
Clinical features and a prognostic nomogram based on the SEER database for hepatoblastoma, hepatocellular carcinoma, and embryonal sarcoma among children and adolescents
Highlight box
Key findings
• Race, household income, surgery, chemotherapy, pathological tissue grading, and tumor node metastasis staging are the independent prognostic factors for malignant liver tumors among children and adolescents.
What is known and what is new?
• Hepatoblastoma, hepatocellular carcinoma, and embryonal sarcoma are the main types of liver tumors among children and adolescents.
• Different kinds of liver tumors have various prognoses and prognostic factors. Race, surgery, and chemotherapy are independent prognostic factors for hepatoblastoma. Pathological tissue grading, tumor node metastasis staging, and surgery are independent prognostic factors for hepatocellular carcinoma. Household income and surgery are independent prognostic factors for embryonal sarcoma.
What are the implications, and what should change now?
• Nomograms were constructed according to the prognostic factors, which can be used to calculate the estimated risk of individual patients and contribute to their treatment.
Introduction
Compared with other malignant conditions in children and adolescents, liver tumors are relatively rare. A previous retrospective analysis suggested that the incidence rate was 1.8/million per year (1). Hepatoblastoma (HB), hepatocellular carcinoma (HCC), and embryonal sarcoma (ES) are the top three most frequent liver tumors in children and adolescents (2-4). Health problems in children cannot be ignored, and the spectrum of liver disease predisposing to liver tumors in children differs from that in adults. In recent years, there has been an increasing amount of literature on the incidence and screening of the prognostic factors in HB, HCC, and ES among children and adolescents. Up to now, Justin’s study has indicated that HB incidence increased, meanwhile, age, race or ethnicity, and stage were the prognostic factors in 5-year relative survival (5). HCC is the second most common primary liver malignancy in a pediatric setting, which is diagnosed more commonly in adolescents (10–14 years). Long term outcomes indicated that survival of younger (0–4 vs. ≥5 years) and male has a better prognosis (6). ES is a rare and aggressive pediatric malignancy. Complete tumor removal remains the key element of its treatment. Combination chemotherapy, as an effective approach to cure children with ES, can facilitate complete surgical resection (7). Thus, clarifying the clinical features and prognostic factors is a promising approach to contribute to the management of liver tumors in this population. There is a growing body of literature that recognizes the importance and convenience of prediction models in childhood and adolescent diseases (8,9). The epidemiology and outcomes for pediatric patients with liver tumors have not been well documented. Therefore, the present article aims to outline the clinical features and prognostic prediction for the three aforementioned types of liver tumors. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-679/rc).
Methods
Data source
We performed a retrospective cohort analysis by querying the Surveillance, Epidemiology, and End Results (SEER) database to identify patients diagnosed with malignant liver tumors from 2000 to 2019 (primary site: C22.0; all patients ≤18 years old). SEER is an authoritative source of information on cancer incidence and survival in the United States, covering approximately 48.0% of the U.S. population. The detailed process of the study is shown in Figure 1. The clinical pathological information is public and anonymous, so our study did not require ethical approval or patient consent. The study method complies with the regulations of the SEER database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
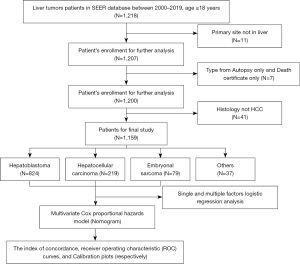
Clinical variables
Patient demographic information (age, sex, race, income), tumor characteristics (tumor grade, cancer-specific factors, tumor size, pathological type, staging), treatment (surgery, radiotherapy, chemotherapy, systemic therapy), and follow-up information (survival status, cause-specific death, survival time) were collected. Annual household income was collected and estimated in a time-dependent manner using data from the US Census American Community Survey 5-year files. The annual median household income was inflation-adjusted to 2018 US dollars and categorized into four groups: <$40,000, $40,000 to $54,999, $55,000 to $69,999, and ≥$70,000. The following exclusion criteria were applied to HCC patients in this study: (I) age >18 years old; (II) those whose tumor was not the first malignant primary indicator; (III) the type of reporting source was only autopsy and death certificate; and (IV) histologic type were not in the liver tumors types. Cases of blank variables were classified as unknown.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (SD) values and analyzed using unpaired t-tests, categorical variables were expressed as the frequencies and proportions, and the Chi-square or Fisher’s exact test was used to compare categorical variables. Survival curves were generated by the Kaplan-Meier method, and the prognostic factors were screened by stepwise regression according to the Akaike information criterion (AIC) (10). The nomogram was constructed using the multivariate Cox proportional hazards model to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), and the risk groups underwent digital quantization using the variable scores. The predictive accuracy and discriminative ability of the nomogram were determined by the concordance index (C‐index) as well as time-dependent receiver operating characteristic (ROC) curves and calibration curves. The C-index and AUC >0.7 were considered to be sufficiently discriminative. The accuracy of the nomogram was tested by calibration plots with 1000 bootstraps resamples. All analyses were conducted using R software version 4.1.1 (www.r-project.org). A two-sided P-value <0.05 was considered statistically significant.
