
Predictive value of serum markers in the operation evaluation of neonatal necrotizing enterocolitis
Highlight box
Key findings
• CRP, PCT, IL-6, I-FABP, and SAA were independent related factors of necrotizing enterocolitis (NEC) surgical treatment. Among these serum markers, I-FABP (≥5.28 ng/mL) and SAA (≥204.89 mg/L) had relatively high sensitivity and specificity in predicting the timing of NEC operation.
What is known and what is new?
• At present, the study of serum markers may have a certain reference value for the choice of NEC surgery opportunity.
• This study aimed to determine the application value of serum markers in selecting operation opportunities for NEC.
What is the implication, and what should change now?
• The clinical value of the serum markers I-FABP, PCT, IL-6, CRP, and SAA in predicting the timing of NEC surgery was investigated in this study to provide a reference for clinically accurate NEC remediation.
Introduction
Neonatal necrotizing enterocolitis (NEC) is a common gastrointestinal emergency in newborns with abdominal distension tenderness, swelling/edema and discoloration as the main clinical manifestations. Currently, the pathogenesis of the disease remains unknown. Studies have associated its occurrence with factors such as premature birth, hypoxia, infection, and intestinal flora disorders. Related studies have shown that the pathogenesis of NEC is not clear, but immature digestive system, mucosal barrier, gastrointestinal dynamics and intestinal bacterial translocation in premature infants are the main causes of necrotizing small intestinal colitis in newborns (1). In recent years, as the survival rate of premature infants, especially those with very low and ultra-low body weight has improved, the incidence of NEC has gradually increased, posing a serious threat to the life and safety of newborns. NEC is a common acute and severe neonatal disease with high mortality. After the remedy, the surviving neonates may also have sequelae such as stenosis, short bowel syndrome, and abnormal growth and development, which seriously affect the life and health of pediatric patients (2,3). Early diagnosis and early remedy are the most effective means to reduce NEC mortality and improve pediatric patients’ prognosis. About 30–50% of clinically diagnosed pediatric patients require surgical remedy, but the choice of surgical opportunity is difficult to determine (4). Ideally, the operation should be performed when the intestinal canal is necrotic but not perforated, which can avoid peritonitis caused by intestinal perforation, relieve the intestinal burden in time, and reduce mortality (5,6). At present, intestinal perforation is the clinical indication of NEC operation, but only a few pediatric patients have typical manifestations of intestinal necrosis. When most pediatric patients show typical symptoms, their condition might have already deteriorated, and it is too late for operation (7).
“The guidelines for the clinical diagnosis of necrotizing enterocolitis in neonates” [2020] (8) recommend interleukin-6 (IL-6), procalcitonin (PCT), and C-reactive protein (CRP) as the diagnostic basis for NEC. Interleukin is a type of cytokine produced by various kinds of cells and plays an essential regulatory role. IL-6 is one of the key mediators of the early inflammatory response and can react with multicellular cytokines with a wide range of biological effects. After bacterial infection, the proliferation of activated T and B cells is stimulated by IL-6. PCT is a propeptide composed of 116 amino acids. Under normal conditions, PCT is not secreted from cells, and blood levels of PCT are less than 0.1 ng/mL. However, when bacteria enter the body, the concentration of PCT in the blood can rise rapidly. During the neonatal period, intestinal development is not mature, and a variety of factors can easily cause the weakening of intestinal peristalsis, which leads to the accumulation of food in the intestinal cavity and then induces intestinal bacterial reproduction. As NEC develops, bacteria and toxins invade the immature intestinal tract, causing damage to the integrity of the intestinal wall, resulting in a widespread inflammatory response. CRP is an endogenous plasma protein secreted by liver cells. CRP levels rise sharply in response to infection and trauma, and whole-blood CRP levels are widely used to assess various types of inflammatory responses. Studies (9,10) have shown that PCT, CRP, and IL-6 levels in patients with stage III NEC are significantly higher than those in patients with stage I–II and thus may serve as a benchmark for choosing a surgical option for NEC.
