
Biological and structural phenotypes associated with neurodevelopmental outcomes in congenital heart disease
Introduction
Congenital heart disease (CHD) is the most common birth defect, affecting 0.6–1.3% of babies born each year (1). It is associated with significant mortality and morbidity (2) with many individuals with CHD requiring lifelong medical care and treatment (3). The cause of most CHD is unknown and attributed to a combination of genetic, epigenetic and environmental factors (4). Following advances in treatment and care, >90% of affected children now survive to adulthood (5), resulting in greater appreciation and interest in longer-term health outcomes, such as neurodevelopment and quality of life (6).
Overview of neurodevelopmental disability in CHD and relevance at all ages
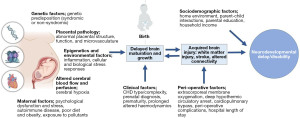
Neurodevelopmental delay and/or disability (NDD) is now recognised as a lifespan issue for many children born with CHD (7). Those with more complex forms of CHD, such as those with single ventricles pathologies or transposition of the great arteries who typically experience more severe disease requiring neonatal bypass operations and on-going monitoring and care, are at a greater risk of worse prognosis (8,9). However, risk factors contributing to more severe or persisting disability are not fully understood and appear multifactorial and synergistic (10). A spectrum of outcomes is observed, and many individuals with CHD demonstrate no impairment and their level of functioning may exceed population norms. However, as many as 50% demonstrate mild to moderate NDD and later cognitive impairment, and a small number demonstrate global cognitive or intellectual disability (7,8,11-15). These challenges have important implications throughout the life course, including on academic achievement, employment opportunities, psychological and social functioning, and overall quality of life (16-19). Established guidelines document the need for routine neurodevelopmental follow-up throughout childhood (7), however, this has not yet translated to standard clinical care in most paediatric centres. Importantly, neuropsychological services for children and adults with CHD are under-resourced in comparison to other clinical paediatric populations, such as those born very preterm, despite similar risk factors and adverse outcomes (11). Understanding biological and structural phenotypes associated with NDD in CHD are necessary to advance clinical care and the development of effective intervention strategies for those at risk. Interventions targeting the earliest stages of development will be vital for optimising developmental outcomes. An overview of the risk factors contributing to NDD in CHD is outlined in Figure 1.
Early manifestations of NDD typically include poor feeding, speech and language delay, challenges with gross and fine motor movement, and early cognitive concerns (20-22). Early speech and language delays more commonly resolve over time (23), whereas motor impairments are a persisting concern throughout childhood (24,25), and the extent of cognitive challenges typically worsen over time (14,21). In infants with more complex CHD, such as those undergoing the single ventricle pathway, the early developmental years are impacted by the need for multiple cardiac surgeries and prolonged exposure to the restrictive and artificial hospital environment. Length of inpatient stay is usually a surrogate marker of more complex diagnoses and post-operative complications and is a strong predictor of long-term neurodevelopmental outcomes (7).
At school age, intelligence quotients are generally within population norms, although typically fall within the low average range (26,27). In contrast, early NDD typically manifests as specific cognitive challenges that have important implications for academic performance and achievement, often requiring additional school provisions and support (19,28). Key challenges include deficits in attention, processing speed, visuospatial skills, memory, social cognition, and executive functioning—that encompasses a range of higher order cognitive skills including working memory, mental flexibility, problem-solving, and inhibitory control (27,29-32). Many children show challenges across multiple domains of functioning, requiring greater intervention and support (21). Behavioural and psychological disorders (e.g., anxiety, depression, and poor behavioural or emotional regulation) are also an important concern (33,34). The extent to which these difficulties are associated with NDD has not yet been established but are anticipated to be linked. In addition, children with CHD are at an increased risk of developing disorders of social functioning, including autism spectrum disorder (35).
Higher-order executive skills continue to develop throughout childhood in conjunction with the maturation of key brain structures, notably the pre-frontal cortex (36), and the full extent of these challenges may not be observed until adolescence or early adulthood. Indeed, studies have shown that executive functioning deficits are among the most prevalent and severe impairments observed in older children and young adults with CHD (8,28,37), which become an increasingly significant challenge alongside greater cognitive demands and the need for increased functional independence that occurs with maturation into adulthood. Cognitive dysfunction persists throughout adulthood (8,38-40), although longer-term outcomes have been less extensively studied. Older adults with a Fontan circulation (up to 50 years old) have been found to have worse neurocognitive dysfunction compared to Fontan adolescents after controlling for age (8), that may suggest a possible worsening of cognitive challenges throughout adulthood in those with highly complex forms of CHD. Although the extent to which these outcomes may reflect an era effect relating to previous surgical strategies and medical care are currently undetermined. Concerningly, adults with all forms of CHD are at a significantly increased risk of dementia, including early onset dementia (41). As the adult CHD population continues to grow, so does the need for a better understanding of the accumulating risk factors that compound early NDD and contribute to worse long-term cognitive outcomes.
Brain structure and function: insights from neuroimaging
In individuals with CHD, NDD is paralleled by abnormalities in brain structure and function across the lifespan (42-44). Neuroimaging studies of the fetal CHD brain have demonstrated that abnormal brain development begins in utero. Altered cortical development and reduced brain size are observed as early as the second trimester of gestation in fetuses with complex cardiac lesions (45-47) and precede anomalous growth patterns and reduced brain volumes, that become progressively more pronounced throughout the third trimester (45,48-51). Fetal brain volume has been shown to predict neurodevelopmental outcomes at 2 years of age in children with CHD (52), suggesting prenatal brain parameters may have prognostic value in identifying infants at risk of early NDD; however, further studies are needed to replicate these findings, and associations with longer-term neurodevelopmental outcomes are yet to be determined.
Post-natal brain development continues to follow an altered growth trajectory, with progressive growth of both regional and global brain volumes occurring at a slower rate compared to typically developing infants (53,54). Consistently, widespread reductions in global and regional brain volumes have been demonstrated pre-operatively in various heterogenous CHD cohorts (54-59). In infants with transposition of the great arteries, the rate of brain growth has been found to significantly increase after surgical correction and normalisation of the cardiac circulation and there may be some ‘catch-up’ growth by 3 years of age (60). In contrast, reduced brain growth and smaller brain volumes persist in infants with hypoplastic left heart syndrome post-operatively (60,61), suggesting that continued haemodynamic instability may have an important contributory role in pervasive post-natal brain development.
In infants requiring cardiac surgery, peri-natal brain immaturity is associated with a greater risk of pre- and post-operative brain injury (62-65), that occurs in as many as 40% and 26–44%, respectively (53,65-68). Predominant lesions include patterns of white matter injury and stroke; the prevalence and severity of which are typically worse in infants with univentricular lesions (65,68). Recently, Peyvandi and colleagues found that moderate to severe peri-operative injury is associated with subsequent reduced brain growth (68), demonstrating a complex interplay between brain development and acquired injury and a possible “two-hit phenomena” (69).
Associations between peri-operative brain injury and early neurodevelopmental outcomes are variable (65,66,68,70,71). The current understanding is that altered brain maturation may be more closely linked to NDD (52,55,72-74). Consistently, significant associations between smaller brain volumes and worse cognitive outcomes are observed throughout childhood and adulthood in various CHD cohorts (8,56,75-79). Alterations in white matter microstructure, that may reflect aberrant white matter maturation, have also shown significant associations with cognitive dysfunction in older children and adults with CHD (80-84), with some variation reported in recent findings (85). In contrast, associations with structural brain injury continue to be broadly inconsistent, despite adolescents and adults demonstrating an alarming rate of injury in some studies (8,42).
Current research has advanced into the field of “connectomics” in an effort to better understand the impact of CHD on structural brain connectivity, which may provide clearer insight into direct structure-function relationships. Emerging studies have demonstrated reduced maturation in the whole brain connectome (i.e., neural connections or networks) and specific brain networks in infants with CHD that is associated with peri-operative white matter injury burden (86-88); however, studies investigating associations with NDD are limited. Ramirez and colleagues have demonstrated that global and regional structural connectivity in infants with CHD predicts early motor development and language outcomes (89). Similarly, Panigraphy and colleagues have found that network topology mediates the differences observed in cognitive functioning in adolescents with CHD compared to healthy controls (90).
While major advances have been made in our understanding of brain development in CHD, much remains unknown and longitudinal brain magnetic resonance imaging (MRI) studies evaluating the developmental trajectory and timing of injury in the CHD brain from gestation onwards are needed to fully elucidate the impact of perinatal brain abnormalities on NDD and longer-term cognitive dysfunction.
Weighing causes and contributors including placental insufficiency
Distinguishing the causes and consequences of acute brain injury, such as stroke, from altered neurodevelopment, which appears to be pre-programmed, will have important implications for the development of targeted NDD interventions. This is made difficult given the considerable overlap between these two entities, particularly in neonates undergoing cardiac surgery, and notably in those on the single ventricle pathway (68). The need for cardio-pulmonary resuscitation (CPR) or extracorporeal membrane oxygenation (ECMO) via the heart-lung machine, more common in the neonatal cardiac surgery population, may be associated with acute brain injury.
