Disorders/differences of sex development (DSDs) for primary care: the approach to the infant with ambiguous genitalia
Introduction
The term ambiguous genitalia encompasses a variety of disorders labeled as DSDs, or disorders of sex development, (alternatively, differences in sex development). DSDs have been defined as congenital conditions in which development of chromosomal, gonadal or anatomical sex is atypical (1-3), and comprise a wide set of metabolic and anatomic defects and variants that often can result in atypical genital appearance and may lead to emotional and psychological distress if not sensitively addressed.
While we have improved our understanding of normal sex development in terms of anatomy and molecular biology of the male and female pathways, our understanding of the defects that can occur along the way is still in process. This particularly holds true with regard to predicting gender expression and identity. We know that pathology can interfere at many different stages in sex determination and development, from early in the differentiation of the bipotential gonad to the final steps of genital formation. Based on gene mutations and/or metabolic defects identified in human and animal models, we have learned from the spectrum of phenotypes that can make up the term ‘ambiguous genitalia’ or alternatively, ‘atypical genitalia’ (AG). We will discuss here various known causes of AG, and important initial steps in the approach and early management. We will conclude with a brief overview of therapy, to help prepare the health care provider in counseling families moving through this potentially tumultuous journey.
Case vignette
A community pediatrician is rounding in the newborn nursery and sees a term newborn that was delivered the previous evening. The neonate is well appearing but the genital exam is abnormal, with a small atypical-looking penis and no palpable testes in the scrotum. The parents are asking why the genitalia look abnormal. Though the parents decided not to share the results of the prenatal ultrasound with friends and relatives, they are now asking why the sonogram had previously suggested they would be having a baby girl.
This scenario would surely add stress to an already highly emotional time for any new parent as well as for the physician attending to them. The potential etiologies and recommended evaluation for genital ambiguity in the newborn will be reviewed along with an approach to the principles of optimal management in this critical period.
Normal sex development
Our understanding of the anatomic and gonadal basis of sex development is ever-expanding, and begins in early embryology. It is important to realize that the gonads as well as internal and external genital structures all originate from the same bipotential embryologic tissues, and only through well-choreographed, well-coordinated and localized expression of genes and their gene products does normal sex differentiation and development progress.
The first migration of bipotential primordial germ cells from the yolk sac endoderm to the urogenital ridges leads to the undifferentiated gonad beginning at 4–6 weeks (4). The first 7 weeks of gestation have been considered the indifferent stage, as male and female fetuses would appear grossly indistinguishable from one another, though histologically, changes are well underway. A clear “switch” or decision point signaling the sex determination then occurs leading to the initiation of testicular or ovarian gonadal differentiation. The young bipotential gonad is able to differentiate towards a dedicated male or female pathway, either testis or ovary, respectively, based initially on factors that reside on the Y chromosome (the SRY gene is the prototypical example). Gonadal differentiation then triggers hormonal responses and along with precise and timely gene activation lead to normal/typical external and internal genital development. We have learned about the role of timely and appropriate gene action in normal sex development through gene defects discovered along the pathways associated with DSDs (5-7).
There is no true “default” pathway, as is historically taught; however, androgen exposure determines the external genitalia phenotype. For example, androgen exposure from testosterone and its more potent metabolite dihydrotestosterone (DHT) leads to phallic enlargement and the formation of the penis and the fusion of the labioscrotal folds into a scrotum. The spectrum of phenotypes relating to androgen exposure is seen in many DSDs with varying degrees of genital ambiguity. Different scales or scores have been developed to assess this spectrum, though the most commonly used is the Prader Scale (4), with some alternative scales being developed more recently (8).
With regard to the internal genitalia, the mesonephric duct develops into the Wollfian duct and the internal male genital organs, and the paramesonephric duct develops into the Müllerian duct and the internal female genital organs. In the presence of anti-Müllerian hormone (AMH) secreted by the Sertoli cells of the newly developed testes, the Müllerian structures regress, leaving only the Wollfian structures-epididymis, vas deferens, seminal vesicles, and ejaculatory ducts. Otherwise, the Müllerian structures persist, developing into the fallopian tubes, uterus, cervix, and upper vagina, while the Wollfian structures regress without testosterone trophic effect.
Causes of ambiguous genitalia
The causes of genital ambiguity in newborn period are listed in Table 1 (9-12). The most common cause of ambiguous genitalia is congenital adrenal hyperplasia. CAH comprises a collection of different adrenal steroid biosynthetic disorders which can result in wide-ranging phenotypes from salt-wasting adrenal crisis in the infant to virilization in young females, delayed puberty in adolescents and even polycystic ovary syndrome (PCOS)-like symptoms (i.e., acne, irregular menses, hirsutism, infertility) in young women. CAH should be the primary consideration in any virilized infant with non-palpable gonads. Adrenal crisis can lead to shock and death if this condition is not promptly recognized and treated.