Results
Epidemiological and clinical features of cancer types
A total of 1,159 patients listed in the SEER dataset met our eligibility criteria. We estimate that there were 824 HB, 219 HCC, and 79 ES patients from 2000 to 2019 (Table 1). There were significantly more males than females in this cohort. Also, new cases of liver tumors were almost consistent in number per year (Figure 2).
Table 1
| Characteristics | Level | Embryonal sarcoma (n=79) | Hepatoblastoma (n=824) | Hepatocellular carcinoma (n=219) | Others (n=37) | P |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 8.67 (3.85) | 1.76 (2.45) | 12.58 (4.79) | 10.32 (6.41) | <0.001 | |
| Gender (%) | Female | 43 (54.4) | 315 (38.2) | 89 (40.6) | 21 (56.8) | 0.007 |
| Male | 36 (45.6) | 509 (61.8) | 130 (59.4) | 16 (43.2) | ||
| Grade (%) | Grade I | 0 (0.0) | 28 (3.4) | 32 (14.6) | 0 (0.0) | <0.001 |
| Grade II | 0 (0.0) | 5 (0.6) | 43 (19.6) | 4 (10.8) | ||
| Grade III | 1 (1.3) | 7 (0.8) | 18 (8.2) | 7 (18.9) | ||
| Grade IV | 60 (75.9) | 15 (1.8) | 3 (1.4) | 2 (5.4) | ||
| NA | 18 (22.8) | 769 (93.3) | 123 (56.2) | 24 (64.9) | ||
| Tumor size (mm), median [IQR] | 148.00 [116.50, 181.00] | 100.00 [70.00, 120.00] | 100.00 [59.00, 145.00] | 87.00 [51.75, 112.50] | <0.001 | |
| AFP (%) | NA | 42 (53.2) | 309 (37.5) | 93 (42.5) | 22 (59.5) | <0.001 |
| Negative | 34 (43.0) | 11 (1.3) | 51 (23.3) | 9 (24.3) | ||
| Positive | 3 (3.8) | 504 (61.2) | 75 (34.2) | 6 (16.2) | ||
| Fibrosis score (%) | Moderate fibrosis | 8 (10.1) | 36 (4.4) | 19 (8.7) | 1 (2.7) | <0.001 |
| NA | 71 (89.9) | 784 (95.1) | 190 (86.8) | 35 (94.6) | ||
| Severe fibrosis | 0 (0.0) | 4 (0.5) | 10 (4.6) | 1 (2.7) | ||
| Lymph nodes surgery (%) | NA | 11 (13.9) | 124 (15.0) | 31 (14.2) | 6 (16.2) | 0.994 |
| None | 44 (55.7) | 473 (57.4) | 123 (56.2) | 21 (56.8) | ||
| Yes | 24 (30.4) | 227 (27.5) | 65 (29.7) | 10 (27.0) | ||
| Radiotherapy (%) | No | 69 (87.3) | 822 (99.8) | 203 (92.7) | 29 (78.4) | <0.001 |
| Yes | 10 (12.7) | 2 (0.2) | 16 (7.3) | 8 (21.6) | ||
| Chemotherapy (%) | No/Unknown | 5 (6.3) | 65 (7.9) | 87 (39.7) | 6 (16.2) | <0.001 |
| Yes | 74 (93.7) | 759 (92.1) | 132 (60.3) | 31 (83.8) | ||
| Systemic therapy (%) | NA | 26 (32.9) | 239 (29.0) | 61 (27.9) | 6 (16.2) | <0.001 |
| No | 4 (5.1) | 111 (13.5) | 107 (48.9) | 15 (40.5) | ||
| Yes | 49 (62.0) | 474 (57.5) | 51 (23.3) | 16 (43.2) | ||
| Race (%) | Black | 7 (8.9) | 71 (8.6) | 18 (8.2) | 6 (16.2) | 0.268 |
| Other (American Indian/AK Native, Asian/Pacific Islander) | 7 (8.9) | 125 (15.2) | 31 (14.2) | 4 (10.8) | ||
| Unknown | 2 (2.5) | 11 (1.3) | 8 (3.7) | 0 (0.0) | ||
| White | 63 (79.7) | 617 (74.9) | 162 (74.0) | 27 (73.0) | ||
| AJCC7 (%) | I | 0 (0.0) | 0 (0.0) | 17 (7.8) | 1 (3.3) | <0.001 |
| II | 0 (0.0) | 0 (0.0) | 9 (4.1) | 0 (0.0) | ||
| III | 0 (0.0) | 0 (0.0) | 10 (4.6) | 0 (0.0) | ||
| IV | 0 (0.0) | 0 (0.0) | 24 (11.0) | 2 (6.7) | ||
| NA | 55 (100.0) | 540 (100.0) | 159 (72.6) | 27 (90.0) | ||
| Survival months, median [IQR] | 79.00 [21.50, 147.50] | 66.50 [17.00, 130.25] | 33.00 [8.00, 83.50] | 13.00 [6.00, 77.00] | <0.001 | |
