential in hopes to help them cope with their stressors. ST motivates patients to recognize that they are the protagonists in their own management plan and this helps then to cope with their treatment challenges in a better way. At times ST therapist may use some explorative and interpretative techniques as indicated (37). DM patients faces multiple changes in their daily life, change in life style, feeling guilt for burden on their loved ones, and dealing with medical and physical issues affecting function and at times causing disability with major medical complications. ST can be especially helpful in the early stages of engaging DM patients to understand the nature and stage of the disease as its non-exploratory and non-judgmental approach may put patients at ease and help patient accept the demands and complexity of treatment regimens. ST focuses on conscious issues apparent to the patient, what symptoms they are dealing with, and how it is affecting their life (37).
Psychodynamic psychotherapy (insight oriented therapy)
Psychodynamic psychotherapy asserts that self-directed anger is a result of difficulties experienced during early childhood and predisposes one to develop depression. The main techniques utilized are interpreting transference, countertransference, resistance, and defense mechanisms. Psychodynamic psychotherapy is beneficial when focusing on the developmental issues, which have impacted a person’s abilities to cope with their current life stressors in a healthy way. Psychodynamic psychotherapy relies heavily on the relationship between therapist and patient relationship and the utilization of free association technique in which the therapist carefully and empathetically listens for the emerging themes and patterns. Success depends on the therapist’s ability to interpret the transference, counter transference, and resistance, expertly to help DM patients understand the underlying subconscious conflicts. As these needs are explored patient’s learn to develop an emotional state where conflicts can be dealt with in a healthy and sustainable manner (38).
Interpersonal therapy (IPT)
IPT is a time limited structured therapy consisting of 16 weeks of weekly sessions addressing four main areas where interpersonal relationship can be challenged and can give rise to persisting depression. The first of the four focus areas is grief, in which IPT may prove to be a very effective way of addressing grief associated with the diagnosis of a chronic disease with a major impact on self-image and lifelong change in life style. IPT can be employed to improve interpersonal and social connectedness difficulties, which are a common area of emotional distress for DM patients. It can also improve self-esteem, self-worth, and alleviate despair and dysphoria. IPT has shown to diminish the helplessness and hopelessness in depressed patients that can lead to suicidal thoughts (39).
Interpersonal and social rhythm therapy (IPSRT)
IPSRT is a combination of IPT and social rhythm therapy (SRT) for the treatment of major depression and bipolar disorder. IPSRT is a time-limited therapy, consisting of 20–24 sessions of individual therapy delivered over a period of 8 months. IPSRT techniques take into account the fact that most relapses of mood disorder occur as a result disruption of interpersonal harmony, non-compliance with treatment and/or interruption of social rhythm (8). The goals of the IPSRT include: increase compliance to pharmacotherapy, develop skills for healthy coping with life-events, and to restore, maintain and monitor of a healthy circadian rhythm (40,41).
Cognitive behavior therapy (CBT)
CBT is based on the information processing theory, which requires a more active role from both therapist and patient. CBT utilizes structured tools and assignments to assess and monitor progress towards therapeutic goals. CBT aims to assess the difficulties encountered in early development as it is considered that these emotional difficulties contribute to cognitive distortions or automatic thoughts impacting a person’s and emotional state and the formation of personality. A distorted or negative cognitive schema about self, future, and the world around, impacts a person’s perceptions resulting in maladaptive and faulty behaviors. CBT was shown to be superior in the treatment of depression in comparison to supportive and family therapy (42,43).
Behavioral therapy (BT)
The premise of BT is that the stressors lead to the development of maladaptive coping mechanisms. This in turn results in negative behavior that consequently forms diminished contingent positive reinforcement. With persistent faulty coping mechanisms the likelihood of developing dysphoria and anxiety increases. This negative feedback loop results in rumination, self-blame, self-criticism, as well as faulty perceptions and behaviors about others (42). BT is rooted in re-routing these negative feedback loops.
Conclusions
Diabetes distress is a rather new front for the health care providers who already struggle with providing the optimal care to their patients. An optimal treatment plan requires paying attention to every nuance of a complex condition like DM in the time constraint environment of the current health care provision. However, recognizing signs of diabetes distress, evaluating patient for it and managing it appropriately may prove to be the best investment of time when developing the long term treatment plan of the patient. Future direction in diabetic care should be to screen patients early and often and prevent the diabetes distress. This should be considered a priority while developing a treatment plan not an afterthought.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011.
- Olerud JE. Diabetes and the skin. In: Porte D Jr, Sherwin RS, Baron A. editors. Ellenburg & Rifkin’s Diabetes Mellitus, 6th ed. New York: McGraw-Hill, 2003:895.
- Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes: association with blood glucose monitoring and glycemic control. J Pediatr Psychol 2010;35:415-25. [Crossref] [PubMed]
- Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069-78. [Crossref] [PubMed]
- Egede LE, Ellis C. Diabetes and depression : Global perspectives. Diabetes Res Clin Pract 2010;87:302-12. [Crossref] [PubMed]
- King H, Aubert R, Herman W. Global Burden of Diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414-31. [Crossref] [PubMed]
- Levenson JL. Psychiatric issues in endocrinology: updates in psychosomatic medicine and consultation-liaison psychiatry. Prim Psychiatry 2006;13:27-30.