Full table
In addition to CAH being often recognized as the most common cause of genital ambiguity in infants and neonates, other notable causes include partial androgen insensitivity syndrome (Partial AIS or PAIS). AIS is caused by mutations in the androgen receptor (AR) gene on the X chromosome, and dictates response to androgen such as testosterone or DHT. Complete AIS (CAIS) refers to a complete defect in AR and thus no response to androgen, whereas PAIS refers to a partial defect and thus partial activity in the AR and the androgen response. CAIS often presents with primary amenorrhea in adolescent female, and since there is no androgen effect it does not lead to genital ambiguity. CAIS cases are characterized by female external genitalia but with 46XY karyotype and elevated levels of testosterone, illustrating the complete androgen resistance resulting from the inactive AR. PAIS, being an intermediate and partial mutation, leads to some androgen effect and thus partial virilization. This phenotype then results in ambiguous genitalia in a 46XY infant.
Unfortunately, a great number of cases do not have an identifiable cause, particularly among 46XY DSD, which highlights the importance of an experienced team with multidisciplinary focus (13). Please note that congenital malformations can also cause genital ambiguity, many of which have a primary genetic cause, and others which have a more structural or developmental cause and do not have any hormonal basis. These include VATER/VACTERL associations or cloacal exstrophy (10).
Terminology
Anatomy definitions
The ability to adequately and confidently assess and then describe any differences seen on the genital exam is helpful in easing new parents. There are a few key terms that are frequently used in describing genital ambiguity and DSDs:
- Micropenis is defined as penile length <2 standard deviations below the mean, and is based on age. For term infants, the commonly used cutoff is <2.0 cm for stretched penile length (SPL). References are available for different ages and gestational ages (14,15). SPL is measured along the dorsal surface of the penis, from pubic symphysis to the tip of the glans, with gentle traction. One method involves use of a firm, flat object, such as a tongue depressor, which is pressed against the pubic bone while the penis is gently stretched along the flat surface. A mark is made where the tip of the glans reaches and this length can then be directly measured.
- Clitoromegaly results from hypertrophy of the clitoris and is seen secondary to androgen exposure (virilization). A commonly used definition is a length >1 cm in term female infants, although width is often measured as well (4).
- Hypospadias is a ventral defect in the penile urethra, and is one of the most common genital defects. Incomplete fusion of the urethral folds will lead to hypospadias. This finding can be described by the location of the defect: penile, penoscrotal, scrotal, perineal, etc.
- Labioscrotal fusion describes the fusion or closure of the labioscrotal folds, and is also seen secondary to androgen exposure (virilization) in the fetus. This phenotype can vary as the fusion effect “zippers” up from posterior (inferior) to anterior (superior), reflecting the magnitude of androgen exposure.
- Bifid scrotum can be a more severe variant of labioscrotal fusion with a clear separation of left and right hemi-scrotum, on each side of the midline scrotal raphe.
- Cryptorchidism, also known as undescended testes, can be unilateral or bilateral, and along with hypospadias, are among the most common genital defects. There are two phases of descent: the first phase (intra-abdominal/inguinal, at 10–15 weeks gestation) is androgen-independent and the second phase (inguinoscrotal, at 26–40 weeks gestation) requires testosterone and DHT (16). Bilaterality can be a clue to more severe hormonal defect or hypogonadism, though unilateral cryptorchidism can be seen in ovotesticular DSDs or mixed gonadal dysgenesis and chromosomal mosaicism.
Revisions of the nomenclature
There have been substantial revisions to what we now refer to as the older nomenclature. This older terminology is now painfully outdated and no longer in use. A re-examination of this old nomenclature was undertaken because of the heightened awareness of the ethical issues and patient advocacy concerns, leading to the International Consensus Meeting on Intersex held in Chicago in 2005 (1-3). This group consisted of international experts specializing in DSDs including endocrinologists, urologists and psychologists, under the auspices of international Pediatric Endocrinology groups such as the Pediatric Endocrine Society (USA/Canada) and the European Society for Paediatric Endocrinology. The findings were deemed of high importance and co-published in three journals (1-3).