| Income (%) | High income | 29 (36.7) | 323 (39.2) | 94 (42.9) | 14 (37.8) | 0.97 |
| Low income | 2 (2.5) | 23 (2.8) | 7 (3.2) | 2 (5.4) | ||
| Median high income | 37 (46.8) | 352 (42.7) | 86 (39.3) | 15 (40.5) | ||
| Median low income | 11 (13.9) | 126 (15.3) | 32 (14.6) | 6 (16.2) | ||
| Surgery (%) | No | 10 (12.7) | 145 (17.6) | 86 (39.3) | 17 (45.9) | <0.001 |
| Yes | 69 (87.3) | 679 (82.4) | 133 (60.7) | 20 (54.1) | ||
| Status (%) | Alive | 67 (84.8) | 660 (80.1) | 105 (47.9) | 16 (43.2) | <0.001 |
| Dead | 12 (15.2) | 164 (19.9) | 114 (52.1) | 21 (56.8) |
SD, standard deviation; NA, not available; IQR, interquartile range; AFP, alpha fetoprotein.
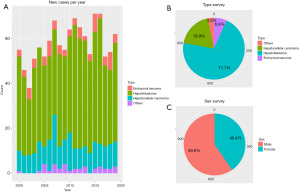
According to the follow-up information, all of the liver tumors could be divided into two groups; in the HB cohort, the median age was 1 year old, and the median survival time was 66.5 months. Patients who survived were more likely to be younger (1.63 vs. 2.30 years, P=0.002), have a smaller tumor size (100 vs. 110 mm, P=0.021), received more effective chemotherapy and systemic therapy (all P<0.001), and have undergone surgery (P<0.001). Here, survival was also related to race (P=0.009) (Table S1).
In the HCC cohort, the median age was 14 years old, and the median survival time was 33 months. Patients who survived were more likely to be younger (11.58 vs. 13.49 years, P=0.003), have a smaller tumor size (81 vs. 124.5 mm, P=0.01), received more effective chemotherapy and systemic therapy (P<0.001, P=0.02, respectively), be at an early stage (P<0.001), and have undergone surgery (P<0.001) (Table S2).
In the ES cohort, the median age was 9 years old, the median survival time was 79 months. Patients who survived were more likely to have a smaller tumor size (140 vs. 188.5 mm, P=0.047), received more effective systemic therapy (P=0.032), and have undergone surgery (P=0.005) (Table S3).
Prognostic factors distinguished by cancer types
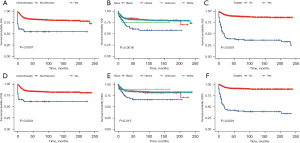
Using univariate and multivariate Cox regression analysis, the predictors of survival among patients in the three types of liver tumors were respectively assessed. According to the AIC (10), there are three factors that play a role in HB, and survival is enhanced significantly in the patients who receive surgery (HR: 0.1021, 95% CI: 0.06458–0.1614, P<0.001) and chemotherapy (HR: 0.27, 95% CI: 0.13605–0.5357, P=0.00018). Meanwhile, survival is significantly worse in Black patients (P=0.0016). In the HCC cohort, survival was markedly better in patients who had undergone surgery (HR: 0.1906, 95% CI: 0.1069–0.3399, P<0.001) but was significantly worse in the patients of an advanced stage (P=0.00061) and pathological tissue grading Ⅲ (P=0.00043). In the ES cohort, survival was notably enhanced in the patients who had undergone surgery (P=0.014) and those from a high-income family (P=0.0007). All of the prognostic factors were validated according to the overall survival (OS) and cancer-specific survival (CSS) (Figures 3-5).