Early assumptions that NDD was due to perinatal neurological compromise or perioperative brain injury are not correct (10,91). The use of deep hypothermic circulatory arrest, a common operative strategy in complex heart operations during which the body is cooled to temperatures ranging from 20–25 ℃ thereby ceasing blood circulation and brain function, was also for a long time, implicated as a cause or contributor to NDD. However, it is likely that deep hypothermic circulatory arrest is just one of many contributors with small effect sizes, including the generation of cerebral microemboli or blood clots by the heart-lung machine and the consequences of the systemic inflammatory response that occurs during surgery. These may contribute to acute brain injury, and may worsen neurodevelopmental trajectory but are not thought of as the primary causes of NDD and best estimates indicate that peri-operative factors explain only 5–8% of the variability in neurodevelopmental outcomes (91-93). Importantly, NDD may occur despite an optimal clinical course of cardiac care, where no complications are observed.
Instead, current thinking implicates impaired fetal brain development (94), placental dysfunction (95,96) and genetic factors (4,97), both fetal and maternal, as predominant causes of NDD. Two important ‘environmental’ stressors affect human fetuses in the context of CHD. The first is a form of placental insufficiency; the placenta may be smaller and structurally abnormal leading to relative substrate deficiency, slower than normal fetal growth, smaller brain volumes, preterm birth, and lower birthweight (96,98) The second is the relative hypoxia encountered by the fetal brain in circumstances where the normal highly oxygenated blood cannot reach the brain because of aortic atresia (99,100), a fundamental component in many single ventricle patients. In this context, blood with a lower-than-normal oxygenation reaches the brain retrograde via the ductus arteriosus and possibly at a lower pressure. These factors may explain lactate accumulation within the brain of CHD fetuses (101), and the relative brain immaturity of neonates with CHD (102). Other environmental factors including maternal obesity, stress, autoimmune disease are also associated with NDD in offspring (103). As well as being implicated in the cause of the CHD (4,97), genetic variants are likely implicated in the adaptive responses to these environmental stresses.
These environmental factors may contribute to the abnormal brain development observed throughout gestation and we lack an understanding of which factors are important at which specific time-points. We also do not know the extent to which amelioration of these factors—including maternal oxygen therapy (104)—might improve developmental trajectories in the child, or whether any of these pathways might be amenable to post-natal intervention.
Genetic contributions and unknowns
NDD in syndromic CHD
That genetics may underpin both heart and brain development is evidenced in the many established genetic syndromes incorporating both heart defects and neurodevelopmental issues as part of the disease spectrum. While the exact cause and/or genetic contribution of the neurodevelopmental phenotype in most established syndromes remains unknown, several candidate genes have been identified in some established syndromes. In syndromes associated with chromosomal alterations and copy number variations, where multiple genes may be implicated, significant headway has been made to understand which genes contribute towards specific phenotypic components, including neurodevelopment. For example, in 22q11.2 deletion syndrome, which encompasses 32 genes all of which are candidates for CHD and NDD, genes contributing towards both heart and brain development [TBX1 (100) and HIRA (105) among others] or solely towards the neurodevelopmental/neurological phenotype (COMT and PRODH (106)), have been identified. Similarly, in Williams syndrome, which typically involves 25–27 genes, the ELN gene is thought to primarily contribute towards the characteristic cardiovascular phenotype, supravalvular aortic stenosis, and the GTF2I and GTF2IRD1 genes towards the neurodevelopmental phenotype (107).
In syndromes caused by variations in single genes, the mechanism by which these genes likely affect two or more organ systems such as the heart and brain among others, is due to their ability to regulate or interact with multiple genes and/or gene pathways. Indeed, chromatin modifying genes KMT2D, CHD7 and CDK13 which cause Kabuki and CHARGE syndrome (108) and CDK13-related disorder (109) respectively, modulate gene expression by altering access to the DNA and thereby disrupting multiple developmental processes, including in the heart and brain. Similarly, transcriptional regulators, including FOXP1 (110), ZEB2 (111) among others, have been implicated in both CHD and NDD.
While NDD is the most common co-morbidity associated with CHD, other extra-cardiac anomalies, such as those affecting the urogenital system (112), are often seen in conjunction with CHD and/or NDD, suggesting that genetic risk may extend beyond heart and brain development to other organ systems.
NDD in non-syndromic CHD
With the exact cause and/or genetic contribution of the neurodevelopmental phenotype in many established syndromes unknown, it is not surprising that understanding the genetic contribution of NDD in non-syndromic CHD poses a significant challenge, not helped by the fact that these represent most presenting cases. Over the last decade; however, advances in genomic technologies combined with large cohorts and sophisticated statistical analyses, have provided important clues to the genetic architecture underlying this patient group. Specifically, these studies identified a 2-fold enrichment in damaging de novo variants in genes highly expressed in the heart in patients with CHD and NDD and a 4.7-fold enrichment in patients with CHD, NDD and other congenital anomalies, suggestive of an additive effect in heart-expressing genes with increasing non-cardiac phenotypic complexity (97). Further, 69 genes harbouring damaging de novo variants, were shared between CHD patients and cohorts comprising patients with isolated NDD, providing additional support for shared genetic aetiologies to heart and brain development. Not surprisingly many of these genes are chromatin modifying genes and transcriptional regulators, likely exerting wide-reaching phenotypic effects. Importantly, CHD patients with damaging de novo variants in these 69 genes, were at significantly increased risk of NDD, providing a basis for future downstream applications of precision medicine approaches.
More recently, a significant overlap of genetic burden has been identified in patients with CHD and patients with autism (113). Enrichments in de novo variants in genes involved in the connectome, have also recently been implicated in patients with CHD (114).
While these studies have provided important insight into disease mechanisms of NDD in CHD, specifically in terms of shared genetic aetiologies, the findings are not readily applicable to the individual patient. Studies assessing the clinical relevance of this information have identified important genetic burden associated with ‘neurotransmitters’, ‘axon guidance’ and ‘RASopathy’ pathways in patients with CHD and NDD; however, no clinically actionable cause for the NDD could be identified (115).
The contribution of common variants in both heart and brain development is becoming increasingly relevant (116,117) and will likely inform the development of polygenic risk scores used to identify those CHD patients most at risk of NDD.
So, while significant headway has been made to unravel the genetic component of NDD in CHD, specifically the contribution of rare and damaging de novo genetic variant burden, which is often in chromatin modifying and connectome genes; additional factors such as common genetic variants, morphology, epigenetics and the environment, are increasingly highlighted as important contributors in the final phenotypic presentation.
Inherited and epigenetic associations in other forms of NDD
Increasing evidence suggests that inflammation, cellular stress, and epigenetic factors at the gene-environment interface play a key role in the pathogenesis of NDDs (118-120). Neurodevelopment begins in-utero and continues to 21 years of age, through a constant process of synaptic pruning and re-wiring (121). Gestation is a particularly sensitive time, as key brain networks are being established in the developing fetus (122). The maternal immune activation hypothesis posits that fetal exposure to inflammation and dysregulated immune milieu adversely affects neurodevelopment (122,123). Many environmental factors, such as poor diet, low exercise, sleep, infection, stress, and pollutants can cause maternal immune activation and have all been associated with increased risk of NDDs in offspring (103,124). Environmental risk factors experienced by the mother during pregnancy are hypothesized to be ‘biologically embedded’ in the cellular and epigenetic architecture of the offspring (125). These inherited vulnerabilities can then be modulated by post-natal risk factors, such as neonatal infections, childhood trauma, or other early life stress (126).
Environmental insults can influence the transcription of susceptibility genes via key inflammatory and cellular stress signalling pathways, including the Toll-like receptor pathway (103), and the integrated stress response (127). These signalling cascades may then modulate the expression of NDDs through finely tuned epigenetic, gene regulation, and post-transcriptional processes (128-130). Epigenetic modifications are chemical or physical changes to chromatin which can either increase or repress gene transcription (130). The four main epigenetic modifying factors are DNA methylation, histone modifications, chromatin modelling, and microRNA. Controlled, yet dynamic, epigenetic patterns are crucial for brain development and are highly sensitive to environmental changes (130).
Preclinical evidence indicates that maternal inflammation during pregnancy can induce long-lasting changes in DNA methylation, histone modifications, and microRNA expression in the offspring brain (131-133). Importantly, animal models have shown that prenatal environmental insults can lead to epigenetic alterations and associated behavioural abnormalities in second—and third-generation offspring, suggesting transferability of epigenetic programming (134,135). Human studies have linked inflammatory risk factors affecting pregnancy, including depression, anxiety, and smoking, to epigenetic changes in the placenta, offspring umbilical cord blood, peripheral blood, and buccal cells (128,136). Furthermore, maternal obesity, gestational diabetes, depression, and asthma, have been associated with epigenetic changes in immune, metabolic, and oxidative stress pathways in offspring umbilical cord blood (137-140). These findings highlight the transgenerational effects and widespread, long-term consequences of disturbances in prenatal programming.
At the post-transcriptional stage, maternal immune activation has been shown to disrupt mRNA translation, ribosome biogenesis, and protein synthesis in rodent brains (127,141,142). These processes are tightly regulated and fundamental to brain development and neural function. Activation of the integrated stress response has been implicated as one mechanism through which these disturbances occur (127); however, this field is very much in its infancy. Therefore, by increasing attention to environmental effects and their epigenetic or cellular consequences, there may be significant opportunities for prevention (e.g., public health measures in pregnancy) or individualised treatments for people with neurodevelopmental disorders.