The older terminology, with phrasing such as ‘hermaphrodite’ and ‘pseudo-hermaphrodite’ was felt to be pejorative, confusing and stigmatizing, and the new classification more sensitive to individuals and families, and also more reflective of our molecular understanding in sex development research and sex differences in the patient. The general principles of the newer terminology are displayed in Table 2.
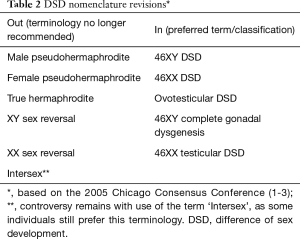
Full table
There is still considerable controversy in the field regarding proper and respectful nomenclature. Even terms like DSD are being revised. DSD as “disorders of sex development” are sometimes being referred to alternatively as differences of sex development (DSDs). To further this point, it has also been pointed out that the term “ambiguous genitalia” also should be used carefully. Genital ambiguity should never, by itself, be a diagnosis (17) and merely a finding on clinical exam (or symptom, perhaps), through which a proper and careful assessment is performed and where hopefully a true diagnosis can be made. Just as a clinician would never just stop at a diagnosis of fever and would search for a cause, the term or diagnosis “Ambiguous Genitalia” should just be used as a temporary diagnostic label until a more specific and accurate diagnosis is made. Examples of a more precise diagnosis could be CAH, or even simply hypospadias and cryptorchidism. Support group websites have been working to address this controversy (18,19), and a recent study surveying caregivers and affected individuals regarding their attitudes on DSD-related nomenclature addresses this controversy as well, showing negative perceptions persist with the terminology used in DSD care (20).
Exam and workup
A thoughtful history and exam, along with an initial workup can be extremely helpful in progressing toward a working diagnosis. Without a diagnosis, it becomes difficult for the medical team to assist the family with proper guidance in assigning a sex of rearing and planning for the future. Though a molecular diagnosis is reached in only a minority of cases, an accurate working diagnosis is not only helpful in planning proper anticipatory guidance with regard to growth and pubertal development, but is also very comforting for the individual and family. A reliable diagnosis is also needed to confidently plan any potential surgical intervention.
Key points in the history and exam are outlined in Table 3 (4,21). After the initial assessment for general symmetry of the genitalia, the presence of gonads and evaluation of the phallic structure are two components of the genital exam that can be assessed quickly and relatively easily. First note if gonads are palpable bilaterally or unilaterally, including palpating the labia majora and inguinal regions for a gonad-like structure. Next, the phallic structure can be described along a continuum from normal/typical male (penis) to normal/typical female (clitoris). Atypical variants can be described as variants of micropenis vs. clitoromegaly. A clinical staging tool called the Prader scale was developed to describe the external genitalia by the degree of virilization, from unvirilized (normal female genitalia) to complete male virilization with penis-size phallus, complete labial fusion, and meatus on the glans (normal male genitalia) (4). A newer scoring system, called the External Masculinization Score, is gaining acceptance as well, and is based on factors such as location of gonads on exam, degree of scrotal fusion, phallus size, and location of urethral meatus (8)

Full table
Mild variation in the clinical exam is common, but an atypical exam can point to the diagnosis. Asymmetry in the genital exam, such as unilateral structures can suggest a sex chromosome DSD such as mixed or partial gonadal dysgenesis, or mosaicism. Examples would include Ovotesticular DSD or 45X/46XY mosaicism. Palpable bilateral gonads are generally associated with a 46 XY karyotype. AG cases with non-palpable gonads should bring up concern for a 46XX infant with virilization (such as CAH, as discussed above), or alternatively could be a 46XY infant with either cryptorchidism or absent gonads (i.e., congenital anorchia; sometimes termed “vanishing testes” syndrome or “testicular regression”).
Further analysis with biochemical and hormone testing, as well as imaging, is usually needed to clear up the diagnostic picture. The minimal initial laboratory and imaging workup for ambiguous genitalia in the infant is listed in Table 4. In cases of non-palpable gonads, the first objective of checking the androgen levels, most importantly 17 hydroxy progesterone, and electrolytes is to evaluate for CAH.
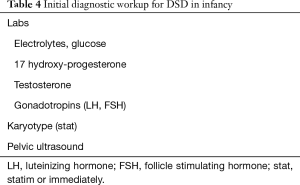
Full table
In cases of genital asymmetry or cryptorchidism, additional testing is often pursued by the specialist team. For example, AMH levels can help detect functional testicular tissue, and the hCG Stimulation Test can be used to assess testicular/gonadal function. This test assesses the ability of the gonad (in this case, presumed testicle) to synthesize and secrete testosterone and other androgens. Cases of partial virilization in a 46XY infant could represent 5 alpha reductase deficiency or could be Partial AIS, and the testosterone and DHT response to human chorionic gonadotropin (hCG) stimulation could help differentiate these two entities.