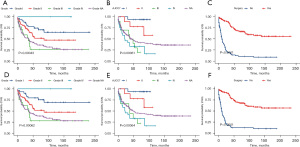
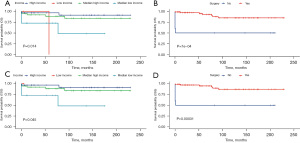
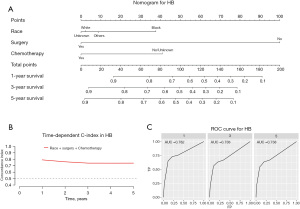
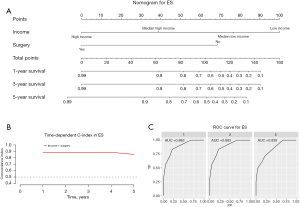
Construction and validation of a prognostic nomogram
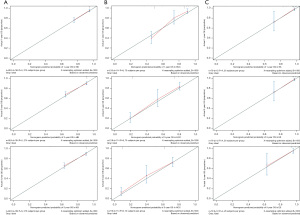
Our prognostic nomogram integrated all of the significant independent factors determined by the multivariate analyses mentioned above. In the HB cohort, The C‐index of the nomogram was 0.747 (95% CI: 0.70584–0.78816), and the nomogram achieved time-dependent ROC-AUCs of 0.782, 0.738, and 0.738 for the prediction of progression risks at 1, 3, and 5 years, respectively (Figure 6). In the HCC cohort, the C‐index of the nomogram was 0.775 (95% CI: 0.73972–0.81028), and the nomogram achieved time-dependent ROC-AUCs of 0.83, 0.821, and 0.812 for the prediction of progression risks at 1, 3, and 5 years, respectively (Figure 7). In the ES cohort, the C‐index of the nomogram was 0.828 (95% CI: 0.70648–0.94952), and the nomogram achieved time-dependent ROC-AUCs of 0.883, 0.883, and 0.839 for the prediction of progression risks at 1, 3, and 5 years, respectively (Figure 8). Moreover, the excellent accuracy of the nomogram’s prediction value was also assessed by the calibration curves at 1, 3, and 5 years of survival, and an optimal consistency between the nomogram-predicted and actual observed values was evident in all three cohorts (Figure 9).

Discussion
Primary liver tumors comprise 1–2% of all pediatric tumors, which can make research delays problematic. An accurate and effective prognostic prediction for cancer patients is very important for clinical treatment and guideline formulation. Herein, we constructed an accurate nomogram based on a large retrospective case series to predict the OS children and adolescents with liver tumors. This novel nomogram provides an important quantitative indicator and reference for clinical decision-making and the management of treatment regimens.
In our cohort, it was obvious that HB was the most common primary liver tumor, accounting for 71.1% of primary hepatic malignancies in children and adolescents. Surgery, chemotherapy, and race were identified as independent prognostic factors in patients with HB. The social factor (race) is an independent prognostic factor for HB. Similar findings were also noted in a previous study conducted in the USA (11). Black patients tend to have poor outcomes. Surgery remains the mainstay of treatment for HB, and complete resection is the only way to achieve a cure (12). The 5-year OS rate for HB is as high as 91% for patients who receive partial hepatectomy (13). However, 40–60% of HB patients are considered inoperable (14). Chemotherapy is a well-known and effective treatment for various kinds of cancers, and platinum-based chemotherapy has provided a foundation for the current management of HB (15). A study has applied a policy of selective preoperative chemotherapy, and 90% of HB are resectable (16).