Serum amyloid A (SAA) is a highly conserved amyloid precursor molecule synthesized by the liver. In the inflammatory response, SAA is enzymatically cleaved to form amyloid proteins and exhibits significant immune activity through the induction of neutrophils and mast cells. It is important to judge the inflammatory process, assess the activity of inflammatory reactions, and monitor the effects of medications. Intestinal fatty acid-binding protein (I-FABP) is a protein secreted by columnar epithelial cells in the intestinal tract and is typically found only in the mucous membranes of the gastrointestinal tract. An increase in gastrointestinal tract loss, when excreted from the body through the kidneys, can be used as an indicator for the early diagnosis of intestinal lesions. Furthermore, recent studies (11,12) have found that the concentrations of I-FABP and SAA are markedly increased in children with NEC, which may reflect the severity of NEC and intestinal injury. Based on these reports, the clinical value of the serum markers I-FABP, PCT, IL-6, CRP, and SAA in predicting the timing of NEC surgery was investigated in this study to provide a reference for clinically accurate NEC remediation. We present the following article in accordance with the STARD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-56/rc).
Methods
Study design
This was a retrospective study with repeated measurements conducted from March 2017 to March 2022. In this retrospective analysis, the operation group was compared with the nonoperation group, and serum sample data for serum CRP, IL-6, SAA, PCT, and I-FABP concentrations were estimated. The differences in overall data and serum markers between the two groups were examined. Independent factors related to surgical treatment in patients with pediatric NEC were analyzed using logistic regression. The utility of serum markers in selecting surgical options in pediatric patients with NEC was analyzed by drawing a receiver operating characteristic (ROC) curve.
Participants and eligibility criteria
The clinical data of 150 pediatric patients with NEC treated at our hospital from March 2017 to March 2022 were analyzed retrospectively. According to surgical treatment, patients were selected into operation (n=58) groups and nonoperation (n=92) groups. Patients were included if they met the clinical diagnostic criteria of NEC (13) and had a Bell stage ≥ II. Meanwhile, patients were excluded if they (I) had other infectious diseases, (II) had metabolic diseases or congenital intestinal malformations, or (III) did not have complete clinical data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and informed consent was taken from the newborns’ guardians. The study was approved by the ethics committee of Maternal and Child Health Hospital of Hubei Province (approval No. 2023IEC017).
Basic data collection
The basic data such as sex, gestational age, mode of delivery, time of starting feeding, birth weight, and day age of diagnosis were collected.
Detection of serum markers
Serum samples were collected before the operation in the operation group and on the day of diagnosis in the nonoperation group. Allow blood to clot for 20–30 minutes at 20–25 ℃. Centrifuge at 1,000 ×g for 10 minutes at 20–25 ℃. Use immediately or aliquot and store at –80 ℃. The concentrations of CRP (EPX01A-10288-901), PCT (EPX01A-12067-901), IL-6 (BMS213-2TEN), I-FABP (EHFABP2), and SAA (KHA0011) in sera were estimated by enzyme-linked immunosorbent assay (ELISA). The coated antibodies were coated in different positions of the 96-well enzyme-labeled plates at 2–8 ℃ overnight. The strong positive sample and the negative control solution (i.e., the sample dilution) were added to different concentrations of the coated antibodies and incubated. The detected antibodies were added separately to the corresponding binding sites of the enzyme plate and incubated for reaction. All steps were followed strictly according to the respective instructions of the manufacturers (ThermoFisher, Massachusetts, USA).
Observation indicators
Observation indicators included in this study included (I) basic data; and (II) the concentrations of serum markers CRP, PCT, IL-6, I-FABP, and SAA.
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used to analyze the data. Measurement data with normal distribution and homogeneous variance were expressed as mean ± standard deviation. The Z test was used to calculate measurement data with nonnormal distributions, which were expressed as medians, while count data were expressed as numbers and percentages and were tested with the χ2 test. Logistic regression was used to identify independent influence factors for NEC surgery. Area under curve (AUC) analysis was used to determine the clinical value of serum markers in predicting the operation opportunity of NEC. Describe a good predictive model with an AUC range of 0.5 to 1.0, a sensitivity of 50% to 100%, and a specificity of 50% to 100%. P<0.05 indicated statistical significance, P<0.05 is two-sided.