In addition to karyotype testing, additional cytogenetic studies can be helpful, such as SRY testing by FISH (fluorescence in-situ hybridization) as well as more advanced techniques such as array CGH (comparative genomic hybridization) and more recently with the use of whole exome sequencing (WES). Significant advances are being made in genome-based testing and other “next-generation sequencing” technologies, such as WES, and some of these techniques may soon become adequately time- and cost- effective to be considered first-tier diagnostic or rule-out testing (22-24).
Numerous different diagnostic algorithms have been previously presented as frameworks for making a clinical diagnosis and are excellent approaches to continue the workup (4,6,25-28).
Initial approach: basic principles of optimal management
The proper management of the infant with ambiguous genitalia requires prompt but thorough evaluation. Lessons have been learned from past negative experiences and the insights made by expert psychologists who study DSDs (29,30). Fundamental principles in the management of DSDs are discussed below.
First conversations with the family
The first conversations with the family need to set a positive tone where the new parents will be respectfully included and guided through the process. In addition, the first impressions tend to have lasting effects in many families. When addressing the infant, it is important to initially be gender-neutral and avoid “he” or “she” pronouns that could inadvertently bias the family towards a gender that may later be reversed. And rather than referring to the child as “it”, warmer terms like “your baby” may promote greater bonding. Staying neutral until the decision is finalized helps prevent misunderstandings and confusion (4).
Perspective is also important, as this may be the child’s only medical issue. Assuming that the medical issues are limited, the medical provider should emphasize that the baby is otherwise healthy, and has the potential to become a healthy and happy, well-adjusted child and adult. The birth of a healthy child is a momentous occasion, and one to be celebrated. Too much emphasis on the genitalia could increase anxiety and stigma within the family (1-3).
Be thoughtful with the sex assignment: do not rush!
Assigning a sex of rearing is a great responsibility and obligation and should not be rushed. Many factors must be taken into account, and thus it is essential to collect all relevant information while still being expeditious. This includes clinical information (genital appearance as reflecting androgen exposure and future sexual function), laboratory (and cytogenetic) data, as well as imaging, as discussed above. Note also that karyotype data alone is insufficient, and can lead the team astray if used in isolation. Parental views and possible cultural practices must also be respected. Additional considerations include prenatal androgen exposure effects, the future need for hormone replacement, surgical options and fertility potential, even if considered remote (31). We now know that the brain should be considered a “sex organ”: early androgen exposure can have long-lasting effects, and the degree of androgen exposure in utero is believed to be predictive of future masculine behavior and gender identity, though evidence is limited (4,32). All of these factors named above must be collectively integrated to formulate the most accurate working diagnosis prior to assigning a sex and gender (5,21,33-36).
Ultimately, an individualized approach is essential since long term outcome data are still quite limited. And though uncertainty in the diagnosis is common, there are some general concepts that are widely accepted. For example, 46XX CAH infants should generally be raised as female, unless markedly virilized, in which case the literature is not clear on outcomes. Also, in general 46XY infants with genital ambiguity should generally be assigned as male, especially if there is reasonable evidence of testicular function and androgen responsiveness. To assign such an individual as female constitutes risk (28). Evidence based guidelines are badly needed and future studies will help give better guidance for specific conditions and clinical scenarios, though multicenter networks are making progress (35).
Transparency and disclosure
Parents must be involved and made to feel included in decision-making. As part of this process, the genital anatomy and pathology should be explained to the family in lay terms to not only help families better understand the dilemma of a complex sex/gender assignment but also to help destigmatize any genital differences. Adequate time must be allotted to answer any questions the family may struggle with. It is imperative that the parents or guardians feel vested in the dialogue. This approach to complex or difficult health care decisions, called “Shared Decision-Making”, helps the family feel more invested in the process, and leads to increased satisfaction (37,38). Note that the child should ideally be informed and involved in the process as well, and information should be provided in an age-appropriate manner with eventual full disclosure to the child.
Be cautious with surgery
Though the history of surgery in DSD is filled with controversy and regret, surgical intervention has an important role in certain circumstances. The major indications for surgery in DSD are gonadectomy (generally to mitigate increased risk of malignancy) and genital reconstructive surgery, and both carry significant controversy. Additional goals of surgery, however, are to optimize future sexual function, facilitate options for future reproduction, minimize risk of urinary tract infection, and to avoid stigmatization related to atypical anatomy (39), though many of these points carry some controversy as well.