HCC is the seventh most frequent cancer among men and women (17) and the second most common malignant liver tumor in children. The spectrum of background liver disease predisposing to HCC in children is different from that in adults; tyrosinemia and perinatally acquired hepatitis B virus (HBV) infection are two major prognostic factors for HCC in children (2). Meanwhile, cirrhosis is one of the most important pathologies of HCC in adults but is absent in 26–62% of childhood cases (18). Surgery is an effective treatment for HCC; a previous report demonstrated that the resection rates in pediatric HCC have improved to 40%, with a median survival time of more than 30 months (19). Liver transplantation is a promising clinical treatment, with a 5-year OS rate of 72–83% (20). Histological grade is a significant predictor of survival in the prognostic evaluation of HCC patients treated with liver translation and liver resection (21). There are significant differences in terms of the histological grades, with a higher histological grade signifying a better OS. It is well known that staging influences mortality but there is currently no uniformly accepted staging system for HCC in children, despite the Barcelona Clinic Liver Cancer score and the tumor node metastasis (TNM) staging system being the main staging systems in HCC.
ES is the third most common type of malignant liver tumor in children and adolescents, which is a rare neoplasm that accounts for 9–15% of pediatric liver malignancies (3). Shi et al. reported an OS of 86% in ES; however, for patients who had undergone surgical resection alone, this rate was 100%, which is a promising approach for ES patients (22). An increasing number of scholars have reported that household income is an independent prognostic factor in various tumors in children and adolescents (23-25). To our knowledge, this study is the first to demonstrate that household income is an independent prognostic factor in patients with ES.
We then integrated the prognostic factors into a nomogram to predict the 1, 3, and 5 years OS. Nomograms are effective and convenient statistical tools that incorporate all prognostic variables and have been generated for a variety of cancer types (26-28). Our nomogram performed well according to the C-index and time-dependent ROC-AUCs. Also, the calibration curves were closely matched to the ideal standard line, which indicated that the nomogram had high predictive power. The main reason for this is that the accuracy of prognostic prediction decreased regardless of the patient’s background and other clinical pathological characteristics (29).
There are several limitations in this study that should be noted. Firstly, this study is a retrospective analysis; therefore, the applicability of the nomogram has not been validated in a separate cohort or at others institutions. Secondly, the critical inclusion and exclusion criteria may have resulted in significant amounts of valuable data being missed. Also, as has been demonstrated in previous studies, the SEER dataset excludes a considerable amount of data relating to several important clinical variables, which contributes to the absence of several important variables in the system, introducing considerable bias (30). For example, it is well known that the American Joint Cancer Committee (AJCC) tumor-node-metastasis (TNM) staging system is commonly used for tumor classification. However, PRETEXT is the only staging system that allows for surgical planning at the time of presentation for HB (31). Finally, multicenter prospective studies are needed to confirm or improve the accuracy of our nomogram.
Conclusions
In summary, this comprehensive analysis of liver tumors in children and adolescents from 2000 to 2019 based on the SEER cancer database showed a continued overall plateau in the incidence of the three main types of malignant liver tumors among children and adolescents. Race, household income, surgery, histological grade, staging, and chemotherapy are independent prognostic factors for the OS of liver tumors patients. Despite the limitations of this study, the nomogram based on these factors presented superior accuracy and applicability in predicting the clinical outcomes of HB, HCC, and ES patients, which could assist in the optimization of clinical decision-making.
Acknowledgments
We sincerely appreciate the statistical and R language assistance from Dr. Jianming Zeng (Faculty of Health Sciences, University of Macau, Taipa, Macau, China).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-679/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-679/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-679/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moreno F, Rose A, Chaplin MA, et al. Childhood liver tumors in Argentina: Incidence trend and survival by treatment center. A report from the national pediatric cancer registry, ROHA network 2000-2015. Pediatr Blood Cancer 2020;67:e28583. [Crossref] [PubMed]
- Kelly D, Sharif K, Brown RM, et al. Hepatocellular carcinoma in children. Clin Liver Dis 2015;19:433-47. [Crossref] [PubMed]
- Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer 1978;42:336-48. [Crossref] [PubMed]
- Weinberg AG, Finegold MJ. Primary hepatic tumors of childhood. Hum Pathol 1983;14:512-37. [Crossref] [PubMed]
- Kahla JA, Siegel DA, Dai S, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer 2022;69:e29763. [Crossref] [PubMed]
- Sergi CM. Carcinoma of the Liver in Children and Adolescents. In: Sergi CM, editor. Liver Cancer. Brisbane (AU): Exon Publications Copyright: The Authors.; 2021.
