
The upregulation of peripheral blood polyamine metabolites spermidine and spermine in children with hand, foot, mouth disease is related to enterovirus 71 capsid protein VP1, but not VP4
Highlight box
Key findings
• In the study, we found that serum polyamine metabolites spermidine (SPD) and spermine (SPM) were elevated in the hand, foot, and mouth disease (HFMD) patients, especially the enterovirus 71 (EV71)-infected children. We also found that the upregulation of peripheral blood metabolites SPD and SPM in children with HFMD was related to the EV71 capsid protein VP1, but not VP4.
What is known and what is new?
• Polyamines are known to be compounds that are ubiquitous in mammalian cells and play a key role in various cellular processes. Several studies have shown that targeting polyamine metabolic pathways can reduce infections caused by viruses.
• However, the significance of polyamine metabolites, such as SPD and SPM, in EV71 infection remains largely unknown. The study adds to the discovery that SPD and SPM may be involved in EV71-induced inflammatory pathogenesis of HFMD, and the upregulation of peripheral blood polyamine metabolites in EV71-infected HFMD children is related to EV71 capsid protein VP1, but not VP4.
What is the implication, and what should change now?
• This study provides insights into the mechanism of EV71 infection and polyamine metabolism and has good reference value for the development of EV71 vaccine.
Introduction
Hand, foot, and mouth disease (HFMD) is a common viral infectious disease that is mainly caused by human enterovirus 71 (EV71) and coxsackievirus A6 (CV-A6), usually occurs in infants and preschool children (aged <5 years), and can cause mild fever, oral ulcers, benign skin lesions, and cardiopulmonary symptoms (1,2). EV71-infected HFMD is characterized by severe central nervous system complications, such as encephalitis, aseptic meningitis, acute flaccid paralysis, and even death (3). It is well known that HFMD is closely related to pro-inflammatory cytokines and anti-inflammatory cytokines (4). A number of studies have reported that interleukin-6 (IL-6), IL-10, and tumor necrosis factor alpha (TNF-α) increase with the severity of HFMD (5-7). Notably, IL-6 is closely related to the severity and pathophysiology of EV71-infected HFMD (4). The levels of IL-6 in EV71-infected patients with HFMD and aseptic meningitis are elevated, which suggests that IL-6 could be used as a diagnostic indicator for aseptic meningitis (8).
EV71 is a single-stranded ribonucleic acid (RNA) virus that contains 3 surface capsid proteins (i.e., VP1, VP2, and VP3), and an internal protein VP4. VP1 plays a key role in virulence and is usually used for virus identification and vaccine targets (9). EV71 VP1 is synthesized during productive infections and may interact closely with specific cellular proteins to cause cytopathic effects and ultimately neurological complications such as acute delayed paralysis and encephalitis. According to reports, VP1 alone can induce a strong cell-mediated immune response (10), while VP4 has a lesser effect on infection but can maintain the stability of the EV71 capsid (11). More importantly, research has shown that the VP4 core sequence induced a cross-protective antibody response in newborn mice (11). However, the pathogenesis of EV71 capsid proteins VP1 and VP4 induced HFMD remains largely unknown.
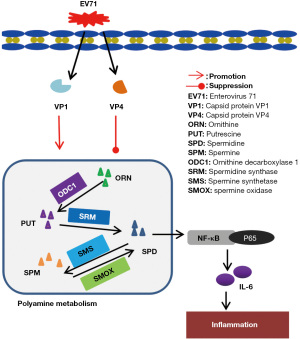
Polyamines, especially spermidine (SPD) and spermine (SPM), are ubiquitous in mammalian cells and play an important role in cell proliferation, cell signal transduction, and protein synthesis (12). The metabolic pathway of polyamines includes ornithine decarboxylase 1 (ODC1), which converts ornithine into putrescine (PUT), which in turn synthesizes SPD under the action of spermidine synthase (SRM) (13). Spermine synthase can convert SPD into SPM. Under the action of spermidine/spermine acetyltransferase, spermine oxidase (SMOX) and polyamine oxidase, SPM is further converted into SPD and PUT (14). In recent years, the role of SPD and SPM in cancer, cardiovascular and cerebrovascular diseases, inflammation, aging, and neurological diseases has been fully demonstrated (13,15-17).