Parental psychological stress, anxiety, and depression
Many complex CHD diagnoses, such as those with single ventricles or transposition of the great arteries, now occur prenatally and coincide with the critical in utero period of neurodevelopment. Severe maternal stress and anxiety surrounding fetal CHD diagnosis is well-established (143-146). Until recently, however, we have had little understanding of the consequences of these maternal symptoms for brain development in CHD.
Threats to the health of the fetus have long been recognized as an important risk factor for maternal psychological disturbance in the perinatal period (147). Parents’ experiences of CHD diagnosis are often associated with enduring psychological distress (2,148,149). Up to 80% of parents report severe psychological distress at some point in their child’s medical trajectory and as many as 50% report anxiety or depressive symptoms indicative of a need for clinical intervention (2,143). These rates far exceed norms for perinatal anxiety and depression in the general population (149); yet the severity and consequences of these symptoms are often under-recognized and under-treated by healthcare professionals. Without timely intervention, parents with higher distress also report poorer physical health (150), greater parenting burden (151), higher health service use (152), more suicidal ideation (153), and poorer quality of life for both themselves and their child with CHD (154,155) compared with parents of sick children with lower distress.
Decades of research demonstrate an association between parent mental health and child neurodevelopment (156-158). Studies of children with CHD and their caregivers suggest that parent psychological distress is one of the strongest predictors of child emotional, behavioural, and developmental outcomes (154,159-161). For example, parent post-traumatic stress, referring to specific psychological and physiological symptoms (e.g., flashbacks, avoidance, hyperarousal) following exposure to a traumatic event (e.g., witnessing their child go into cardiac arrest), is associated with lower psychosocial functioning (160) and quality of life (155) for children and adolescents with CHD. While the mechanisms underlying these associations are not fully understood, family environment and parent-child interactions likely play an important role (148,162). There is also evidence that high and persistent maternal psychological stress and anxiety during pregnancy can alter fetal brain development in both healthy fetuses and fetuses with CHD (147).
Animal studies have shown that stress-elicited perturbations in maternal prenatal biology (e.g., hypothalamic-pituitary-adrenal axis function) affect offspring development, including stress reactivity (163). Studies of psychological stress in pregnant women largely mirror these findings, showing links to long-term cognitive, behavioral, and emotional dysfunction in children across development (164-168). High maternal stress during the middle-second and third trimesters - a time when the fetal brain is highly susceptible to modification by environmental factors and when CHD diagnosis is also most common—may be associated with the greatest neurodevelopmental impact (169). These effects persist after controlling for family socioeconomic status and maternal postnatal mental health, indicating a window of opportunity during fetal development when we may be able to intervene to prevent or minimize adverse child neurodevelopmental outcomes (170).
Maternal prenatal stress is linked to altered fetal brain development
Maternal prenatal stress, anxiety or depression has been linked with cortical thinning (171), altered brain microstructure (172,173), altered amygdala (174) and hippocampal (175) growth, and alterations in functional neural networks (i.e., the connectome) (176-178) in offspring with (179) and without CHD. Altered placental function (180,181), including decreased perfusion (182) and placental expression of neurotropic precursors, such as 11-beta-hydroxysteroid dehydrogenase type 2 (11β-HSD2), are potential mechanisms (183). Decreased 11β-HSD2 expression may increase fetal cortisol exposure (184,185), affecting gene expression in fetal brain cells (186), such that small changes to early developmental trajectories may have life-altering consequences for neurodevelopment (55) and mental health outcomes (174,181,187-189). These findings underscore the importance of targeted therapies aimed at reducing prenatal stress to optimize both maternal and fetal wellbeing.
If maternal distress elicited by prenatal cardiac diagnosis negatively impacts fetal brain development, we are obliged to understand these ‘off-target’ effects and initiate prenatal neuroprotective therapies to improve long-term outcomes. Expectant mothers are especially motivated for self-care (190), and studies in other populations show prenatal interventions, including interpersonal psychotherapy (191-193), effectively prevent or reduce maternal anxiety and depression (191,192,194-197). In CHD, one postnatally-delivered psychological intervention targeting mothers demonstrated efficacy in improving infant mental development at age 6 months (198), but this benefit was not sustained (159). Neurocognitive interventions delivered later in life, for children or adolescents with CHD, have had only limited success (199-201).
‘Brain in a dish’ and insights from related and unrelated neurological disease
The ability to deconstruct the biological and molecular complexities of human neurodevelopmental disease and translate findings into the clinic is only made possible through the multidisciplinary approach between basic science researchers and clinicians. While animal models have been instrumental in advancing our understanding of the molecular mechanisms of several genetic or extrinsic factors, extrapolating these findings to human disease are largely ineffective (202-204). This underscores a critical need for models that can leverage the disparities between the human and non-human brain with specific regard to early developmental processes that can broadly impact NDD. Human pluripotent stem cells (hPSCs), including both embryonic- and patient-derived induced pluripotent stem cells, have been a widely utilized model to satisfy the necessity for analysing human cells and methods to direct hPSCs towards specific cell fates in-vitro has demonstrated to be an excellent tool for studying human development programs and disease etiology (205-209).
Several strategies for differentiation of hPSCs into specific neuronal subtypes include transcriptional programming, small molecule and growth factor directed differentiation in both monolayer, and three-dimensional mini-brain organoids. While all methods will generate the neuronal cell types of interest, factors related to the speed and efficiency of cell generation, developmental accuracy, and reproducibility need to be considered for downstream applications. Advantages of transcriptional programming include the rapid differentiation (~5–10 days) from hPSCs resulting in a near homogenous population of cells that represent the final product for analysis such as excitatory neurons and astrocytes (209). However, questions regarding how development of the neuron or astrocyte differentiate are limited as well as the study of specific subtypes of neurons or astrocytes may be limited due to a largely generic cell identity. The directed differentiation of hPSCs recapitulates aspects of brain development such as corticogenesis and spinal cord development which can illuminate the process of differentiation which provides an additional layer of analysis for neurodevelopmental disorders. Recently, hPSCs have been differentiated as three-dimensional neural spheroids or cerebral organoids to highlight the remarkable self-organizing properties of cortical development (210,211). Importantly, cerebral organoids are similar to traditional monolayer differentiation where one can analyze cortical differentiation; however, they can also recreate the cortical architecture of the human brain, providing additional insight into human development, albeit with increased cellular heterogeneity and variability (205).
Brain models derived from hPSCs have been extensively used for various disease modelling, including neurodevelopmental disorders including autism and schizophrenia (212) assessing early stages of neurogenesis (213-216). In the CHD field, this approach has been used to define molecular mechanisms of functional alterations evident in two-dimensional (2D) cultures and organoids derived from patients with 22q11.2 deletion syndrome (217). This deletion is associated with cardiac outflow tract malformations. Transcriptional profiling over 100 days demonstrated predictable differentiation and alterations in neuronal excitability genes, emulating cortical brain regions. These changes were evident in both 2D and organoid cultures and correction of haploinsufficient gene dosage was able to correct the disease phenotype. It is reassuring that a disease phenotype was evident and correctable in patient iPSC cells, highlighting the value of this approach in assessing mechanisms of disease. The impact of a chromosomal deletion may exceed the impact of one or several single nucleotide variants expected in NDD, and the cellular phenotype may also be more subtle. Nevertheless, expression profiling, functional testing and correlation of cell phenotype with patient phenotype is likely to discriminate and allow prioritization of individual genes and gene pathways that can be related back to the genomic sequence of the patients involved to corroborate individual variants and burden testing in gene set pathways.
Using a hPSC model to study NDD is advantageous as it accounts for the complexity of human brain development and eliminates confounding differences between human and rodent brain development (218). As a model to study NDD, the use of organoids permits the characterization of fetal brain development in-vitro using patient derived hPSC in an environment that is similar to what would be encountered in-vivo. With advancement of existing technologies, patient derived hPSC can be corrected using CRISPR-Cas9 system, allowing for reversion of disease-causing gene mutations (219). However, manipulating the progenitor populations may prove to be a more effective strategy to obtain clinical relevance. As autologous transplantation becomes more widely studied, novel therapies based on replacement of genetically corrected cell populations may be utilized to treat genetic brain disease (220).
Conclusions
Clinical applications and future directions
While research into improving current diagnostic, prognostic and treatment options for NDD in CHD is on-going, there are a number of practical ways in which neurodevelopmental outcomes can be maximised for the CHD patient (69).
The importance of prenatal diagnosis of CHD in reducing the risk of NDD through optimisation of perinatal care has been demonstrated (221) and presents an important modifiable factor in improving long-term neurodevelopmental outcomes for CHD patients (222). Individualised inpatient developmental care implemented in the intensive care unit have been associated with better neurodevelopmental outcomes, family functioning, and greater school attendance (223). The inclusion of parent-focused psychoeducation in such programs have demonstrated a positive impact on psychological, emotional and social development in the child with CHD (223) and extends to improved maternal psychological functioning and coping (198).
Similarly, genetic testing may identify patients at high risk of NDD, and enable early interventions aimed at optimising NDD outcomes for patients—which may be particularly important in centres where neurodevelopmental follow-up for infants with CHD is not part of standardised care. The distinction between syndromic and non-syndromic forms of CHD can be subtle and may be viewed as a continuum or spectrum of disease. The importance of a genetic assessment to identify subtle extracardiac features and possible genetic contributors to guide clinical management should be considered by physicians involved in the care of CHD patients. The European Society of Cardiology and American Heart Associations have released recommendations for genetic testing in patients with CHD (224,225). Notably, they recommend that patients with CHD and extracardiac anomalies, including significant neurodevelopmental abnormalities, should have chromosomal microarray testing, followed by trio exome or genome sequencing due to the reported high diagnostic yield of 25–40% in this patient group (226,227).