Gonadectomy
There is increased risk of neoplasm in dysgenetic gonads carrying Y chromosome material, and in sex chromosome DSDs such as mixed/partial gonadal dysgenesis. Malignant transformation can occur if resection is not pursued in a timely manner. While resection of the gonads to prevent the formation of these gonadal tumors (i.e., gonadoblastoma or dysgerminoma) is a reasonable consideration, there is believed to be time to deliberate since they usually do not occur in the first few years of life. In addition, potential function of that gonad (from a hormone production and fertility perspective) should be considered. In some cases, for example, waiting until adolescence can allow for spontaneous puberty to occur and avoid the need for pubertal induction hormone therapy. Importantly though, there may be future ethical or medicolegal implications, since it can be viewed as a sterilization procedure (21,37,38,40,41).
Reconstructive surgery
This type of surgery includes feminizing procedures for CAH, such as clitoral reduction and vaginoplasty, but also includes corrective repair in the male, for hypospadias and cryptorchidism. Previous guidelines (including the 2005 Chicago consensus) emphasized that feminizing reconstructive surgery should only be considered for more severe cases of virilization (Prader scores III, IV and V) (1-3). However, more recently some human rights groups have called for a moratorium on genital surgery (40), with the general recommendation to defer surgery until the individual is of age to decide for themselves. This complex topic will continue to foster controversy, since there is limited evidence for both sides of the debate, and this highlights the importance of open and transparent dialogue between the individual, family and team (21,37,38,40,41).
Prior to undergoing any procedure, it is important to have a thorough and open dialogue with the family (and patient, whenever possible) to avoid unnecessary and irreversible surgery. In addition, clear communication is needed to prevent unrealistic expectations regarding the timing of surgery and of anatomy following reconstruction (1-3).
A team-based approach is optimal
Because of the unique challenges and resources needed in cases of atypical sex development, a management team comprised of specialists from numerous disciplines has been deemed essential. As a new standard of care according to the consensus statements, this multidisciplinary team should at minimum include representatives from endocrinology, urology (and/or surgery), social work & behavioral health (psychology/psychiatry), and nursing, and have access to experts in gynecology, bioethics, neonatology, and child life (1-3,5,13,33,38,42,43).
For example, at our institution the THRIVE program (Team-driven Health care that Respects the Individual and Values Emotions) was created to address the specialized care required for children and adolescents with differences/DSD, complex urological conditions, and gender concerns. Major institutions across the Americas and Europe now frequently employ a multi-disciplinary team strategy in addressing the many needs of this population. A recent international survey (mostly comprising European centers) showed the majority of centers implementing a multi-disciplinary team, and with 41% of centers surveyed showing a team participating within the first week after presentation (44). In addition to the expertise that can assist in formulating a diagnosis and sex assignment, the benefits to the family are innumerable and transcend all phases of care, from the initial interactions in the neonatal period, through the processing of the diagnosis and treatment plans, and all transitions through adulthood. This is all collectively part of the new holistic approach that provides important continuity of care.
Psychological issues: optimizing psychosocial and psychosexual well-being
The need for broad and longitudinal psychological support cannot be overstated. Psychological issues can be dynamic along the age scale through infant, childhood, adolescent and adult developmental phases. Beginning in infancy, the confusion surrounding the diagnosis and sex assignment is long lasting and can be traumatic. Then later in childhood and progressing through adolescence and adulthood, themes of stigma, secrecy, and shame are common and intervention by experienced mental health providers for individual and family can provide much needed support.
Perhaps the ultimate goal of the sex assignment is that it eventually aligns with the individual’s gender identity, in order to foster a happy, productive and well-adjusted adult. Ideally this goal should also include optimized fertility potential and adult sexual function, though historically, we have done poorly. Fertility prognosis has generally been dismal for many DSDs, though assisted reproductive technologies continue to improve and a recent study suggested that germ cells may be present in many infants with a variety of DSDs (31). Further, sexual function has often been compromised, sometimes thought to be related to surgical intervention. In addition to issues of stigmatization, these factors have contributed to high rates of dissatisfaction with medical and surgical care, leading to mistrust as well as increased depression and other mental health concerns. Parental stress has also been increasingly identified as a critical factor as well (45). For these reasons psychological support is crucial. In addition to resources within the multidisciplinary clinic team, there are many support groups available (21,32,35,36,38,45).