Polyamines have also been reported to play a role in viral infections. Targeting polyamine metabolic pathways may be a potential method to control human viral infections (14). For example, inhibition of polyamine biosynthesis by difluoromethylornithine (DFMO) affected viral protease activities (2A and 3C) and promoted the cleavage of eIF4G during viral infection, thereby inhibiting the replication of Coxsackie virus B3 (CVB3). In addition, Mounce et al. showed that reducing SPD and SPM by upregulating spermidine/spermine N1-acetyltransferase 1 (SAT1) can limit the replication of the Chikungunya and Zika viruses, which suggests that polyamines are essential for viral infection in vitro (18). Research has shown that hepatitis C virus in human hepatoma Huh7 cells inhibits ODC1 and SAT1 levels and increases SOMX levels, resulting in a decrease in the concentration of the biological polyamines SPD and SPM (19).
However, the significance of polyamine metabolites, such as SPD and SPM, in EV71 infection remains largely unknown. Different from previous studies, our study aims to explore the role and mechanism of polyamine metabolites in EV71 infection. In this study, we found that the serum polyamine metabolites SPD and SPM were elevated in the HFMD patients, especially the EV71-infected children. We also found that the upregulation of peripheral blood metabolites SPD and SPM in children with HFMD was related to EV71 capsid protein VP1, but not VP4. We present the following article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-41/rc).
Methods
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Internal Review and Ethics Boards of Dongguan Maternal and Child Health Care Hospital (No. 2015-8) and Guangzhou First People’s Hospital (No. GFPH0787), and informed consent was obtained from the parents of each of the enrolled children.
Subjects
A total of 82 children with HFMD and 70 healthy volunteers (HVs) admitted to Guangzhou First People’s Hospital and Dongguan Maternal and Child Health Care Hospital between October 2016 and July 2017 were included in this study. We adopted the diagnostic criteria for HFMD disease with EV71 infection set out in the Chinese guidelines for the diagnosis and treatment of HFMD (2018 edition) (20). Reverse transcription polymerase chain reaction (RT-PCR) was used to conduct the stool test to determine if the participants were positive for the EV71 virus as previously reported (6,21). The demographic and clinical characteristics of all the study participants are set out in Table 1.
Table 1
| Groups | HFMD patients | HV (n=70) | |
|---|---|---|---|
| EV71 (n=25) | Un-EV71 (n=57) | ||
| Female/male | 11/14 | 24/33 | 34/36 |
| Age (years), mean ± SD | 3.61±1.48 | 2.34±1.29 | 3.52±1.08 |
| Maximum body temperature (℃), mean ± SD | 39.11±0.73 | 39.01±0.70 | – |
| Vomiting, n (%) | 9 (36.00) | 21 (36.84) | – |
| Skin rash, n (%) | 23 (92.00) | 54 (94.74) | – |
| Oral ulcer, n (%) | 24 (96.00) | 55 (96.49) | – |
| WBC (×109/L), mean ± SD | 10.60±2.73 | 10.43±3.20 | – |
HFMD, hand, foot, and mouth disease; EV71, enterovirus 71; HV, healthy volunteers; SD, standard deviation; WBC, white blood cell.
Determination of serum polyamine metabolites
The serum samples were collected from venous blood at room temperature and stored at –80 ℃ for later use. Polyamine standards were purchased from Sigma-Aldrich, USA. We referred to and improved the experimental procedures of Wang et al. and Ducros et al. (22,23). First, the protein of the serum sample was precipitated with 5% perchloric acid. The sample was then vortexed, mixed, and centrifuged at 4 ℃ for 10 min at 12,000 rpm, and the supernatant was collected for derivatization. Derivatized dansyl chloride (Dns-Cl) (5 mg/mL acetonitrile solution) (Sigma-Aldrich, USA) was used for the derivatization in a 60 ℃ water bath for 45 minutes. The excess Dns-Cl was removed with ammonium hydroxide and left to cool at room temperature. It was extracted with chloroform, and the organic phase was evaporated to dryness at 40 ℃ under a gentle stream of nitrogen. Finally, the residue was dissolved in 500 µL of acetonitrile, filtered through a 0.22-µm filter membrane, and detected by reversed-phase high performance liquid chromatography (RP-HPLC) (LC-20AT, Shimadzu, JPN). The mobile phase was A: ultra-pure water and B: HPLC-grade acetonitrile. Gradient elution was selected at 60–80%B for 0–3 min, 80–95%B for 3–12 min, 95–95%B for 12–21 min, 95–60%B for 21–22 min, and 60%B for 22–25 min. The flow rate was 1.0 mL/min, and the fluorescence detection wavelengths were set at 340 and 510 nm.
Cytokine assays
The human IL-6 enzyme-linked immunosorbent assay kit (Ray Biotech, Inc.) was used to measure IL-6 levels in the serum and culture supernatant in accordance with the manufacturer’s instructions (6).