Further, they recommend that a prenatal diagnosis of CHD, should be accompanied by fetal chromosomal microarray testing and trio fetal exome and genome sequencing should be considered in any patients with complex CHD and/or extracardiac anomalies (224,225) Early diagnosis of a genetic syndrome provides important information in terms of managing associated NDD which is present in up to 75% of children with a diagnosed genetic syndrome (228).
It is important to note; however, that the absence of a genetic diagnosis or an uninformative genetic test result does not minimise the risk of NDD or preclude its development; and regular neurodevelopmental follow-up throughout childhood should be part of best care practice for all patients with CHD (7) Not all children will develop NDD; however, without regular neurodevelopmental assessments, those with issues, particularly those with minor issues, will be overlooked and opportunities for intervention and/or additional support, missed (229).
Similarly, there is growing support for increased monitoring for brain injury pre- and post-cardiac surgery that may serve as a prognostic indicator for new or worsening NDD (68). A high proportion of peri-operative brain injury is ‘silent’, evidenced by the disparity between the rate of brain injury identified in clinical practice (230), whereby brain imaging typically occurs in response to a clinical event, compared to studies that have included neuroimaging in an entire cohort of CHD patients (53,65-68). Routine pre- and post-operative neuroimaging may be warranted in infants with severe CHD (231). However, is generally limited by available resources and typically includes head ultrasound—that may miss up to 80% of lesions detected by MRI, particularly white matter injury (232). Better understanding of the sensitive interplay between brain injury and NDD trajectories in CHD, may inform future clinical practice in this regard.
Additional factors, including peri-operative course and socio-economic status may be stronger predictors of long-term NDD compared to conventional brain MRI (233) and these data should also be considered when determining a patient’s risk profile.
Longer-term neurodevelopmental and cognitive follow-up extending beyond the early childhood years are recommended (7); however poor accessibility to neuropsychological services for older children and adults with CHD remains an important issue. Interventions to minimise new and accumulating risk factors occurring throughout the CHD life course are required to mitigate the persisting impact of NDD beyond the early childhood years.
In summary, current understanding and treatment options for NDD in CHD are still in their infancy and the coming years will likely see significant advances in the field. Clinical trials, prospective clinical studies using novel and sophisticated imaging technologies, as well as large genomic analyses and functional experiments will provide important insight into disease mechanisms, development, and progression as well as possible interventions and treatment. Indeed, examples of how genetic information can inform personalised therapies already exist in syndromes with neurodevelopmental phenotypes such as Kabuki syndrome (234). Simple dietary modifications, inducing epigenetic changes, have been shown to significantly improve cognitive function through more effective neuronal development in these patients (235).
Further, networks such as the Cardiac Neurodevelopmental Outcome Collaborative (cardiacneuro.org) are enabling large multinational, multicentre collaborations to inform future research efforts and provide important longitudinal data.
Clinically, the rapidly evolving field of fetal cardiac interventions, may be an important avenue to optimise neurodevelopmental outcomes, especially in fetuses with single ventricle pathologies (236).
Finally, polygenic risk scores, may in time provide important information to determine which CHD patients are at increased risk of developing NDD. Using prenatal genetic testing to determine individual polygenic risk scores, would enable early and on-going interventions to maximise neurodevelopmental outcomes in at risk patients.
Acknowledgments
Funding: This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Autism Research Program (Award No. W81XWH-21-ARP-CDA to J Tchieu).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Antonio F. Corno and Jorge D. Salazar) for the column “Pediatric Heart” published in Translational Pediatrics. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-687/coif). The column “Pediatric Heart” was commissioned by the editorial office without any funding or sponsorship. JT was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Autism Research Program (Award No. W81XWH-21-ARP-CDA). NAK receives funding from the National Heart Foundation of Australia, Additional Ventures and the National Health and Medical Research Council of Australia (research grant only, payment made to the institution). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900. [Crossref] [PubMed]
- Kasparian NA, Winlaw DS, Sholler GF. "Congenital heart health": how psychological care can make a difference. Med J Aust 2016;205:104-7. [Crossref] [PubMed]
- McCracken C, Spector LG, Menk JS, et al. Mortality Following Pediatric Congenital Heart Surgery: An Analysis of the Causes of Death Derived From the National Death Index. J Am Heart Assoc 2018;7:e010624. [Crossref] [PubMed]
- Morton SU, Quiat D, Seidman JG, et al. Genomic frontiers in congenital heart disease. Nat Rev Cardiol 2022;19:26-42. [Crossref] [PubMed]
- Brida M, Gatzoulis MA. Adult congenital heart disease: Past, present and future. Acta Paediatr 2019;108:1757-64. [Crossref] [PubMed]
- Kovacs AH, Bellinger DC. Neurocognitive and psychosocial outcomes in adult congenital heart disease: a lifespan approach. Heart 2021;107:159-67. [Crossref] [PubMed]
- Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 2012;126:1143-72. [Crossref] [PubMed]
- Verrall CE, Yang JYM, Chen J, et al. Neurocognitive Dysfunction and Smaller Brain Volumes in Adolescents and Adults With a Fontan Circulation. Circulation 2021;143:878-91. [Crossref] [PubMed]
- Calderon J, Newburger JW, Rollins CK. Neurodevelopmental and Mental Health Outcomes in Patients With Fontan Circulation: A State-of-the-Art Review. Front Pediatr 2022;10:826349. [Crossref] [PubMed]
- Verrall CE, Blue GM, Loughran-Fowlds A, et al. 'Big issues' in neurodevelopment for children and adults with congenital heart disease. Open Heart 2019;6:e000998. [Crossref] [PubMed]
- Wehrle FM, Bartal T, Adams M, et al. Similarities and Differences in the Neurodevelopmental Outcome of Children with Congenital Heart Disease and Children Born Very Preterm at School Entry. J Pediatr 2022;250:29-37.e1. [Crossref] [PubMed]
- Hövels-Gürich HH, Seghaye MC, Schnitker R, et al. Long-term neurodevelopmental outcomes in school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg 2002;124:448-58. [Crossref] [PubMed]
- Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003;126:1385-96. [Crossref] [PubMed]
- Brosig CL, Bear L, Allen S, et al. Neurodevelopmental outcomes at 2 and 4 years in children with congenital heart disease. Congenit Heart Dis 2018;13:700-5. [Crossref] [PubMed]
- Brosig CL, Bear L, Allen S, et al. Preschool Neurodevelopmental Outcomes in Children with Congenital Heart Disease. J Pediatr 2017;183:80-86.e1. [Crossref] [PubMed]
- Riehle-Colarusso T, Autry A, Razzaghi H, et al. Congenital Heart Defects and Receipt of Special Education Services. Pediatrics 2015;136:496-504. [Crossref] [PubMed]
- Kasparian NA, Kovacs AH. Quality of Life and Other Patient-Reported Outcomes Across the Life Span Among People With Fontan Palliation. Can J Cardiol 2022;38:963-76. [Crossref] [PubMed]
- Marshall KH, D'Udekem Y, Sholler GF, et al. Health-Related Quality of Life in Children, Adolescents, and Adults With a Fontan Circulation: A Meta-Analysis. J Am Heart Assoc 2020;9:e014172. [Crossref] [PubMed]
- Girouard HS, Kovacs AH. Congenital heart disease: Education and employment considerations and outcomes. Int J Cardiol Congenit Heart Dis 2020;1:100005. [Crossref]
- Jones CE, Desai H, Fogel JL, et al. Disruptions in the development of feeding for infants with congenital heart disease. Cardiol Young 2021;31:589-96. [Crossref] [PubMed]
- Latal B. Neurodevelopmental Outcomes of the Child with Congenital Heart Disease. Clin Perinatol 2016;43:173-85. [Crossref] [PubMed]
- Walker K, Badawi N, Halliday R, et al. Early developmental outcomes following major noncardiac and cardiac surgery in term infants: a population-based study. J Pediatr 2012;161:748-752.e1. [Crossref] [PubMed]
- Gaudet I, Paquette N, Bernard C, et al. Neurodevelopmental Outcome of Children with Congenital Heart Disease: A Cohort Study from Infancy to Preschool Age. J Pediatr 2021;239:126-135.e5. [Crossref] [PubMed]
- Bolduc ME, Dionne E, Gagnon I, et al. Motor Impairment in Children With Congenital Heart Defects: A Systematic Review. Pediatrics 2020;146:e20200083. [Crossref] [PubMed]
- Majnemer A, Limperopoulos C, Shevell M, et al. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr 2006;148:72-7. [Crossref] [PubMed]
- Feldmann M, Bataillard C, Ehrler M, et al. Cognitive and Executive Function in Congenital Heart Disease: A Meta-analysis. Pediatrics 2021;148:e2021050875. [Crossref] [PubMed]
- Naef N, Liamlahi R, Beck I, et al. Neurodevelopmental Profiles of Children with Congenital Heart Disease at School Age. J Pediatr 2017;188:75-81. [Crossref] [PubMed]
- Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation 2011;124:1361-9. [Crossref] [PubMed]
- Bellinger DC, Watson CG, Rivkin MJ, et al. Neuropsychological Status and Structural Brain Imaging in Adolescents With Single Ventricle Who Underwent the Fontan Procedure. J Am Heart Assoc 2015;4:e002302. [Crossref] [PubMed]
- Calderon J, Jambaqué I, Bonnet D, et al. Executive functions development in 5- to 7-year-old children with transposition of the great arteries: a longitudinal study. Dev Neuropsychol 2014;39:365-84. [Crossref] [PubMed]
- Miatton M, De Wolf D, François K, et al. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr 2007;151:73-8, 78.e1.