With regard to psychosexual development, it is now becoming more understood that sex and gender, while having much overlap, is not the same. In addition, the concept of gender itself is being broadened, and reference has been made to a spectrum of gender expression and identity. The so-called “genderbread person”, an infographic and learning tool popularized on social media is one model to understand this wide phenotypic variation (46). While designed as a free online resource for understanding and conceptualizing differences and variations in gender identity, gender expression, and anatomical sex, it can also help facilitate a discussion of gender vs. sex either in the context of transgender medical care or in DSDs (46). Cases of either dissatisfaction with assigned gender or gender dysphoria are not rare, and these require the care of specialists experienced in managing gender dysphoria. Key points of advice for health care providers are summarized in Table 5.

Full table
Referral
Urgent referral or consultation with pediatric endocrinology (and eventually the entire multidisciplinary team) is essential if genital ambiguity is noted in the neonatal period, in order to expedite an informed sex assignment. While ambiguous genitalia may not be a medical emergency, it certainly is a potential social or psychological emergency, perhaps a “gender” emergency. As such, rapid mobilization of resources (as discussed above) is necessary to adequately address the sex/gender determination question. Even if the AG is noted later in infancy or in childhood, a prompt referral to endocrinology is still worthwhile, as many parents become anxious regarding genital abnormalities and this stigmatization can lead to consequences in child development. Isolated hypospadias or simple unilateral cryptorchidism may only require urology referral.
In addition, pediatricians often are asked questions regarding their child’s genital development, such as variations in penis size or labial appearance. When there is a concern, cases such as these can benefit from pediatric endocrinology or urology consultation, as these genital variations can often be addressed and destigmatized.
Returning to the case vignette
This scenario involved a well appearing newborn with an abnormal genital exam- small abnormal penis, and no palpable testes in a hypoplastic scrotum. Parents were anxious since the prenatal ultrasound suggested they would be having a baby girl but this infant looked like a partially virilized male. In this case, a small phallic structure should bring up the question of micropenis vs. clitoromegaly. And in particular, these findings along with non-palpable gonads should raise suspicion for congenital adrenal hyperplasia in a virilized female infant (with clitoromegaly). Further workup confirmed 46XX karyotype, and pelvic ultrasound confirmed female internal structures (Müllerian structures, such as uterus), though ovaries were not visualized. Of note, this is not necessarily a concerning finding as gonads can be difficult to visualize at this age (25). In addition, newborn screen results revealed elevated 17 hydroxy progesterone (17OHP) levels, and an immediate call placed to endocrinology for referral. These findings all helped to confirm congenital adrenal hyperplasia and hyperandrogenemia as the cause of the genital ambiguity.
By far the most common form of CAH encountered is 21 hydroxylase deficiency, which results in defective conversion of 17 hydroxy progesterone to 11 deoxycortisol and cortisol. This leads to high levels of 17OHP and subsequent elevations in the androgenic metabolites androstenedione and testosterone as well. The high androgen levels virilize the infant, and the block in cortisol synthesis leads to adrenal insufficiency which risks electrolyte imbalance, hyponatremia, hyperkalemia, hypotension, vomiting, and if untreated, cardiovascular collapse, shock and death.
As with most enzymatic defects, the 21-hydroxylase deficiency form of CAH is inherited in an autosomal recessive manner. The carrier rates can be high, especially in some populations.
Treatment for CAH is steroid replacement (usually hydrocortisone in the childhood years), in addition to the synthetic mineralocorticoid fludrocortisone, which helps maintain adequate electrolyte levels and salt balance. As part of chronic management, appropriate counseling must be given with regard to stress-dosing for physiological stressors, such as illness and surgical procedures. During early infancy, sodium chloride/salt replacement (typically using a liquid formulation) is often needed to ensure adequate salt intake.
Achieving a balance between adequate and excessive steroid dosing in CAH can be a challenge. Chronic over-dosing of steroid replacement risks Cushingoid effects, leading to excess weight gain, poor linear growth (stunting), hypertension, and low bone mineral density (4). Inadequate steroid replacement will lead to inadequate metabolic control of androgen production, resulting in excessive virilization and precocious puberty, as well as rapid bone age advancement and eventual short stature. Optimal care in CAH, as with other DSDs (as described above), requires a multidisciplinary team, including urologists, geneticists, social workers, psychologists (42,47).
Concluding thoughts
While clinical and molecular biology research have propelled us in breaking down the complexities of male and female sex development, it has been significantly shaped by decades of practical medical experience and the lessons learned from painful personal, family, and individual experiences. Together, these lessons have honed our ability to provide affirming care that is transparent, with full disclosure, and focused on a more shared decision-making process. Nurturing the individual and family through a team-based approach can be the most rewarding.