Peripheral blood mononuclear cells (PBMCs) isolation and cell culture
The PBMCs were separated by density gradient centrifugation through Ficoll-Paque (TBD, Shanghai), retrieved, and cultured in Roswell Park Memorial Institute medium 1640 containing 10% fetal bovine serum, 1% penicillin, and streptomycin in 6-well tissue culture dishes at 37 ℃ in a 5% carbon dioxide atmosphere. The PBMCs were then treated with EV71 VP1 (2.5 µg/mL, provided by Nansha Center for Disease Control and Prevention) or 2.5 µg/mL, and 5 µg/mL EV71 VP4 (Bioss, Beijing) dissolved with phosphate buffered saline (pH =7.4). THP-1 was induced to adhere to the wall with PMA (100 ng/mL) for 4 hours before treatment in vitro, and VP1 and VP4 were treated as described above. After 24 hours of incubation, the cells and supernatant were collected for further analysis.
Western blot analysis
The cells were lysed and homogenized using radioimmunoprecipitation assay buffer (Cell Signaling Technology), and the protein concentration was measured by an Enhanced Bicinchoninic acid Protein Assay Kit (Beyotime). Equal amounts of protein extracts were separated by electrophoresis on a 12% NuPAGE Bis-Tris Mini Gel (Invitrogen) and then transferred to nitrocellulose membranes (GE Healthcare) (24). The membranes were blocked for 1 hour in Tris-buffered saline containing 5 % bovine serum albumin and then probed with antibodies. Antibodies against SRM (Cat#: 19858), ODC1 (Cat#: 17003), SMOX (Cat#: 15052), iNOS (Cat#: 18985), IL-6 (Cat#: 21865), and GAPDH (Cat#: 60004) were purchased from Proteintech, and nuclear factor kappa B (NF-κB) p65 (Cat#: 8242S) was purchased from Cell Signaling Technology. The membranes were then probed with secondary antibodies conjugated to horseradish peroxidase. Next, the immunoblots were developed as recommended in the ECL Advance Western blot detection kit (Amersham Biosciences).
Statistical analysis
The data from at least 3 independent experiments were analyzed using GraphPad Prism 7.0 software (USA). The Student’s t-test was used to determine statistical differences for the 2-group comparisons and a 1-way analysis of variance was used for the multiple comparisons. The correlations were evaluated using Spearman’s rank correlation test. A P value <0.05 was considered statistically significant.
Results
Serum SPD and SPM were elevated in the HFMD patients, especially the EV71-infected children
Polyamines, including putrescine, SPD, and SPM, are small positively charged amine molecules in mammalian cells. Studies have reported that the replication of the EV71 virus in host cells relies on polyamines (14,25). However, little is known about the level of polyamines in the peripheral blood of EV71-infected HFMD patients, and whether it is related to EV71 infection.
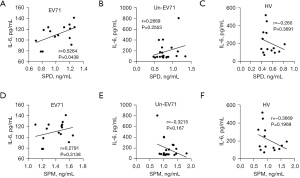
We used RP-HPLC to detect the levels of the polyamine metabolites SPD and SPM in the peripheral blood of the EV71-infected and uncertain or non-EV71 (un-EV71)-infected HFMD patients (n=82) and healthy individual volunteers (HVs) (n=70). The results showed that the serum SPD and SPM levels of the HFMD patients were significantly higher than those of the HVs (P<0.0001; Figure 1A,1B). Moreover, a strong positive correlation was found between the SPD and SPM levels in the HFMD patients and HVs (Figure 1C,1D).

Based on the fecal EV71 test results, the HFMD patients were divided into the EV71-infected HFMD group and the un-EV71-infected HFMD group. The results showed that the serum SPD and SPM levels of the EV71-infected HFMD patients were significantly higher than those of the un-EV71-infected HFMD patients (Figure 1E,1F). In addition, a strong correlation was found between the levels of SPD and SPM in the EV71-infected HFMD patients (Figure 1G), but not in the un-EV71-infected HFMD patients (Figure 1H). These results suggest that the upregulated polyamine metabolites in the peripheral blood of the EV71-infected HFMD patients was related to EV71 infection.
Serum SPD was positively correlated with IL-6 in the EV71-infected HFMD children
IL-6 is produced rapidly during infection and tissue damage and promotes the host’s defenses by stimulating acute phase reactions, and hematopoietic and immune responses. We previously showed that the serum IL-6 level of EV71-infected patients was significantly higher than that of un-EV71-infected patients or HVs and was associated with the pathogenesis of EV71 infection (6). In this study, we further examined the relationship between serum IL-6 and polyamine metabolites in EV71-infected patients, un-EV71-infected patients, and HVs and found that IL-6 and SPD were positively correlated in the EV71-infected patients, but not in un-EV71-infected patients or HVs (Figure 2). However, we did not observe any correlation between IL-6 and SPM among the EV71-infected patients, un-EV71-infected patients, or HVs (Figure 2). These results suggest that the rise of IL-6 caused by EV71 infection may be related to SPD.