Results
Comparison of basic data between the 2 groups
The data of sex, gestational age, mode of delivery, time of starting feeding, birth weight, and day age of diagnosis were not significantly different between the operation group and the nonoperation group (P>0.05), as shown in Table 1 and Figures 1,2.
Table 1
| Grouping | N | Gender (male/female) (n) |
Gestational age (weeks) |
Natural production/cesarean section (n) | Start of feeding time (days) | Birth weight (g) |
Age at diagnosis (days) |
|---|---|---|---|---|---|---|---|
| Operation group | 58 | 25/33 | 32.5±3.5 | 13/45 | 3.5±1.2 | 1,580.5±440.2 | 15.2±2.8 |
| Nonoperation group | 92 | 50/42 | 32.1±3.6 | 20/72 | 3.3±1.0 | 1,602.1±435.4 | 14.8±2.5 |
| χ2/t value | 1.799* | 0.670 | 0.009* | 1.103 | 0.295 | 0.911 | |
| P value | 0.180 | 0.504 | 0.923 | 0.272 | 0.769 | 0.364 |
Data are presented as mean ± standard deviation if not otherwise specified. *, indicates χ2 value.
Comparison of serum markers between the 2 groups
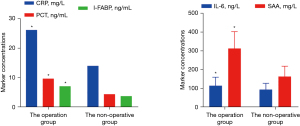
Serum CRP, PCT, IL-6, I-FABP, and SAA concentrations in the operation group were markedly higher than those in the nonoperation group (P<0.05), as shown in Table 2 and Figure 3.
Table 2
| Grouping | N | CRP (mg/L) | PCT (ng/mL) | IL-6 (ng/L) | I-FABP (ng/mL) | SAA (mg/L) |
|---|---|---|---|---|---|---|
| Operation group | 58 | 26.24* | 9.71* | 116.59±44.80 | 7.17* | 313.29±90.23 |
| Nonoperation group | 92 | 14.07* | 4.47* | 95.01±34.16 | 3.82* | 164.48±55.17 |
| Z/t value | 9.459 | 4.119 | 3.334 | 6.185 | 12.543 | |
| P value | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
Data are presented as mean ± standard deviation if not otherwise specified. *, indicates median. CRP, C-reactive protein; I-FABP, intestinal fatty acid-binding protein; IL-6, interleukin 6; PCT, procalcitonin; SAA, serum amyloid A.
Multifactor logistic regression analysis
Multivariate logistic regression analysis was performed with CRP, I-FABP, IL-6, PCT, and SAA as independent variables and with the surgical remedy as the dependent variable. The results confirmed that serum CRP, I-FABP, IL-6, PCT, and SAA were independently associated with the surgical remedy of NEC (P<0.05), as shown in Table 3.
Table 3
| Serum markers | β | SE | Wald χ2 | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| CRP (mg/L) | 0.127 | 0.054 | 5.531 | 1.135 | 1.021–1.262 | 0.019 |
| I-FABP (ng/mL) | 0.436 | 0.150 | 8.449 | 1.547 | 1.153–2.075 | 0.004 |
| IL-6 (ng/L) | 0.163 | 0.067 | 5.919 | 1.177 | 1.032–1.342 | 0.015 |
| PCT (ng/mL) | 0.085 | 0.030 | 8.028 | 1.089 | 1.027–1.155 | 0.005 |
| SAA (mg/L) | 1.015 | 0.428 | 5.524 | 2.759 | 1.193–6.385 | 0.018 |
CI, confidence interval; CRP, C-reactive protein; I-FABP, intestinal fatty acid-binding protein; IL-6, interleukin 6; OR, odds ratio; PCT, procalcitonin; SAA, serum amyloid A; SE, standard error.