- Sanz JH, Berl MM, Armour AC, et al. Prevalence and pattern of executive dysfunction in school age children with congenital heart disease. Congenit Heart Dis 2017;12:202-9. [Crossref] [PubMed]
- Clancy T, Jordan B, de Weerth C, et al. Early Emotional, Behavioural and Social Development of Infants and Young Children with Congenital Heart Disease: A Systematic Review. J Clin Psychol Med Settings 2020;27:686-703. [Crossref] [PubMed]
- Finkel GG, Sun LS, Jackson WM. Children with Congenital Heart Disease Show Increased Behavioral Problems Compared to Healthy Peers: A Systematic Review and Meta-Analysis. Pediatr Cardiol 2023;44:116-23. [Crossref] [PubMed]
- Sigmon ER, Kelleman M, Susi A, et al. Congenital Heart Disease and Autism: A Case-Control Study. Pediatrics 2019;144:e20184114. [Crossref] [PubMed]
- Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat 2013;9:449-61. [PubMed]
- Cassidy AR, White MT, DeMaso DR, et al. Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. J Int Neuropsychol Soc 2015;21:34-49. [Crossref] [PubMed]
- Perrotta ML, Saha P, Zawadzki R, et al. Adults With Mild-to-Moderate Congenital Heart Disease Demonstrate Measurable Neurocognitive Deficits. J Am Heart Assoc 2020;9:e015379. [Crossref] [PubMed]
- Schlosser L, Kessler N, Feldmann M, et al. Neurocognitive functioning in young adults with congenital heart disease: insights from a case-control study. Cardiol Young 2022;32:694-701. [Crossref] [PubMed]
- Ilardi D, Ono KE, McCartney R, et al. Neurocognitive functioning in adults with congenital heart disease. Congenit Heart Dis 2017;12:166-73. [Crossref] [PubMed]
- Bagge CN, Henderson VW, Laursen HB, et al. Risk of Dementia in Adults With Congenital Heart Disease: Population-Based Cohort Study. Circulation 2018;137:1912-20. [Crossref] [PubMed]
- Brossard-Racine M, Panigrahy A. Structural Brain Alterations and Their Associations With Function in Children, Adolescents, and Young Adults With Congenital Heart Disease. Can J Cardiol 2023;39:123-32. [Crossref] [PubMed]
- Marelli A, Miller SP, Marino BS, et al. Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation 2016;133:1951-62. [Crossref] [PubMed]
- Selvanathan T, Smith JMC, Miller SP, et al. Neurodevelopment and Cognition Across the Lifespan in Patients With Single-Ventricle Physiology: Abnormal Brain Maturation and Accumulation of Brain Injuries. Can J Cardiol 2022;38:977-87. [Crossref] [PubMed]
- Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex 2013;23:2932-43. [Crossref] [PubMed]
- Lauridsen MH, Uldbjerg N, Petersen OB, et al. Fetal Heart Defects and Measures of Cerebral Size. J Pediatr 2019;210:146-53. [Crossref] [PubMed]
- Masoller N, Martínez JM, Gómez O, et al. Evidence of second-trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2014;44:182-7. [Crossref] [PubMed]
- Rajagopalan V, Votava-Smith JK, Zhuang X, et al. Fetuses with single ventricle congenital heart disease manifest impairment of regional brain growth. Prenat Diagn 2018;38:1042-8. [Crossref] [PubMed]
- Rollins CK, Ortinau CM, Stopp C, et al. Regional Brain Growth Trajectories in Fetuses with Congenital Heart Disease. Ann Neurol 2021;89:143-57. [Crossref] [PubMed]
- Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010;121:26-33. [Crossref] [PubMed]
- Ortinau CM, Rollins CK, Gholipour A, et al. Early-Emerging Sulcal Patterns Are Atypical in Fetuses with Congenital Heart Disease. Cereb Cortex 2019;29:3605-16. [Crossref] [PubMed]
- Sadhwani A, Wypij D, Rofeberg V, et al. Fetal Brain Volume Predicts Neurodevelopment in Congenital Heart Disease. Circulation 2022;145:1108-19. [Crossref] [PubMed]
- Ortinau CM, Mangin-Heimos K, Moen J, et al. Prenatal to postnatal trajectory of brain growth in complex congenital heart disease. Neuroimage Clin 2018;20:913-22. [Crossref] [PubMed]
- Ortinau C, Beca J, Lambeth J, et al. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J Thorac Cardiovasc Surg 2012;143:1264-70. [Crossref] [PubMed]
- Meuwly E, Feldmann M, Knirsch W, et al. Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Sci Rep 2019;9:10885. [Crossref] [PubMed]
- von Rhein M, Buchmann A, Hagmann C, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain 2014;137:268-76. [Crossref] [PubMed]
- Hagmann C, Singer J, Latal B, et al. Regional Microstructural and Volumetric Magnetic Resonance Imaging (MRI) Abnormalities in the Corpus Callosum of Neonates With Congenital Heart Defect Undergoing Cardiac Surgery. J Child Neurol 2016;31:300-8. [Crossref] [PubMed]
- Claessens NH, Moeskops P, Buchmann A, et al. Delayed cortical gray matter development in neonates with severe congenital heart disease. Pediatr Res 2016;80:668-74. [Crossref] [PubMed]
- Ng IHX, Bonthrone AF, Kelly CJ, et al. Investigating altered brain development in infants with congenital heart disease using tensor-based morphometry. Sci Rep 2020;10:14909. [Crossref] [PubMed]
- Ibuki K, Watanabe K, Yoshimura N, et al. The improvement of hypoxia correlates with neuroanatomic and developmental outcomes: comparison of midterm outcomes in infants with transposition of the great arteries or single-ventricle physiology. J Thorac Cardiovasc Surg 2012;143:1077-85. [Crossref] [PubMed]
- Peyvandi S, Kim H, Lau J, et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J Thorac Cardiovasc Surg 2018;155:291-300.e3. [Crossref] [PubMed]
- Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg 2010;139:543-56. [Crossref] [PubMed]
- Goff DA, Shera DM, Tang S, et al. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surg 2014;147:1312-8. [Crossref] [PubMed]
- Brossard-Racine M, du Plessis A, Vezina G, et al. Brain Injury in Neonates with Complex Congenital Heart Disease: What Is the Predictive Value of MRI in the Fetal Period? AJNR Am J Neuroradiol 2016;37:1338-46. [Crossref] [PubMed]
- Beca J, Gunn JK, Coleman L, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation 2013;127:971-9. [Crossref] [PubMed]
- Claessens NHP, Algra SO, Ouwehand TL, et al. Perioperative neonatal brain injury is associated with worse school-age neurodevelopment in children with critical congenital heart disease. Dev Med Child Neurol 2018;60:1052-8. [Crossref] [PubMed]
- Dimitropoulos A, McQuillen PS, Sethi V, et al. Brain injury and development in newborns with critical congenital heart disease. Neurology 2013;81:241-8. [Crossref] [PubMed]
- Peyvandi S, Chau V, Guo T, et al. Neonatal Brain Injury and Timing of Neurodevelopmental Assessment in Patients With Congenital Heart Disease. J Am Coll Cardiol 2018;71:1986-96. [Crossref] [PubMed]
- Ortinau CM, Smyser CD, Arthur L, et al. Optimizing Neurodevelopmental Outcomes in Neonates With Congenital Heart Disease. Pediatrics 2022;150:e2022056415L. [Crossref] [PubMed]
- Andropoulos DB, Easley RB, Brady K, et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg 2012;94:1250-5; discussion 1255-6. [Crossref] [PubMed]
- Andropoulos DB, Ahmad HB, Haq T, et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth 2014;24:266-74. [Crossref] [PubMed]
- Rollins CK, Asaro LA, Akhondi-Asl A, et al. White Matter Volume Predicts Language Development in Congenital Heart Disease. J Pediatr 2017;181:42-48.e2. [Crossref] [PubMed]
- Wong A, Chavez T, O'Neil S, et al. Synchronous Aberrant Cerebellar and Opercular Development in Fetuses and Neonates with Congenital Heart Disease: Correlation with Early Communicative Neurodevelopmental Outcomes, Initial Experience. AJP Rep 2017;7:e17-27. [Crossref] [PubMed]
- Owen M, Shevell M, Donofrio M, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr 2014;164:1121-1127.e1. [Crossref] [PubMed]
- Naef N, Schlosser L, Brugger P, et al. Brain volumes in adults with congenital heart disease correlate with executive function abilities. Brain Imaging Behav 2021;15:2308-16. [Crossref] [PubMed]
- Latal B, Patel P, Liamlahi R, et al. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr Res 2016;80:531-7. [Crossref] [PubMed]
- Fontes K, Rohlicek CV, Saint-Martin C, et al. Hippocampal alterations and functional correlates in adolescents and young adults with congenital heart disease. Hum Brain Mapp 2019;40:3548-60. [Crossref] [PubMed]
- Badaly D, Beers SR, Ceschin R, et al. Cerebellar and Prefrontal Structures Associated With Executive Functioning in Pediatric Patients With Congenital Heart Defects. Front Neurol 2022;13:827780. [Crossref] [PubMed]
- Pike NA, Roy B, Moye S, et al. Reduced hippocampal volumes and memory deficits in adolescents with single ventricle heart disease. Brain Behav 2021;11:e01977. [Crossref] [PubMed]
- Ehrler M, Schlosser L, Brugger P, et al. Altered white matter microstructure is related to cognition in adults with congenital heart disease. Brain Commun 2021;3:fcaa224. [Crossref] [PubMed]
- Ehrler M, Latal B, Kretschmar O, et al. Altered frontal white matter microstructure is associated with working memory impairments in adolescents with congenital heart disease: A diffusion tensor imaging study. Neuroimage Clin 2020;25:102123. [Crossref] [PubMed]
- Rollins CK, Watson CG, Asaro LA, et al. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr 2014;165:936-44.e1-2.