The upregulation of SPD and SPM in the EV71-infected HFMD children was related to EV71 capsid protein VP1, but not VP4
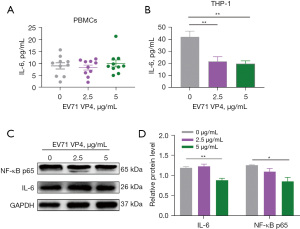
Several studies have shown that targeting polyamine metabolic pathways can reduce infections caused by viruses (14,26). The above results suggested that EV71 infection could also affect the level of peripheral blood polyamine metabolites. To further examine how EV71 infection regulates polyamine metabolism, we used the EV71 capsid proteins VP1 and VP4 to stimulate the PBMCs and PMA-induced THP-1 cells, and then detected the expression of polyamine metabolism-related enzymes and the production of polyamine metabolites. We found that VP1 upregulated and promoted the production of both SPD and SPM in the PBMC (Figure 3A) and THP-1 (Figure 3B) cells. We also found that VP1 significantly upregulated the expression of SRM, ODC1, SMOX, and iNOS in the THP-1 cells (Figure 3C). Notably, after VP4 stimulation, the production of SPD and SPM in the PBMC (Figure 4A) and THP-1 (Figure 4B) cells was reduced, which is opposite to the result of VP1 stimulation (Figure 3A,3B).
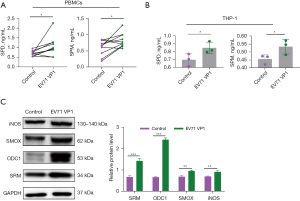

VP1 is the major virulence determinant of EV71 responsible for virus genotyping and virus entry (27,28). VP4 is essential for infectivity and interacts with the membrane structure of the endocytic vesicle, resulting in the breaking of that vesicle (29). Notably, VP4 upregulated the expression of SMOX and iNOS, but downregulated the expression of SRM and ODC1 in the THP-1 cells (Figure 4C). These results suggest that the upregulation of peripheral blood polyamine metabolites in EV71-infected patients is related to the EV71 capsid protein VP1, but not VP4.
VP1 promoted and VP4 inhibited the SPD/NF-кB/IL-6 signaling pathway
The SPM oxidase SMOX catalyzes the oxidation of SPM to generate SPD, hydrogen peroxide, and 3-aminopropanal. The above results indicated that both VP1 and VP4 upregulated the expression of SMOX (Figures 3C,4C), and a positive correlation between SPD and IL-6 was found in the EV71-infected patients (Figure 2). Choi et al. showed that SPD treatment attenuated the production of IL-6, and appeared to involve the suppression of translocation of NF-κB p65 subunit into the nucleus in lipopolysaccharide-stimulated murine BV2 microglia cells (30). Morón et al. found that SPD treatment alone did not increase the level of IL-6 in THP-1 cells, but SPD did upregulate the level of IL-6 in THP-1 cells in an interferon-gamma (IFN-γ)–dependent manner (31). Several studies have shown that VP1 stimulation upregulates the expression of IFN-γ in host cells (10,32).
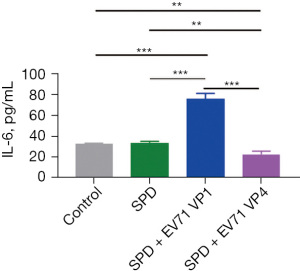
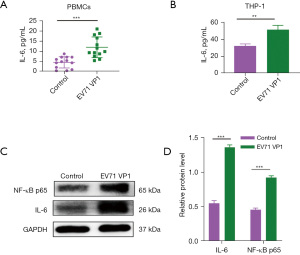
We also found that SPD treatment did not increase the level of IL-6 in host cells (Figure 5). However, in the SPD combined with VP1 and VP1 alone treatment groups, the level of IL-6 in the host cells was significantly increased, while in the SPD combined with VP4 and VP4 alone treatment groups, the level of IL-6 in the host cells was significantly decreased (Figures 5-7). Research has shown that the expression of VP1 and IL-6 in host cells increases simultaneously after EV71 infection (33-35). Our results also showed that VP1 treatment promoted the production of IL-6 in the PBMC and THP-1 cells (Figure 6A,6B), and upregulated the expression of NF-κB p65 in the THP-1 cells (Figure 6C). These results suggest that VP4 treatment inhibited the production of SPD and SPM in the PBMC and THP-1 cells (Figure 4). Notably, VP4 did not affect the production of IL-6 in PBMC cells (Figure 7A), but it did downregulate the expression of IL-6 and NF-κB p65 in the THP-1 cells (Figure 7B,7C). These results suggest that VP1 promotes and VP4 inhibits the SPD/NF-кB/IL-6 signaling pathway, thereby initiating the inflammatory response regulated by polyamine metabolism after EV71 infection.