Application value of serum markers in the timing of NEC operation
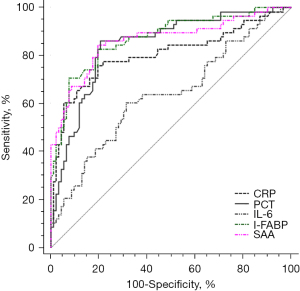
The ROC curve results yielded AUC values of serum CRP, PCT, IL-6, I-FABP, and SAA for predicting NEC operation opportunity of 0.805, 0.844, 0.635, 0.872, and 0.864, respectively; sensitivities of 75.90%, 86.20%, 60.30%, 82.80%, and 84.50%, respectively; and specificities of 80.40%, 79.30%, 68.35%, 80.40%, and 80.55%, respectively. As shown in Table 4 and Figure 4.
Table 4
| Serum markers | AUC value | Best truncation value | Sensitivity (%) | Specificity (%) | 95% CI | P value |
|---|---|---|---|---|---|---|
| CRP (mg/L) | 0.805 | 17.96 | 75.90 | 80.40 | 0.733–0.865 | <0.001 |
| PCT (ng/mL) | 0.844 | 4.82 | 86.20 | 79.30 | 0.776–0.898 | <0.001 |
| IL-6 (ng/L) | 0.635 | 108.00 | 60.30 | 68.35 | 0.553–0.927 | <0.001 |
| I-FABP (ng/mL) | 0.872 | 5.28 | 82.80 | 80.40 | 0.808–0.921 | <0.001 |
| SAA (mg/L) | 0.864 | 204.89 | 84.50 | 80.55 | 0.798–0.914 | <0.001 |
AUC, area under the curve; CI, confidence interval; CRP, C-reactive protein; I-FABP, intestinal fatty acid-binding protein; IL-6, interleukin 6; NEC, necrotizing enterocolitis; PCT, procalcitonin; SAA, serum amyloid A.
Discussion
The main clinical manifestations of NEC are intestinal perforation, abdominal distension, apnea, peritonitis, vomiting, and hematochezia. In severe cases, multiple organ failure or shock may occur (14,15). Clinical observation has confirmed that the inflammatory reaction caused by NEC can cause the body to produce high immune reactivity, thus damaging immune function. Moreover, the inflammation will also affect the nervous system. Even if pediatric patients are cured, the risk of intestinal stenosis, nerve retardation, and other sequelae will increase (16). NEC is characterized by rapid progression, multiple complications, and poor prognosis. Therefore, early diagnosis and early cure are the keys to NEC treatment. Generally, conservative medical intervention is recommended for pediatric patients with stage I–II NEC, while surgical treatment is recommended for pediatric patients with stage III NEC. Due to the rapid progress of NEC, 30–50% of pediatric patients need surgical intervention (17). NEC intestinal perforation is the absolute indication of surgical treatment, but the surgical indication and timing of children without intestinal perforation are still controversial (18). Thus, a means for doctors to accurately identify the indications of NEC surgery in a timely fashion and choose the best operation opportunity is urgently needed to improve the prognosis of pediatric patients to the greatest extent.
The characteristics of sex, gestational age, mode of delivery, time to start feeding, birth weight, and day of diagnosis were similar between the operation and nonoperation groups. One study (19) found that the incidence of NEC is related to the gestational age of newborns. The earlier the birth, the higher the incidence, which is not related to whether surgical intervention occurred. The pathogenesis of NEC has not been fully clarified yet, but it has been reported to be related to immune disorders, acute inflammatory cascade reactions, and mucosal barrier function damage (20). This study found that the concentrations of CRP, I-FABP, IL-6, PCT, and SAA in the operation group were notably higher, indicating that the inflammatory reaction and intestinal mucosa damage in NEC patients were more serious. CRP is an acute phase reactive protein, which is synthesized in large quantities when inflammation, injury, or infection occurs in the body, and the serum CRP content increases rapidly within 6–8 hours and reaches its peak at 24–48 hours. The higher its content is, the more serious the inflammatory reaction (21). PCT is a precursor of calcitonin and a specific marker of bacterial infection, which can reach its peak within 6–8 hours of systemic bacterial infection (22). Some scholars have reported that the sensitivity of PCT in the diagnosis of NEC is higher than that of CRP and that both can be used for the early diagnosis and curative effect evaluation of NEC (23). IL-6 is a typical inflammatory mediator and an important factor involved in inflammatory “waterfall” reactions, which can reflect the severity of NEC (24). Although CRP, PCT, and IL-6 have a certain reference value in evaluating the changes of NEC, they are all nonspecific inflammatory markers with limited sensitivity. In recent years, SAA was discovered as an index of the acute inflammatory response, and its sensitivity and specificity are higher than those of CRP. It can be used to assist in the diagnosis of various neonatal acute diseases (25). I-FABP is a protein distributed in the intestinal mucosa with high tissue specificity. When the intestinal mucosa is damaged, the cell permeability increases, I-FABP can enter the blood circulation, and its serum content can reflect the damage to the small intestine (26). A recent study (27) found that I-FABP and SAA can be used as effective indicators to judge the severity of NEC II–III.