- Brewster RC, King TZ, Burns TG, et al. White Matter Integrity Dissociates Verbal Memory and Auditory Attention Span in Emerging Adults with Congenital Heart Disease. J Int Neuropsychol Soc 2015;21:22-33. [Crossref] [PubMed]
- Watson CG, Stopp C, Wypij D, et al. Altered White Matter Microstructure Correlates with IQ and Processing Speed in Children and Adolescents Post-Fontan. J Pediatr 2018;200:140-149.e4. [Crossref] [PubMed]
- Verrall CE, Chen J, Yeh CH, et al. A diffusion MRI study of brain white matter microstructure in adolescents and adults with a Fontan circulation: Investigating associations with resting and peak exercise oxygen saturations and cognition. Neuroimage Clin 2022;36:103151. [Crossref] [PubMed]
- Ní Bhroin M, Abo Seada S, Bonthrone AF, et al. Reduced structural connectivity in cortico-striatal-thalamic network in neonates with congenital heart disease. Neuroimage Clin 2020;28:102423. [Crossref] [PubMed]
- Feldmann M, Guo T, Miller SP, et al. Delayed maturation of the structural brain connectome in neonates with congenital heart disease. Brain Commun 2020;2:fcaa209. [Crossref] [PubMed]
- Schmithorst VJ, Votava-Smith JK, Tran N, et al. Structural network topology correlates of microstructural brain dysmaturation in term infants with congenital heart disease. Hum Brain Mapp 2018;39:4593-610. [Crossref] [PubMed]
- Ramirez A, Peyvandi S, Cox S, et al. Neonatal brain injury influences structural connectivity and childhood functional outcomes. PLoS One 2022;17:e0262310. [Crossref] [PubMed]
- Panigrahy A, Schmithorst VJ, Wisnowski JL, et al. Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. Neuroimage Clin 2015;7:438-48. [Crossref] [PubMed]
- Gaynor JW, Wernovsky G, Jarvik GP, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg 2007;133:1344-53, 1353.e1-3.
- Impact of Operative and Postoperative Factors on Neurodevelopmental Outcomes After Cardiac Operations. Ann Thorac Surg 2016;102:843-9. [Crossref] [PubMed]
- Hirsch JC, Jacobs ML, Andropoulos D, et al. Protecting the infant brain during cardiac surgery: a systematic review. Ann Thorac Surg 2012;94:1365-73; discussion 1373. [Crossref] [PubMed]
- Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg 2009;137:529-36; discussion 536-7. [Crossref] [PubMed]
- Courtney JA, Cnota JF, Jones HN. The Role of Abnormal Placentation in Congenital Heart Disease; Cause, Correlate, or Consequence? Front Physiol 2018;9:1045. [Crossref] [PubMed]
- Rychik J, Goff D, McKay E, et al. Characterization of the Placenta in the Newborn with Congenital Heart Disease: Distinctions Based on Type of Cardiac Malformation. Pediatr Cardiol 2018;39:1165-71. [Crossref] [PubMed]
- Homsy J, Zaidi S, Shen Y, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015;350:1262-6. [Crossref] [PubMed]
- Lawrence KM, McGovern PE, Mejaddam A, et al. Chronic intrauterine hypoxia alters neurodevelopment in fetal sheep. J Thorac Cardiovasc Surg 2019;157:1982-91. [Crossref] [PubMed]
- Lim JM, Porayette P, Marini D, et al. Associations Between Age at Arterial Switch Operation, Brain Growth, and Development in Infants With Transposition of the Great Arteries. Circulation 2019;139:2728-38. [Crossref] [PubMed]
- Yagi H, Furutani Y, Hamada H, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet 2003;362:1366-73. [Crossref] [PubMed]
- Sun L, Macgowan CK, Sled JG, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015;131:1313-23. [Crossref] [PubMed]
- Birca A, Vakorin VA, Porayette P, et al. Interplay of brain structure and function in neonatal congenital heart disease. Ann Clin Transl Neurol 2016;3:708-22. [Crossref] [PubMed]
- Han VX, Patel S, Jones HF, et al. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol 2021;17:564-79. [Crossref] [PubMed]
- Lee FT, Marini D, Seed M, et al. Maternal hyperoxygenation in congenital heart disease. Transl Pediatr 2021;10:2197-209. [Crossref] [PubMed]
- Jeanne M, Vuillaume ML, Ung DC, et al. Haploinsufficiency of the HIRA gene located in the 22q11 deletion syndrome region is associated with abnormal neurodevelopment and impaired dendritic outgrowth. Hum Genet 2021;140:885-96. [Crossref] [PubMed]
- Carmel M, Zarchi O, Michaelovsky E, et al. Association of COMT and PRODH gene variants with intelligence quotient (IQ) and executive functions in 22q11.2DS subjects. J Psychiatr Res 2014;56:28-35. [Crossref] [PubMed]
- Kozel BA, Barak B, Kim CA, et al. Williams syndrome. Nat Rev Dis Primers 2021;7:42. [Crossref] [PubMed]
- Butcher DT, Cytrynbaum C, Turinsky AL, et al. CHARGE and Kabuki Syndromes: Gene-Specific DNA Methylation Signatures Identify Epigenetic Mechanisms Linking These Clinically Overlapping Conditions. Am J Hum Genet 2017;100:773-88. [Crossref] [PubMed]
- Sifrim A, Hitz MP, Wilsdon A, et al. Distinct genetic architectures for syndromic and nonsyndromic congenital heart defects identified by exome sequencing. Nat Genet 2016;48:1060-5. [Crossref] [PubMed]
- Lozano R, Gbekie C, Siper PM, et al. FOXP1 syndrome: a review of the literature and practice parameters for medical assessment and monitoring. J Neurodev Disord 2021;13:18. [Crossref] [PubMed]
- Garavelli L, Mainardi PC. Mowat-Wilson syndrome. Orphanet J Rare Dis 2007;2:42. [Crossref] [PubMed]
- Dovjak GO, Zalewski T, Seidl-Mlczoch E, et al. Abnormal Extracardiac Development in Fetuses With Congenital Heart Disease. J Am Coll Cardiol 2021;78:2312-22. [Crossref] [PubMed]
- Jin SC, Homsy J, Zaidi S, et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet 2017;49:1593-601. [Crossref] [PubMed]
- Ji W, Ferdman D, Copel J, et al. De novo damaging variants associated with congenital heart diseases contribute to the connectome. Sci Rep 2020;10:7046. [Crossref] [PubMed]
- Blue GM, Ip E, Walker K, et al. Genetic burden and associations with adverse neurodevelopment in neonates with congenital heart disease. Am Heart J 2018;201:33-9. [Crossref] [PubMed]
- Gaynor JW, Kim DS, Arrington CB, et al. Validation of association of the apolipoprotein E ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. J Thorac Cardiovasc Surg 2014;148:2560-6. [Crossref] [PubMed]
- Škorić-Milosavljević D, Lahrouchi N, Bosada FM, et al. Rare variants in KDR, encoding VEGF Receptor 2, are associated with tetralogy of Fallot. Genet Med 2021;23:1952-60. [Crossref] [PubMed]
- Lennington JB, Coppola G, Kataoka-Sasaki Y, et al. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biol Psychiatry 2016;79:372-82. [Crossref] [PubMed]
- Nardone S, Sams DS, Reuveni E, et al. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl Psychiatry 2014;4:e433. [Crossref] [PubMed]
- Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011;474:380-4. [Crossref] [PubMed]
- Pujol J, Vendrell P, Junqué C, et al. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol 1993;34:71-5. [Crossref] [PubMed]
- Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016;353:772-7. [Crossref] [PubMed]
- Knuesel I, Chicha L, Britschgi M, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 2014;10:643-60. [Crossref] [PubMed]
- Han VX, Patel S, Jones HF, et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry 2021;11:71. [Crossref] [PubMed]
- Aristizabal MJ, Anreiter I, Halldorsdottir T, et al. Biological embedding of experience: A primer on epigenetics. Proc Natl Acad Sci U S A 2020;117:23261-9. [Crossref] [PubMed]
- Meyer U. Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci 2019;42:793-806. [Crossref] [PubMed]
- Kalish BT, Kim E, Finander B, et al. Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat Neurosci 2021;24:204-13. [Crossref] [PubMed]
- Banik A, Kandilya D, Ramya S, et al. Maternal Factors that Induce Epigenetic Changes Contribute to Neurological Disorders in Offspring. Genes (Basel) 2017;8:150. [Crossref] [PubMed]
- Renz H, Holt PG, Inouye M, et al. An exposome perspective: Early-life events and immune development in a changing world. J Allergy Clin Immunol 2017;140:24-40. [Crossref] [PubMed]
- Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med 2009;27:351-7. [Crossref] [PubMed]
- Nardone S, Elliott E. The Interaction between the Immune System and Epigenetics in the Etiology of Autism Spectrum Disorders. Front Neurosci 2016;10:329. [Crossref] [PubMed]
- Basil P, Li Q, Dempster EL, et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry 2014;4:e434. [Crossref] [PubMed]
- Labouesse MA, Dong E, Grayson DR, et al. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics 2015;10:1143-55. [Crossref] [PubMed]