Discussion
This study investigated the levels of polyamine metabolites in children with HFMD caused by EV71 infection. During the analysis, significant changes were observed in the metabolic profile, especially the metabolites SPD and SPM. Notably, the levels of the serum polyamines (SPD and SPM) in patients with HFMD were elevated, especially in patients with EV71 infection, which suggests that polyamines (SPD and SPM) may be related to EV71 virus infection. We also found that in the EV71 group, the IL-6 levels were related to the SPD levels, but not the SPM levels. This suggests that serum SPD and IL-6 could serve as disease monitoring indicators for the EV71 infection of HFMD.
Previously, Els et al. reported that SPD levels in the blood of patients with focal cerebral ischemia are elevated (36). In addition, SPD has been shown to be correlated with clinical results in the first 48 hours and infarct volume on days 4 to 6 (36). Interestingly, exogenous polyamines or polyamine inhibitors can affect a range of physiological activities. For example, research showed that the pretreatment of SPD or SPM significantly inhibited the apoptosis of the ischemia/reperfusion cells that restrained the expression of caspase-3 and caspase-9, suppressed the release of cytochrome c, upregulated the expression of Bcl-2, and decreased [Ca2+] (37). In a hypertension-induced congestive heart failure model, SPD feeding decreased blood pressure, increased titin phosphorylation, and prevented cardiac hypertrophy, which in turn may have delayed the progression of heart failure (38).
In human colon carcinoma COLO 320 cells, polyamine depletion led to a >90% decrease in the expression of a key gene, c-myc proto-oncogene, which maintains cell growth (39). Further, ODC1, a key enzyme of polyamines, is important for tumor progression. The overexpression of ODC1 profoundly interferes with skin homeostasis and can lead to tumor development in mice (40-43). Nilsson et al. (44) inhibited ODC1 by difluoromethylornithine (DFMO) affected Myc-mediated proliferation during tumorigenesis and markedly delayed the development of lymphoma, which suggests that ODC1 could be an effective target for cancer chemoprevention.
In the current study, we found that SPD and SPM are highly expressed in HFMD, especially after EV71 infection, which indicates that the polyamine metabolism mechanism of the EV71-infected HFMD patients is different to that of other non-EV71-infected HFMD patients or healthy individuals. Thus, polyamines could serve as novel target molecules for the prevention and diagnosis of HFMD.
Further, it has been reported that serum IL-6 levels are increased in patients with severe HFMD with comorbidities but are decreased in patients with mild HFMD and during the HFMD recovery period (5-8,45,46). To clarify the relationship between disease severity and polyamine metabolism, we further tested the correlation between IL-6 concentration and polyamine levels. Our results indicate that the level of IL-6 is related to the level of SPD in EV71-infected patients with HFMD.
In addition, the expression levels of polyamine metabolizing enzymes (iNOS, ODC1, SRM, and SMOX) are increased after EV71 VP1 stimulation, which leads to increased SPD levels. However, the results after EV71 VP4 stimulation were opposite to those after VP1 stimulation; that is, the level of SPD and SPM and the expression of ODC1 and SRM decreased. In summary, it can be inferred that EV71 VP1 enhances SPD and SPM and their metabolic enzymes due to the main effect of its virulence protein, while the differential effect of VP4 is mainly due to its distribution in the location of the virus, which may not be related to virulence.
Using EV71 VP1 to enhance the expression of SRM and SMOX, we found that the level of SPD increased, and the expression of NF-кB/p65 and IL-6 increased. It is well known that NF-кB/p65 is one of the main modulators regulating IL-6 secretion (47). Additionally, EV71 infection causes the activation of NF-кB, which then leads to viral replication and the secretion of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 (48-51). Blocking the NF-кB pathway effectively inhibited EV71 replication and cytokine production (35,51). Conversely, polyamines affect cell growth and death through the signal transduction pathway of NF-Кb (52).