- Techavichit P, Masand PM, Himes RW, et al. Undifferentiated Embryonal Sarcoma of the Liver (UESL): A Single-Center Experience and Review of the Literature. J Pediatr Hematol Oncol 2016;38:261-8. [Crossref] [PubMed]
- Wu X, Niu Z, Zhu Y, et al. Peripheral biomarkers to predict the diagnosis of bipolar disorder from major depressive disorder in adolescents. Eur Arch Psychiatry Clin Neurosci 2022;272:817-26. [Crossref] [PubMed]
- Lacomba-Trejo L, Valero-Moreno S, Montoya-Castilla I, et al. Predicting health-related quality of life in Spanish adolescents with allergic rhinoconjunctivitis and bronchial asthma. Psychol Health Med 2022;27:613-25. [Crossref] [PubMed]
- Sakamoto Y, Ishiguro M, Kitagawa G. Akaike information criterion statistics. Dordrecht, The Netherlands: D Reidel 1986;81:26853.
- Janitz AE, Ramachandran G, Tomlinson GE, et al. Maternal and paternal occupational exposures and hepatoblastoma: results from the HOPE study through the Children's Oncology Group. J Expo Sci Environ Epidemiol 2017;27:359-64. [Crossref] [PubMed]
- Malogolowkin MH, Katzenstein HM, Meyers RL, et al. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol 2011;29:3301-6. [Crossref] [PubMed]
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration. Lancet Oncol 2017;18:122-31. [Crossref] [PubMed]
- Stocker JT. Hepatic tumors in children. Clin Liver Dis 2001;5:259-81. viii-ix. [Crossref] [PubMed]
- Czauderna P, Haeberle B, Hiyama E, et al. The Children's Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer 2016;52:92-101. [Crossref] [PubMed]
- Stringer MD, Hennayake S, Howard ER, et al. Improved outcome for children with hepatoblastoma. Br J Surg 1995;82:386-91. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Moore SW, Millar AJ, Hadley GP, et al. Hepatocellular carcinoma and liver tumors in South African children: a case for increased prevalence. Cancer 2004;101:642-9. [Crossref] [PubMed]
- Wang J, Mao Y, Liu Y, et al. Hepatocellular Carcinoma in Children and Adolescents: Clinical Characteristics and Treatment. J Gastrointest Surg 2017;21:1128-35. [Crossref] [PubMed]
- Beaunoyer M, Vanatta JM, Ogihara M, et al. Outcomes of transplantation in children with primary hepatic malignancy. Pediatr Transplant 2007;11:655-60. [Crossref] [PubMed]
- Martins-Filho SN, Paiva C, Azevedo RS, et al. Histological Grading of Hepatocellular Carcinoma-A Systematic Review of Literature. Front Med (Lausanne) 2017;4:193. [Crossref] [PubMed]
- Shi Y, Rojas Y, Zhang W, et al. Characteristics and outcomes in children with undifferentiated embryonal sarcoma of the liver: A report from the National Cancer Database. Pediatr Blood Cancer 2017;64:e26272. [Crossref] [PubMed]
- Bona K, London WB, Guo D, et al. Trajectory of Material Hardship and Income Poverty in Families of Children Undergoing Chemotherapy: A Prospective Cohort Study. Pediatr Blood Cancer 2016;63:105-11. [Crossref] [PubMed]
- Sharma M, Sonig A, Ambekar S, et al. Discharge dispositions, complications, and costs of hospitalization in spinal cord tumor surgery: analysis of data from the United States Nationwide Inpatient Sample, 2003-2010. J Neurosurg Spine 2014;20:125-41. [Crossref] [PubMed]
- Wong RJ, Kim D, Ahmed A, et al. Patients with hepatocellular carcinoma from more rural and lower-income households have more advanced tumor stage at diagnosis and significantly higher mortality. Cancer 2020;127:45-55. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Wang R, Dai W, Gong J, et al. Development of a novel combined nomogram model integrating deep learning-pathomics, radiomics and immunoscore to predict postoperative outcome of colorectal cancer lung metastasis patients. J Hematol Oncol 2022;15:11. [Crossref] [PubMed]
- Zaorsky NG, Wang X, Garrett SM, et al. Pan-cancer analysis of prognostic metastatic phenotypes. Int J Cancer 2022;150:132-41. [Crossref] [PubMed]
- Chun YH, Kim SU, Park JY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer 2011;47:2568-75.
- Jeong CW, Washington SL 3rd, Herlemann A, et al. The New Surveillance, Epidemiology, and End Results Prostate with Watchful Waiting Database: Opportunities and Limitations. Eur Urol 2020;78:335-44. [Crossref] [PubMed]
- Roebuck DJ, Aronson D, Clapuyt P, et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol 2007;37:123-32; quiz 249-50. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