In our research, through further logistic regression analysis, it was demonstrated that serum CRP, PCT, IL-6, I-FABP, and SAA were independently correlated with NEC surgery, suggesting that CRP, PCT, IL-6, I-FABP, and SAA can be used as potential serum indicators to predict the opportunity of NEC surgery. It is well known that the occurrence and development of NEC are closely related to the excessive inflammatory response. Inflammation-related factors can cause intestinal mucosal injury and destroy intestinal mucosa integrity. At this time, the intestinal mucosa cannot completely repair itself, which leads to the progression of NEC. In addition, inflammation leads to decreased intestinal mucosal blood perfusion, resulting in intestinal ischemia, which further aggravates the condition of NEC (28,29). Therefore, serum CRP, PCT, IL-6, I-FABP, and SAA can reflect intestinal injury in pediatric patients with NEC, which may have a certain guiding value in predicting the opportunity of NEC operation. By drawing a ROC curve, we found that the AUC values of serum CRP, PCT, IL-6, I-FABP, and SAA for predicting the operation opportunity of NEC were 0.805, 0.844, 0.635, 0.872, and 0.864 respectively, indicating that the predictive value of IL-6 was low, while the others had moderate predictive value. Among these serum markers, I-FABP (≥5.28 ng/mL) and SAA (≥204.89 mg/L) had relatively high sensitivity and specificity in predicting the timing of NEC operation, which were 82.80%, 80.40% and 84.50%, 80.55%, respectively.
In addition to the relevant serological indicators in this study, it was pointed out that the serum corticosteroid-binding globulin (CBG), SAA and HSP-70 levels in the observation group were significantly higher than those in the control group and showed high expression, suggesting that the intensification of the inflammatory response in NEC can cause the secretion of CBG, SAA and HSP-70, which are involved in the occurrence and development of NEC and can be used as indicators for the diagnosis of early NEC (30).
The analysis of a study showed that various serum markers have some significance in guiding the diagnosis and treatment, disease progression and prognosis of NEC (31). I-FABP, SAA, and intestinal trefoil factor (ITF) have good sensitivity and specificity in predicting the diagnosis and severity of NEC. The sensitivity and specificity of CRP are good (32); increased CRP is a high-risk factor in children with NEC requiring surgical treatment (33). PCT is a diagnostic marker for detecting bacterial infections (34); a severe decrease in serum protein and platelet (PLT) indicates a deterioration of NEC and even the possibility of perforation (35). The timeliness and validity of these serological indices have irreplaceable advantages and facilitate repeated monitoring, so they have good application prospects. However, based on the small sample size of this study and the fact that it is a retrospective analysis, some bias in the results may be caused, so it is necessary to further confirm the findings of this study through multicenter clinical trials while expanding the sample size in the future.
Conclusions
Serum markers CRP, PCT, I-FABP, and SAA have certain predictive values in judging the operation opportunity of NEC; however, the AUC value of IL-6 was not sufficiently high for predicting the operation opportunity and thus has limited application value. In addition, multicenter and large-sample experiments need to be conducted to confirm our findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-56/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-56/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-56/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and informed consent was taken from the newborns’ guardians. The study was approved by the ethics committee of Maternal and Child Health Hospital of Hubei Province (approval No. 2023IEC017).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu N, Pan WK, Zhang L, et al. Diagnostic value of bedside high-frequency ultrasound and removable digital X-ray in neonatal necrotizing small bowel colitis. Journal of Chinese Practical Diagnosis and Therapy 2018;32:901-3.