A study reported that oxidative stress in human liver cancer HUH7 cells activated NF-кB, which in turn upregulated ODC1 and SAT1, leading to increased polyamine levels (53). In diabetic ischemia reperfusion injury, the expression of SAT1 and NF-кB and the level of SPD was increased (54), which indicated a connection between polyamines (especially SPD) and NF-кB. It was reported years ago that ODC1 messenger RNA was accumulated and polyamine levels were elevated in TNF-ɑ-activated macrophages (55). After adding ODC1 inhibitors, DFMO, and polyamine inhibitors, methylglyoxal bis (guanylhydrazone) (MGBG) hindered the activation of macrophages. Notably, the addition of exogenous polyamines reversed the inhibitory effect of MGBG. EV71 VP4 has diverse effects; however, we are still of the view that EV71 infection may result in an increase of polyamines (SPD and SPM), which in turn activates the NF-кB/p65 pathway to promote the secretion of IL-6, leading to inflammation.
Our study had some limitations. First, we can only speculate that the metabolic changes of polyamine metabolites in children with HFMD can be used as disease monitoring indicators, and specific diagnostic conditions need to be explored. Second, this study separately explored the effects of VP1 and VP4 on EV71, but this limited analysis cannot represent EV71 entirely. However, our results are insightful, as they indicate that VP1 and VP4 may play completely opposite roles in the EV71 virus, and provide a theoretical basis for follow-up research. Further research should be conducted to evaluate the function of polyamines in larger sample sizes or in vitro experiments.
Conclusions
Our findings suggest that SPD and SPM may be involved in the inflammatory pathogenesis of HFMD induced by EV71, and serum SPD and SPM levels may be important diagnostic indicators. Therefore, we believe that SPD and SPM may be candidate targets for the treatment of hand, foot and mouth disease. We also showed that the upregulation of peripheral blood polyamine metabolites in EV71-infected HFMD children is related to EV71 capsid protein VP1, but not VP4. VP1 may promote the expression of polyamine metabolism-related enzymes and promote the production of polyamine metabolites, thereby upregulating the SPD/NF-кB/IL-16 signaling pathway (Figure 8). However, VP4 has the opposite effect in this process (Figure 8).
Acknowledgments
Funding: This study was supported by grants from the Natural Science Foundation of Guangdong Province (Nos. 2021A1515012054 and 2021B1515140066), Guangzhou Health Technology Project (No. 20201A010009), the Characteristic Innovation Experimental Project of Ordinary Universities in Guangdong Province (No. 2020KTSCX044), the Discipline Construction Project of Guangdong Medical University (No. 4SG21278P), the Medical Science Foundation of Guangdong Province (Nos. A2020425 and A2021438), the Guangdong Medical University Student Innovation Experimental Project (No. 202110571012), and the Guangdong Province Science and Technology Innovation Strategy Special Fund (No. pdjh2021b0222).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-41/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-41/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-41/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-41/coif). JZ reports that since the initial planning of the work, this study was supported by grants from the Natural Science Foundation of Guangdong Province, Guangzhou Health Technology Project, the Characteristic Innovation Experimental Project of Ordinary Universities in Guangdong Province, the Discipline Construction Project of Guangdong Medical University, the Medical Science Foundation of Guangdong Province, the Guangdong Medical University Student Innovation Experimental Project, and the Guangdong Province Science and Technology Innovation Strategy Special Fund. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Internal Review and Ethics Boards of Dongguan Maternal and Child Health Care Hospital (No. 2015-8) and Guangzhou First People’s Hospital (No. GFPH0787), and informed consent was obtained from the parents of each of the enrolled children.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cox JA, Hiscox JA, Solomon T, et al. Immunopathogenesis and Virus-Host Interactions of Enterovirus 71 in Patients with Hand, Foot and Mouth Disease. Front Microbiol 2017;8:2249. [Crossref] [PubMed]
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr 2022;11:1502-9. [Crossref] [PubMed]
- Griffiths MJ, Ooi MH, Wong SC, et al. In enterovirus 71 encephalitis with cardio-respiratory compromise, elevated interleukin 1β, interleukin 1 receptor antagonist, and granulocyte colony-stimulating factor levels are markers of poor prognosis. J Infect Dis 2012;206:881-92. [Crossref] [PubMed]
- Zhang W, Huang Z, Huang M, et al. Predicting Severe Enterovirus 71-Infected Hand, Foot, and Mouth Disease: Cytokines and Chemokines. Mediators Inflamm 2020;2020:9273241. [Crossref] [PubMed]
- Duan G, Yang H, Shi L, et al. Serum inflammatory cytokine levels correlate with hand-foot-mouth disease severity: a nested serial case-control study. PLoS One 2014;9:e112676. [Crossref] [PubMed]
- Chen Z, Li R, Xie Z, et al. IL-6, IL-10 and IL-13 are associated with pathogenesis in children with Enterovirus 71 infection. Int J Clin Exp Med 2014;7:2718-23.