- Weber-Stadlbauer U. Epigenetic and transgenerational mechanisms in infection-mediated neurodevelopmental disorders. Transl Psychiatry 2017;7:e1113. [Crossref] [PubMed]
- Ronovsky M, Berger S, Zambon A, et al. Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav Immun 2017;63:127-36. [Crossref] [PubMed]
- Hodyl NA, Roberts CT, Bianco-Miotto T. Cord Blood DNA Methylation Biomarkers for Predicting Neurodevelopmental Outcomes. Genes (Basel) 2016;7:117. [Crossref] [PubMed]
- Wu S, Gennings C, Wright RJ, et al. Prenatal Stress, Methylation in Inflammation-Related Genes, and Adiposity Measures in Early Childhood: the Programming Research in Obesity, Growth Environment and Social Stress Cohort Study. Psychosom Med 2018;80:34-41. [Crossref] [PubMed]
- Ruchat SM, Houde AA, Voisin G, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics 2013;8:935-43. [Crossref] [PubMed]
- Gunawardhana LP, Gibson PG, Simpson JL, et al. Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clin Exp Allergy 2014;44:47-57. [Crossref] [PubMed]
- Nemoda Z, Massart R, Suderman M, et al. Maternal depression is associated with DNA methylation changes in cord blood T lymphocytes and adult hippocampi. Transl Psychiatry 2015;5:e545. [Crossref] [PubMed]
- Lombardo MV, Moon HM, Su J, et al. Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol Psychiatry 2018;23:1001-13. [Crossref] [PubMed]
- Tsivion-Visbord H, Kopel E, Feiglin A, et al. Increased RNA editing in maternal immune activation model of neurodevelopmental disease. Nat Commun 2020;11:5236. [Crossref] [PubMed]
- Woolf-King SE, Anger A, Arnold EA, et al. Mental Health Among Parents of Children With Critical Congenital Heart Defects: A Systematic Review. J Am Heart Assoc 2017;6:e004862. [Crossref] [PubMed]
- Rychik J, Donaghue DD, Levy S, et al. Maternal psychological stress after prenatal diagnosis of congenital heart disease. J Pediatr 2013;162:302-7.e1. [Crossref] [PubMed]
- Brosig CL, Whitstone BN, Frommelt MA, et al. Psychological distress in parents of children with severe congenital heart disease: the impact of prenatal versus postnatal diagnosis. J Perinatol 2007;27:687-92. [Crossref] [PubMed]
- Kasparian NA, Kan JM, Sood E, et al. Mental health care for parents of babies with congenital heart disease during intensive care unit admission: Systematic review and statement of best practice. Early Hum Dev 2019;139:104837. [Crossref] [PubMed]
- Kasparian NA. Heart care before birth: A psychobiological perspective on fetal cardiac diagnosis. Prog Pediatr Cardiol 2019;54:101142. [Crossref]
- McWhorter LG, Christofferson J, Neely T, et al. Parental post-traumatic stress, overprotective parenting, and emotional and behavioural problems for children with critical congenital heart disease. Cardiol Young 2022;32:738-45. [Crossref] [PubMed]
- Kasparian N, Barnett B, Winlaw D, et al. When hearts and minds meet: A psychological perspective on fetal cardiac diagnosis. Infant Ment Health J 2010;32:104.
- Pinelli J, Saigal S, Wu YWB, et al. Patterns of change in family functioning, resources, coping and parental depression in mothers and fathers of sick newborns over the first year of life. J Neonatal Nurs 2008;14:156-65. [Crossref]
- Lawoko S. Factors influencing satisfaction and well-being among parents of congenital heart disease children: development of a conceptual model based on the literature review. Scand J Caring Sci 2007;21:106-17. [Crossref] [PubMed]
- Werner H, Latal B, Valsangiacomo Buechel E, et al. The impact of an infant's severe congenital heart disease on the family: a prospective cohort study. Congenit Heart Dis 2014;9:203-10. [Crossref] [PubMed]
- Lawoko S, Soares JJ. Distress and hopelessness among parents of children with congenital heart disease, parents of children with other diseases, and parents of healthy children. J Psychosom Res 2002;52:193-208. [Crossref] [PubMed]
- Denniss DL, Sholler GF, Costa DSJ, et al. Need for Routine Screening of Health-Related Quality of Life in Families of Young Children with Complex Congenital Heart Disease. J Pediatr 2019;205:21-28.e2. [Crossref] [PubMed]
- Ernst MM, Marino BS, Cassedy A, et al. Biopsychosocial Predictors of Quality of Life Outcomes in Pediatric Congenital Heart Disease. Pediatr Cardiol 2018;39:79-88. [Crossref] [PubMed]
- Grigoriadis S. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry 2013;74:e321-41. [Crossref] [PubMed]
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 2010;67:1012-24. [Crossref] [PubMed]
- Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014;384:1800-19. [Crossref] [PubMed]
- McCusker CG, Armstrong MP, Mullen M, et al. A sibling-controlled, prospective study of outcomes at home and school in children with severe congenital heart disease. Cardiol Young 2013;23:507-16. [Crossref] [PubMed]
- DeMaso DR, Labella M, Taylor GA, et al. Psychiatric disorders and function in adolescents with d-transposition of the great arteries. J Pediatr 2014;165:760-6. [Crossref] [PubMed]
- Goldberg S, Janus M, Washington J, et al. Prediction of preschool behavioral problems in healthy and pediatric samples. J Dev Behav Pediatr 1997;18:304-13. [Crossref] [PubMed]
- Tesson S, Butow PN, Marshall K, et al. Parent-child bonding and attachment during pregnancy and early childhood following congenital heart disease diagnosis. Health Psychol Rev 2022;16:378-411. [Crossref] [PubMed]
- Maccari S, Darnaudery M, Morley-Fletcher S, et al. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev 2003;27:119-27. [Crossref] [PubMed]
- Barker DJ, Gluckman PD, Godfrey KM, et al. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993;341:938-41. [Crossref] [PubMed]
- Van den Bergh BRH, van den Heuvel MI, Lahti M, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev 2020;117:26-64. [Crossref] [PubMed]
- Cruceanu C, Matosin N, Binder EB. Interactions of early-life stress with the genome and epigenome: From prenatal stress to psychiatric disorders. Curr Opin Behav Sci 2017;14:167-71. [Crossref]
- Van den Bergh BR, Mulder EJ, Mennes M, et al. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev 2005;29:237-58. [Crossref] [PubMed]
- O'Donnell KJ, Glover V, Barker ED, et al. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev Psychopathol 2014;26:393-403. [Crossref] [PubMed]
- Kinney DK, Munir KM, Crowley DJ, et al. Prenatal stress and risk for autism. Neurosci Biobehav Rev 2008;32:1519-32. [Crossref] [PubMed]
- Hay DF, Pawlby S, Sharp D, et al. Intellectual problems shown by 11-year-old children whose mothers had postnatal depression. J Child Psychol Psychiatry 2001;42:871-89. [Crossref] [PubMed]
- Sandman CA, Buss C, Head K, et al. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry 2015;77:324-34. [Crossref] [PubMed]
- Rifkin-Graboi A, Meaney MJ, Chen H, et al. Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J Am Acad Child Adolesc Psychiatry 2015;54:313-21.e2. [Crossref] [PubMed]
- Rifkin-Graboi A, Bai J, Chen H, et al. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry 2013;74:837-44. [Crossref] [PubMed]
- Buss C, Davis EP, Shahbaba B, et al. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A 2012;109:E1312-9. [Crossref] [PubMed]
- Qiu A, Rifkin-Graboi A, Chen H, et al. Maternal anxiety and infants' hippocampal development: timing matters. Transl Psychiatry 2013;3:e306. [Crossref] [PubMed]
- Qiu A, Anh TT, Li Y, et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry 2015;5:e508. [Crossref] [PubMed]
- Scheinost D, Kwon SH, Lacadie C, et al. Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage Clin 2016;12:381-8. [Crossref] [PubMed]
- Scheinost D, Sinha R, Cross SN, et al. Does prenatal stress alter the developing connectome? Pediatr Res 2017;81:214-26. [Crossref] [PubMed]
- Wu Y, Lu YC, Jacobs M, et al. Association of Prenatal Maternal Psychological Distress With Fetal Brain Growth, Metabolism, and Cortical Maturation. JAMA Netw Open 2020;3:e1919940. [Crossref] [PubMed]
- Paquette AG, Houseman EA, Green BB, et al. Regions of variable DNA methylation in human placenta associated with newborn neurobehavior. Epigenetics 2016;11:603-13. [Crossref] [PubMed]
- Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Dev Psychopathol 2012;24:1361-76. [Crossref] [PubMed]
- Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318:153-7. [Crossref] [PubMed]
- O'Donnell KJ, Bugge Jensen A, Freeman L, et al. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology 2012;37:818-26. [Crossref] [PubMed]