- Jiang S, Yan W, Li S, et al. Mortality and Morbidity in Infants <34 Weeks' Gestation in 25 NICUs in China: A Prospective Cohort Study. Front Pediatr 2020;8:33. [Crossref] [PubMed]
- Kelleher ST, McMahon CJ, James A. Necrotizing Enterocolitis in Children with Congenital Heart Disease: A Literature Review. Pediatr Cardiol 2021;42:1688-99. [Crossref] [PubMed]
- El Manouni El Hassani S, Niemarkt HJ, Derikx JPM, et al. Predictive factors for surgical treatment in preterm neonates with necrotizing enterocolitis: a multicenter case-control study. Eur J Pediatr 2021;180:617-25. [Crossref] [PubMed]
- Moschino L, Duci M, Fascetti Leon F, et al. Optimizing Nutritional Strategies to Prevent Necrotizing Enterocolitis and Growth Failure after Bowel Resection. Nutrients 2021;13:340. [Crossref] [PubMed]
- GaŁĄzka P. Chrzanowska M, StyczyŃski J. Clinical Spectrum and Outcomes of Neonatal Necrotizing Enterocolitis. In Vivo 2021;35:585-91. [Crossref] [PubMed]
- Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev 2015;CD003481. [Crossref] [PubMed]
- Evidence-Based Medicine Group. Clinical guidelines for the diagnosis and treatment of neonatal necrotizing enterocolitis (2020). Zhongguo Dang Dai Er Ke Za Zhi 2021;23:1-11. [PubMed]
- Wen JY, Li MX, Lin DY, et al. Dynamic Changes and Significance of Serum CRP Levels in Neonatal Necrotizing Enterocolitis. Medical Innovation of China 2020;141-4.
- Perrone S, Cremonini I, Marinelli F, et al. New Strategies for Necrotizing Enterocolitis Diagnosis and Prevention in Newborns. Curr Pediatr Rev 2021;17:191-200. [Crossref] [PubMed]
- Yang J, Wu Y, Xu Y, et al. Dexmedetomidine Resists Intestinal Ischemia-Reperfusion Injury by Inhibiting TLR4/MyD88/NF-κB Signaling. J Surg Res 2021;260:350-8. [Crossref] [PubMed]
- Huo R, Liu H, Chen J, et al. Serum HMGB1 level is correlated with serum I-FABP level in neonatal patients with necrotizing enterocolitis. BMC Pediatr 2021;21:355. [Crossref] [PubMed]
- Çağlar Ö, Cesur E, Sade R, et al. Dual energy CT in necrotizing enterocolitis; a novel diagnostic approach. Turk J Med Sci 2021;51:2575-83. [Crossref] [PubMed]
- Xiong T, Maheshwari A, Neu J, et al. An Overview of Systematic Reviews of Randomized-Controlled Trials for Preventing Necrotizing Enterocolitis in Preterm Infants. Neonatology 2020;117:46-56. [Crossref] [PubMed]
- Celis Castañeda LA, Morales Camacho WJ, Durán Ochoa NM. Sepsis due to Lactobacillus reuteri in an extreme preterm newborn: case report. Arch Argent Pediatr 2019;117:e509-13. [PubMed]
- Hwang M, Tierradentro-García LO, Dennis RA, et al. The role of ultrasound in necrotizing enterocolitis. Pediatr Radiol 2022;52:702-15. [Crossref] [PubMed]
- Tarnow-Mordi WO, Abdel-Latif ME, Martin A, et al. The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): a multicentre, double-blind, randomised controlled trial. Lancet Child Adolesc Health 2020;4:444-54. [Crossref] [PubMed]
- Koivusalo A, Karila K, Pakarinen M. Late Abdominal Reoperations after Surgery for Necrotizing Enterocolitis and Spontaneous Intestinal Perforation. Eur J Pediatr Surg 2021;31:535-40. [Crossref] [PubMed]
- Garg PM, Paschal JL, Ware J, et al. Gestational age-specific hematological patterns in preterm infants following necrotizing enterocolitis. J Matern Fetal Neonatal Med 2022;35:10093-102. [Crossref] [PubMed]