- Sun JF, Li HL, Sun BX. Correlation analysis on serum inflammatory cytokine level and neurogenic pulmonary edema for children with severe hand-foot-mouth disease. Eur J Med Res 2018;23:21. [Crossref] [PubMed]
- Lee JY, Son M, Kang JH, et al. Serum interleukin-6 levels as an indicator of aseptic meningitis among children with enterovirus 71-induced hand, foot and mouth disease. Postgrad Med 2018;130:258-63. [Crossref] [PubMed]
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 2002;26:91-107. [Crossref] [PubMed]
- Issaro N, Wu F, Weng L, et al. Induction of immune responses by a novel recombinant fusion protein of enterovirus A71 in BALB/c mice. Mol Immunol 2019;105:1-8. [Crossref] [PubMed]
- Zhao M, Bai Y, Liu W, et al. Immunization of N terminus of enterovirus 71 VP4 elicits cross-protective antibody responses. BMC Microbiol 2013;13:287. [Crossref] [PubMed]
- Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol 2010;42:39-51. [Crossref] [PubMed]
- Park MH, Igarashi K. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol Ther (Seoul) 2013;21:1-9. [Crossref] [PubMed]
- Huang M, Zhang W, Chen H, et al. Targeting Polyamine Metabolism for Control of Human Viral Diseases. Infect Drug Resist 2020;13:4335-46. [Crossref] [PubMed]
- Lian J, Liang Y, Zhang H, et al. The role of polyamine metabolism in remodeling immune responses and blocking therapy within the tumor immune microenvironment. Front Immunol 2022;13:912279. [Crossref] [PubMed]
- Miller-Fleming L, Olin-Sandoval V, Campbell K, et al. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J Mol Biol 2015;427:3389-406. [Crossref] [PubMed]
- Madeo F, Eisenberg T, Pietrocola F, et al. Spermidine in health and disease. Science 2018;359:eaan2788. [Crossref] [PubMed]
- Mounce BC, Poirier EZ, Passoni G, et al. Interferon-Induced Spermidine-Spermine Acetyltransferase and Polyamine Depletion Restrict Zika and Chikungunya Viruses. Cell Host Microbe 2016;20:167-77. [Crossref] [PubMed]
- Smirnova OA, Keinanen TA, Ivanova ON, et al. Hepatitis C virus alters metabolism of biogenic polyamines by affecting expression of key enzymes of their metabolism. Biochem Biophys Res Commun 2017;483:904-9. [Crossref] [PubMed]
- Li XW, Ni X, Qian SY, et al. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition). World J Pediatr 2018;14:437-47. [Crossref] [PubMed]
- Huang M, Du W, Liu J, et al. Interleukin-27 as a Novel Biomarker for Early Cardiopulmonary Failure in Enterovirus 71-Infected Children with Central Nervous System Involvement. Mediators Inflamm 2016;2016:4025167. [Crossref] [PubMed]
- Wang X, Liang Y, Wang Y, et al. Simultaneous determination of 10 kinds of biogenic amines in rat plasma using high-performance liquid chromatography coupled with fluorescence detection. Biomed Chromatogr 2018;32:e4211. [Crossref] [PubMed]
- Ducros V, Ruffieux D, Belva-Besnet H, et al. Determination of dansylated polyamines in red blood cells by liquid chromatography-tandem mass spectrometry. Anal Biochem 2009;390:46-51. [Crossref] [PubMed]
- Ren D, Lin B, Zhang X, et al. Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget 2017;8:49807-23. [Crossref] [PubMed]
- Mounce BC, Cesaro T, Moratorio G, et al. Inhibition of Polyamine Biosynthesis Is a Broad-Spectrum Strategy against RNA Viruses. J Virol 2016;90:9683-92. [Crossref] [PubMed]
- Firpo MR, Mounce BC. Diverse Functions of Polyamines in Virus Infection. Biomolecules 2020;10:628. [Crossref] [PubMed]
- Tee KK, Lam TT, Chan YF, et al. Evolutionary genetics of human enterovirus 71: origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J Virol 2010;84:3339-50. [Crossref] [PubMed]
- Huang SW, Wang YF, Yu CK, et al. Mutations in VP2 and VP1 capsid proteins increase infectivity and mouse lethality of enterovirus 71 by virus binding and RNA accumulation enhancement. Virology 2012;422:132-43. [Crossref] [PubMed]
- Cao J, Qu M, Liu H, et al. Myristoylation of EV71 VP4 is Essential for Infectivity and Interaction with Membrane Structure. Virol Sin 2020;35:599-613. [Crossref] [PubMed]