- O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci 2009;31:285-92. [Crossref] [PubMed]
- Field T, Diego M, Dieter J, et al. Prenatal depression effects on the fetus and the newborn. Infant Beh Dev 2004;27:216-29. [Crossref]
- Salaria S, Chana G, Caldara F, et al. Microarray analysis of cultured human brain aggregates following cortisol exposure: implications for cellular functions relevant to mood disorders. Neurobiol Dis 2006;23:630-6. [Crossref] [PubMed]
- Conradt E, Lester BM, Appleton AA, et al. The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics 2013;8:1321-9. [Crossref] [PubMed]
- Marsit CJ, Maccani MA, Padbury JF, et al. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One 2012;7:e33794. [Crossref] [PubMed]
- Huizink AC, Robles de Medina PG, Mulder EJ, et al. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry 2003;44:810-8. [Crossref] [PubMed]
- Kost K, Landry DJ, Darroch JE. Predicting maternal behaviors during pregnancy: does intention status matter? Fam Plann Perspect 1998;30:79-88. [Crossref] [PubMed]
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affect Disord 2018;232:316-28. [Crossref] [PubMed]
- US Preventive Services Task Force. Interventions to Prevent Perinatal Depression: US Preventive Services Task Force Recommendation Statement. JAMA 2019;321:580-7. [Crossref] [PubMed]
- Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev 2013;CD001134. [Crossref] [PubMed]
- Werner E, Miller M, Osborne LM, et al. Preventing postpartum depression: review and recommendations. Arch Womens Ment Health 2015;18:41-60. [Crossref] [PubMed]
- O'Connor E, Senger CA, Henninger ML, et al. Interventions to Prevent Perinatal Depression: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019;321:588-601. [Crossref] [PubMed]
- Field T. Prenatal Depression Risk Factors, Developmental Effects and Interventions: A Review. J Pregnancy Child Health 2017;4:301. [Crossref] [PubMed]
- Loughnan SA, Wallace M, Joubert AE, et al. A systematic review of psychological treatments for clinical anxiety during the perinatal period. Arch Womens Ment Health 2018;21:481-90. [Crossref] [PubMed]
- McCusker CG, Doherty NN, Molloy B, et al. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev 2010;36:110-7. [Crossref] [PubMed]
- Calderon J, Wypij D, Rofeberg V, et al. Randomized Controlled Trial of Working Memory Intervention in Congenital Heart Disease. J Pediatr 2020;227:191-198.e3. [Crossref] [PubMed]
- Jordan LC, Siciliano RE, Cole DA, et al. Cognitive training in children with hypoplastic left heart syndrome: A pilot randomized trial. Prog Pediatr Cardiol 2020;57:101185. [Crossref]
- Fourdain S, Caron-Desrochers L, Simard MN, et al. Impacts of an Interdisciplinary Developmental Follow-Up Program on Neurodevelopment in Congenital Heart Disease: The CINC Study. Front Pediatr 2020;8:539451. [Crossref] [PubMed]
- Mullard A. Parsing clinical success rates. Nat Rev Drug Discov 2016;15:447. [PubMed]
- Yamaguchi S, Kaneko M, Narukawa M. Approval success rates of drug candidates based on target, action, modality, application, and their combinations. Clin Transl Sci 2021;14:1113-22. [Crossref] [PubMed]
- Sun D, Gao W, Hu H, et al. Why 90% of clinical drug development fails and how to improve it? Acta Pharm Sin B 2022;12:3049-62. [Crossref] [PubMed]
- Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373-9. [Crossref] [PubMed]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72. [Crossref] [PubMed]
- Tapia N, Schöler HR. Molecular Obstacles to Clinical Translation of iPSCs. Cell Stem Cell 2016;19:298-309. [Crossref] [PubMed]
- Sidhaye J, Knoblich JA. Brain organoids: an ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ 2021;28:52-67. [Crossref] [PubMed]
- Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 2014;9:2329-40. [Crossref] [PubMed]
- Paşca AM, Sloan SA, Clarke LE, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 2015;12:671-8. [Crossref] [PubMed]
- Birey F, Andersen J, Makinson CD, et al. Assembly of functionally integrated human forebrain spheroids. Nature 2017;545:54-9. [Crossref] [PubMed]
- Kathuria A, Lopez-Lengowski K, Jagtap SS, et al. Transcriptomic Landscape and Functional Characterization of Induced Pluripotent Stem Cell-Derived Cerebral Organoids in Schizophrenia. JAMA Psychiatry 2020;77:745-54. [Crossref] [PubMed]
- Clevers H. COVID-19: organoids go viral. Nat Rev Mol Cell Biol 2020;21:355-6. [Crossref] [PubMed]
- Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020;181:905-913.e7. [Crossref] [PubMed]
- Mariani J, Coppola G, Zhang P, et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015;162:375-90. [Crossref] [PubMed]
- Kim NS, Wen Z, Liu J, et al. Pharmacological rescue in patient iPSC and mouse models with a rare DISC1 mutation. Nat Commun 2021;12:1398. [Crossref] [PubMed]
- Khan TA, Revah O, Gordon A, et al. Neuronal defects in a human cellular model of 22q11.2 deletion syndrome. Nat Med 2020;26:1888-98. [Crossref] [PubMed]
- Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell 2008;134:877-86. [Crossref] [PubMed]
- Wang P, Mokhtari R, Pedrosa E, et al. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells. Mol Autism 2017;8:11. [Crossref] [PubMed]
- Quadrato G, Brown J, Arlotta P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat Med 2016;22:1220-8. [Crossref] [PubMed]
- Calderon J, Angeard N, Moutier S, et al. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. J Pediatr 2012;161:94-8.e1. [Crossref] [PubMed]
- Sethi N, Carpenter JL, Donofrio MT. Impact of perinatal management on neurodevelopmental outcomes in congenital heart disease. Semin Perinatol 2022;46:151582. [Crossref] [PubMed]
- Cassidy AR, Butler SC, Briend J, et al. Neurodevelopmental and psychosocial interventions for individuals with CHD: a research agenda and recommendations from the Cardiac Neurodevelopmental Outcome Collaborative. Cardiol Young 2021;31:888-99. [Crossref] [PubMed]
- Wilde AAM, Semsarian C, Márquez MF, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. Europace 2022;24:1307-67. [Crossref] [PubMed]
- Landstrom AP, Kim JJ, Gelb BD, et al. Genetic Testing for Heritable Cardiovascular Diseases in Pediatric Patients: A Scientific Statement From the American Heart Association. Circ Genom Precis Med 2021;14:e000086. [Crossref] [PubMed]
- Morrish AM, Smith J, Enriquez A, et al. A new era of genetic testing in congenital heart disease: A review. Trends Cardiovasc Med 2022;32:311-9. [Crossref] [PubMed]
- Blue GM, Kirk EP, Giannoulatou E, et al. Advances in the Genetics of Congenital Heart Disease: A Clinician's Guide. J Am Coll Cardiol 2017;69:859-70. [Crossref] [PubMed]
- Mussatto KA, Hoffmann RG, Hoffman GM, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics 2014;133:e570-7. [Crossref] [PubMed]
- Ricci MF, Moddemann D, Garcia Guerra G, et al. A Practical Approach to Optimizing Neurodevelopment in Children With Congenital Heart Disease. Can J Cardiol 2023;39:156-8. [Crossref] [PubMed]
- Verrall CE, Walker K, Loughran-Fowlds A, et al. Contemporary incidence of stroke (focal infarct and/or haemorrhage) determined by neuroimaging and neurodevelopmental disability at 12 months of age in neonates undergoing cardiac surgery utilizing cardiopulmonary bypass. Interact Cardiovasc Thorac Surg 2018;26:644-50. [Crossref] [PubMed]
- Bhutta AT. Peri-Operative Brain MRI in Children Undergoing Congenital Heart Surgery: Is it Time for Routine Imaging? J Am Coll Cardiol 2018;71:1997-8. [Crossref] [PubMed]
- Rios DR, Welty SE, Gunn JK, et al. Usefulness of routine head ultrasound scans before surgery for congenital heart disease. Pediatrics 2013;131:e1765-70. [Crossref] [PubMed]
- Claessens NHP, Breur JMPJ, Groenendaal F, et al. Brain microstructural development in neonates with critical congenital heart disease: An atlas-based diffusion tensor imaging study. Neuroimage Clin 2019;21:101672. [Crossref] [PubMed]
- Boniel S, Szymańska K, Śmigiel R, et al. Kabuki Syndrome-Clinical Review with Molecular Aspects. Genes (Basel) 2021;12:468. [Crossref] [PubMed]
- Benjamin JS, Pilarowski GO, Carosso GA, et al. A ketogenic diet rescues hippocampal memory defects in a mouse model of Kabuki syndrome. Proc Natl Acad Sci U S A 2017;114:125-30. [Crossref] [PubMed]
- Sharma C, Burns J, Joshi K, et al. Advances in the Prenatal Management of Fetal Cardiac Disease. Children (Basel) 2022;9:812. [Crossref] [PubMed]