- Palleri E, Wackernagel D, Wester T, et al. Low Splanchnic Oxygenation and Risk for Necrotizing Enterocolitis in Extremely Preterm Newborns. J Pediatr Gastroenterol Nutr 2020;71:401-6. [Crossref] [PubMed]
- Barseghyan K, Gayer C, Azhibekov T. Differences in Serum Alkaline Phosphatase Levels in Infants with Spontaneous Intestinal Perforation versus Necrotizing Enterocolitis with Perforation. Neonatology 2020;117:349-57. [Crossref] [PubMed]
- Li Y, Min L, Zhang X. Usefulness of procalcitonin (PCT), C-reactive protein (CRP), and white blood cell (WBC) levels in the differential diagnosis of acute bacterial, viral, and mycoplasmal respiratory tract infections in children. BMC Pulm Med 2021;21:386. [Crossref] [PubMed]
- Wang L, Ni SW, Zhu KR, et al. Changes in C-reactive protein and procalcitonin levels in neonates with necrotizing enterocolitis and their clinical significance. Chinese Journal of Contemporary Pediatrics 2018;20:825-30. [PubMed]
- Liu Y, Wang Z, Huang H, et al. miR-200a-3p improves neonatal necrotizing enterocolitis by regulating RIPK1. Am J Transl Res 2021;13:12662-72. [PubMed]
- Zhu H, Lin Y, Liu Y. miR-34a increases inflammation and oxidative stress levels in patients with necrotizing enterocolitis by downregulating SIRT1 expression. Mol Med Rep 2021;24:664. [Crossref] [PubMed]
- Su W, Li Y, Wang Y, et al. Correlation between 1-FABP, Blood Routine and Grading of Necrotising Enterocolitis. J Coll Physicians Surg Pak 2021;31:238-9. [Crossref] [PubMed]
- Coufal S, Kokesova A, Tlaskalova-Hogenova H, et al. Urinary I-FABP, L-FABP, TFF-3, and SAA Can Diagnose and Predict the Disease Course in Necrotizing Enterocolitis at the Early Stage of Disease. J Immunol Res 2020;2020:3074313. [Crossref] [PubMed]
- El-Abd Ahmed A, Hassan MH, Abo-Halawa N, et al. Lactate and intestinal fatty acid binding protein as essential biomarkers in neonates with necrotizing enterocolitis: ultrasonographic and surgical considerations. Pediatr Neonatol 2020;61:481-9. [Crossref] [PubMed]
- Shores DR, Fundora J, Go M, et al. Normative values for circulating intestinal fatty acid binding protein and calprotectin across gestational ages. BMC Pediatr 2020;20:250. [Crossref] [PubMed]
- Yan LJ, Ruan XY, Wu BF. Analysis of serum markers for early diagnosis of necrotizing small intestinal colitis in neonates. Maternal and Child Health Care of China 2020;23:4488-91.
- Markel TA, Engelstad H, Poindexter BB. Predicting disease severity of necrotizing enterocolitis: how to identify infants for future novel therapies. J Clin Neonatol 2014;3:1-9. [Crossref] [PubMed]
- Gollin G, Stadie D, Mayhew J, et al. Early detection of impending necrotizing enterocolitis with urinary intestinal fatty acid-binding protein. Neonatology 2014;106:195-200. [Crossref] [PubMed]
- Duci M, Fascetti-Leon F, Erculiani M, et al. Neonatal independent predictors of severe NEC. Pediatr Surg Int 2018;34:663-9. [Crossref] [PubMed]
- Turner D, Hammerman C, Rudensky B, et al. Low levels of procalcitonin during episodes of necrotizing enterocolitis. Dig Dis Sci 2007;52:2972-6. [Crossref] [PubMed]
- Chen XX, Chen Q, Cao ZJ, et al. Effects of different surgical procedures on serological markers of neonatal necrotizing small bowel colitis caused by Ileal perforation by different surgical procedures on serological markers. Chinese Journal of Practical Medicine 2018;8-11.
(English Language Editor: J. Gray)