Acknowledgements
The author gratefully acknowledges the editorial assistance of Dr. Rebecca Slaunwhite and the administrative assistance of Ms. Leah Yodzis.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Lee PA, Houk CP, Ahmed SF, et al. Consensus statement on management of intersex disorders. Pediatrics 2006;118:e488-500. [Crossref] [PubMed]
- Hughes IA, Houk C, Ahmed SF, et al. Consensus statement on management of intersex disorders. J Pediatr Urol 2006;2:148-62. [Crossref] [PubMed]
- Hughes IA, Houk C, Ahmed SF, et al. Consensus statement on management of intersex disorders. Arch Dis Child 2006;91:554-63. [Crossref] [PubMed]
- Sperling M. Pediatric Endocrinology. 4th ed. Philadelphia, Pennsylvania: Saunders Elsevier, 2014.
- Ahmed SF, Achermann JC, Arlt W, et al. UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development. Clin Endocrinol (Oxf) 2011;75:12-26. [Crossref] [PubMed]
- Yatsenko SA, Witchel SF. Genetic approach to ambiguous genitalia and disorders of sex development: What clinicians need to know. Semin Perinatol 2017;41:232-43. [Crossref] [PubMed]
- MacLaughlin DT, Donahoe PK. Sex Determination and Differentiation. N Engl J Med 2004;350:367-78. [Crossref] [PubMed]
- Ahmed SF, Khwaja O, Hughes IA. The role of a clinical score in the assessment of ambiguous genitalia. BJU Int 2000;85:120-4. [Crossref] [PubMed]
- Chi C, Lee HC, Neely EK. Ambiguous Genitalia in the Newborn. Neoreviews 2008;9:e78-84. [Crossref]
- Hutson JM, Grover SR, O'Connell M, et al. Malformation syndromes associated with disorders of sex development. Nat Rev Endocrinol 2014;10:476-87. [Crossref] [PubMed]
- Esen I, Demirel F. Preterm ovarian hyperstimulation. N Engl J Med 2015;372:2336. [Crossref] [PubMed]
- Greaves R, Kanumakala S, Read A, et al. Genital abnormalities mimicking congenital adrenal hyperplasia in premature infants. J Paediatr Child Health 2004;40:233-6. [Crossref] [PubMed]
- Parisi MA, Ramsdell LA, Burns MW, et al. A gender assessment team: Experience with 250 patients over a period of 25 years. Genet Med 2007;9:348-57. [Crossref] [PubMed]
- Schonfield WA. Primary and secondary sexual characteristics. Am J Dis Child 1943;65:535-49. [Crossref]
- Feldman KW, Smith DW. Fetal phallic growth and penile standards for newborn male infants. J Pediatr 1975;86:395-8. [Crossref] [PubMed]
- Thorup J, McLachlan R, Cortes D, et al. What is new in cryptorchidism and hypospadias—a critical review on the testicular dysgenesis hypothesis. J Pediatr Surg 2010;45:2074-86. [Crossref] [PubMed]
- Dreger AD. "Ambiguous sex"--or ambivalent medicine? Ethical issues in the treatment of intersexuality. Hastings Cent Rep 1998;28:24-35. [Crossref] [PubMed]
- Androgen Insensitivity Syndrome Support Group. Available online: http://www.aissg.org. Accessed September 12, 2017
- Intersex Society of North America. Available online: http://www.isna.org. Accessed September 12, 2017
- Johnson EK, Rosoklija I, Finlayson C, et al. Attitudes towards “disorders of sex development” nomenclature among affected individuals. J Pediatr Urol 2017. [Epub ahead of print].