- Brown LC, Majumdar SR, Newman SC, et al. History of depression increases risk of type 2 diabetes in younger adults. Diabetes Care 2005;28:1063-7. [Crossref] [PubMed]
- Li C, Barker L, Ford ES, et al. Diabetes and anxiety in US adults: findings from the 2006 Behavioral Risk Factor Surveillance System. Diabet Med 2008;25:878-81. [Crossref] [PubMed]
- Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 2002;25:464-70. [Crossref] [PubMed]
- Mezuk B, Albrecht W, Golden S, et al. Depression and Type 2 Diabetes Over the Lifespan. Diabetes Care 2008;31:2383-90. [Crossref] [PubMed]
- Réus GZ, Augusta M, Santos B, et al. Pathophysiological mechanisms involved in the relationship between diabetes and major depressive disorder. Life Sci 2017;183:78-82. [Crossref] [PubMed]
- Knol MJ, Twisk JW, Beekman AT, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006;49:837-45. [Crossref] [PubMed]
- Tareen RS. Psychoneuroimmunology and other interactions between skin and psyche. In: Tareen RS, Greydanus DE, Jafferney M, et al. editors. Pediatric Psychodermatology: A Clinical Manual of Child and Adolescent Psychocutaneous Disorders. Berline 2012:69-80.
- Kyrou I, Tsigos C. Hypothalamic-pituitary-adrenal axis. In: Linos D, Heerden J. editors. Adrenal Glands ed. Berlin: Springer Berlin Heidelberg, 2005:19-32.
- Golden SH. A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev 2007;3:252-9. [Crossref] [PubMed]
- Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci 2017;1391:20-34. [Crossref] [PubMed]
- Delahanty LM, Grant RW, Wittenberg E, et al. Association of diabetes-related emotional distress with diabetes treatment in primary care patients with Type 2 diabetes. Diabet Med 2007;24:48-54. [Crossref] [PubMed]
- Joensen LE, Tapager I, Willaing I. Diabetes distress in Type 1 diabetes--a new measurement fit for purpose. Diabet Med 2013;30:1132-9. [Crossref] [PubMed]
- Fisher L, Russell E, Strycker L. The Relationship Between Diabetes Distress and Clinical Depression With Glycemic Control Among Patients With Type 2 Diabetes. Diabetes Care 2010;33:1034-6. [Crossref] [PubMed]
- Fisher L, Danielle M, Polonsky WH, et al. When Is Diabetes Distress Clinically Meaningful ?: Establishing cut points for the Diabetes Distress. Diabetes Care. 2012;35:259-64. [Crossref] [PubMed]
- Fisher L, Skaff MM, Mullan JT, et al. Psychological Issues A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diet Med 2008;1096-101.
- Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications 2015;29:572-7. [Crossref] [PubMed]
- Beverly EA, Ivanov NN, Court AB, et al. Is diabetes distress on your radar screen? J Fam Pract 2017;66:9-14. [PubMed]
- Zhou H, Zhu J, Liu L, et al. Diabetes-related distress and its associated factors among patients with type 2 diabetes mellitus in China. Psychiatry Res 2017;252:45-50. [Crossref] [PubMed]
- Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626-31. [Crossref] [PubMed]
- Jablon SL, Nalibof BD, Gilmore SL. Effects of Relaxation Training on Glucose Tolerance and Diabetic Control in Type II Diabetes. Applied Psychophysiology and Biofeedback 1997;22:155-69. [Crossref] [PubMed]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edn, text rev (DSM-IV-TR). Washington, DC: APA, 2000.
- Jones JM, Lawon ML, Daneman D, et al. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ 2000;320:1563-6. [Crossref] [PubMed]
- Davidson J. Diabulimia: how eating disorders can affect adolescents with diabetes. Nurs Stand 2014;29:44-9. [Crossref] [PubMed]
- Bryden KS, Neil A, Mayou RA, et al. Eating habits, body weight, and insulin misuse: A longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care 1999;22:1956-60. [Crossref] [PubMed]
- Rydall AC, Rodin GM, Olmsted MP, et al. Disordered eating behavior and microvascular complications in young women with insulin-dependent diabetes mellitus. N Engl J Med 1997;336:1849-54. [Crossref] [PubMed]
- Northam EA, Anderson PJ, Werther GA, et al. Predictors of change in the neuropsychological profiles of children with type 1 diabetes 2 years after disease onset. Diabetes Care 1999;22:1438-44. [Crossref] [PubMed]
- Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34:383-96. [Crossref] [PubMed]
- Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Current Opinion in Psychiatry 2006;19:438-43. [Crossref] [PubMed]
- Graue M, Wentzel-Larsen T, Bru E, et al. The coping styles of adolescents with type 1 diabetes are associated with degree of metabolic control. Diabetes Care 2004;27:1313-7. [Crossref] [PubMed]
- Dewald PA. Principles of supportive psychotherapy. Am J Psychother 1994;48:505-18. [PubMed]
- Stein DJ, Kupfer DJ, Schatzber AF. editors. Psychoanalytic and psychodynamic psychotherapy for depression and dysthymia. In: The American Psychiatric Publishing Textbook of Mood Disorders. American Psychiatric Publishing, 2007.
- Robertson M, Rushton P, Wurm C. Interpersonal Psychotherapy: An overview. Psychother Aust 2008;14:46-55.
- Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry 2005;62:996-1004. [Crossref] [PubMed]
- Bouwkamp CG, de Kruiff ME, van Troost TM, et al. Interpersonal and social rhythm group therapy for patients with bipolar disorder. Int J Group Psychother 2013;63:97-115. [Crossref] [PubMed]
- Wroe AL, Rennie EW, Sollesse S, et al. Is Cognitive Behavioural Therapy focusing on Depression and Anxiety Effective for People with Long-Term Physical Health Conditions? A Controlled Trial in the Context of Type 2 Diabetes Mellitus. Behav Cogn Psychother 2017.1-19. [Crossref] [PubMed]
- Dobson KS. editor. Problem-solving therapy. In: Handbook of Cognitive-Behavioral Therapies. 3rd edition. New York, NY: Guilford Press, 2010.