- Lee PA, Nordenström A, Houk CP, et al. Global disorders of sex development update since 2006: Perceptions, approach and care. Horm Res Paediatr 2016;85:158-80. [Crossref] [PubMed]
- Baxter RM, Arboleda VA, Lee H, et al. Exome sequencing for the diagnosis of 46,XY disorders of sex development. J Clin Endocrinol Metab 2015;100:E333-44. [Crossref] [PubMed]
- Eggers S, Sadedin S, van den Bergen JA, et al. Disorders of sex development: insights from targeted gene sequencing of a large international patient cohort. Genome Biol 2016;17:243. [Crossref] [PubMed]
- Alhomaidah D, McGowan R, Ahmed SF. The current state of diagnostic genetics for conditions affecting sex development. Clin Genet 2017;91:157-62. [Crossref] [PubMed]
- Chavhan GB, Parra DA, Oudjhane K, et al. Imaging of Ambiguous Genitalia: Classification and Diagnostic Approach. Radiographics 2008;28:1891-904. [Crossref] [PubMed]
- Ahmed SF, Rodie M. Investigation and initial management of ambiguous genitalia. Best Pract Res Clin Endocrinol Metab 2010;24:197-218. [Crossref] [PubMed]
- Moshiri M, Chapman T, Fechner PY, et al. Evaluation and management of disorders of sex development: Multidisciplinary approach to a complex diagnosis. Radiographics 2012;32:1599-618. [Crossref] [PubMed]
- Bangalore Krishna K, Houk CP, Lee PA. Pragmatic approach to intersex, including genital ambiguity, in the newborn. Semin Perinatol 2017;41:244-51. [Crossref] [PubMed]
- Glassberg KI. The intersex infant: Early gender assignment and surgical reconstruction. J Pediatr Adolesc Gynecol 1998;11:151-4. [Crossref] [PubMed]
- Bakula DM, Mullins AJ, Sharkey CM, et al. Gender identity outcomes in children with disorders/differences of sex development: Predictive factors. Semin Perinatol 2017;41:214-7. [Crossref] [PubMed]
- Finlayson C, Fritsch MK, Johnson EK, et al. Presence of germ cells in disorders of sex development: Implications for fertility potential and preservation. J Urol 2017;197:937-43. [Crossref] [PubMed]
- Arboleda VA, Sandberg DE, Vilain E. DSDs: genetics, underlying pathologies and psychosexual differentiation. Nat Rev Endocrinol 2014;10:603-15. [Crossref] [PubMed]
- Mieszczak J, Houk CP, Lee PA. Assignment of the sex of rearing in the neonate with a disorder of sex development. Curr Opin Pediatr 2009;21:541-7. [Crossref] [PubMed]
- Douglas G, Axelrad ME, Brandt ML, et al. Consensus in Guidelines for Evaluation of DSD by the Texas Children's Hospital Multidisciplinary Gender Medicine Team. Int J Pediatr Endocrinol 2010;2010:919707. [Crossref] [PubMed]
- Lee PA, Wisniewski AB, Baskin L, et al. Advances in diagnosis and care of persons with DSD over the last decade. Int J Pediatr Endocrinol 2014;2014:19. [Crossref]
- Meyer-Bahlburg HF, Baratz Dalke K, Berenbaum SA, et al. Gender assignment, reassignment and outcome in disorders of sex development: Update of the 2005 consensus conference. Horm Res Paediatr 2016;85:112-8. [Crossref] [PubMed]
- Karkazis K, Tamar-Mattis A, Kon AA. Genital Surgery for Disorders of Sex Development: Implementing a Shared Decision-Making Approach. J Pediatr Endocrinol Metab 2010;23:789-805. [Crossref] [PubMed]
- Karkazis K, Rossi WC. Ethics for the Pediatrician: Disorders of Sex Development: Optimizing Care. Pediatr Rev 2010;31:e82-5. [Crossref] [PubMed]
- Mouriquand PD, Gorduza DB, Gay CL, et al. Surgery in disorders of sex development (DSD) with a gender issue: If (why), when, and how? J Pediatr Urol 2016;12:139-49. [Crossref] [PubMed]
- Human Rights Watch and InterACT: “I Want to be Like Nature Made Me”: Medically Unnecessary Surgeries on Intersex children in the US. Available online: https://www.hrw.org/report/2017/07/25/i-want-be-nature-made-me/medically-unnecessary-surgeries-intersex-children-us. Accessed September 12, 2017.
- Karkazis KA. Early genital surgery to remain controversial. Pediatrics 2006;118:814-5. [Crossref] [PubMed]
- Brain CE, Creighton SM, Mushtaq I, et al. Holistic management of DSD. Best Pract Res Clin Endocrinol Metab 2010;24:335-54. [Crossref] [PubMed]
- Consortium on the Management of Disorders of Sex Development. Clinical guidelines for the management of disorders of sex development in childhood. Intersex Society of North America. Rohnert Park, 2006.
- Kyriakou A, Dessens A, Bryce J, et al. Current models of care for disorders of sex development – results from an International survey of specialist centres. Orphanet J Rare Dis 2016;11:155. [Crossref] [PubMed]
- Wisniewski AB, Sandberg DE. Parenting Children with Disorders of Sex Development (DSD): A Developmental Perspective Beyond Gender. Horm Metab Res 2015;47:375-9. [Crossref] [PubMed]
- The Genderbread Person. Available online: https://www.genderbread.org. Accessed September 12, 2017.
- Speiser PW, Azziz R, Baskin LS, et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2010;95:4133-60. [Crossref] [PubMed]