- Choi YH, Park HY. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J Biomed Sci 2012;19:31. [Crossref] [PubMed]
- Morón B, Spalinger M, Kasper S, et al. Activation of protein tyrosine phosphatase non-receptor type 2 by spermidine exerts anti-inflammatory effects in human THP-1 monocytes and in a mouse model of acute colitis. PLoS One 2013;8:e73703. [Crossref] [PubMed]
- Wu CN, Lin YC, Fann C, et al. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine 2001;20:895-904. [Crossref] [PubMed]
- Zhang Y, Han H, Sun L, et al. Antiviral activity of shikonin ester derivative PMM-034 against enterovirus 71 in vitro. Braz J Med Biol Res 2017;50:e6586. [Crossref] [PubMed]
- Li B, Zheng J. MicroR-9-5p suppresses EV71 replication through targeting NFκB of the RIG-I-mediated innate immune response. FEBS Open Bio 2018;8:1457-70. [Crossref] [PubMed]
- Zhang L, Li Y, Gu Z, et al. Resveratrol inhibits enterovirus 71 replication and pro-inflammatory cytokine secretion in rhabdosarcoma cells through blocking IKKs/NF-κB signaling pathway. PLoS One 2015;10:e0116879. [Crossref] [PubMed]
- Els T, Bruckmann J, Röhn G, et al. Spermidine: A predictor for neurological outcome and infarct size in focal cerebral ischemia? Stroke 2001;32:43-6. [Crossref] [PubMed]
- Han L, Xu C, Jiang C, et al. Effects of polyamines on apoptosis induced by simulated ischemia/reperfusion injury in cultured neonatal rat cardiomyocytes. Cell Biol Int 2007;31:1345-52. [Crossref] [PubMed]
- Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 2016;22:1428-38. [Crossref] [PubMed]
- Celano P, Baylin SB, Giardiello FM, et al. Effect of polyamine depletion on c-myc expression in human colon carcinoma cells. J Biol Chem 1988;263:5491-4.
- Megosh L, Gilmour SK, Rosson D, et al. Increased frequency of spontaneous skin tumors in transgenic mice which overexpress ornithine decarboxylase. Cancer Res 1995;55:4205-9.
- Auvinen M, Laine A, Paasinen-Sohns A, et al. Human ornithine decarboxylase-overproducing NIH3T3 cells induce rapidly growing, highly vascularized tumors in nude mice. Cancer Res 1997;57:3016-25.
- O'Brien TG, Megosh LC, Gilliard G, et al. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res 1997;57:2630-7.
- Guo Y, Zhao J, Sawicki J, et al. Conversion of C57Bl/6 mice from a tumor promotion-resistant to a -sensitive phenotype by enhanced ornithine decarboxylase expression. Mol Carcinog 1999;26:32-6.
- Nilsson JA, Keller UB, Baudino TA, et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell 2005;7:433-44. [Crossref] [PubMed]
- Khong WX, Foo DG, Trasti SL, et al. Sustained high levels of interleukin-6 contribute to the pathogenesis of enterovirus 71 in a neonate mouse model. J Virol 2011;85:3067-76. [Crossref] [PubMed]
- Yuan A, Li J, Liu P, et al. Association of interleukin-6-572C/G gene polymorphism and serum or cerebrospinal fluid interleukin-6 level with enterovirus 71 encephalitis in Chinese Han patients with hand, foot, and mouth disease. Inflammation 2015;38:728-35. [Crossref] [PubMed]
- He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res 2011;21:159-68. [Crossref] [PubMed]
- Tung WH, Hsieh HL, Lee IT, et al. Enterovirus 71 modulates a COX-2/PGE2/cAMP-dependent viral replication in human neuroblastoma cells: role of the c-Src/EGFR/p42/p44 MAPK/CREB signaling pathway. J Cell Biochem 2011;112:559-70. [Crossref] [PubMed]
- Wang C, Gao L, Jin Y, et al. Regulation of host responses and viral replication by the mitogen-activated protein kinases in intestinal epithelial cells infected with Enterovirus 71. Virus Res 2015;197:75-84. [Crossref] [PubMed]
- Luo Z, Ge M, Chen J, et al. HRS plays an important role for TLR7 signaling to orchestrate inflammation and innate immunity upon EV71 infection. PLoS Pathog 2017;13:e1006585. [Crossref] [PubMed]
- Chen D, Tian X, Zou X, et al. Harmine, a small molecule derived from natural sources, inhibits enterovirus 71 replication by targeting NF-κB pathway. Int Immunopharmacol 2018;60:111-20. [Crossref] [PubMed]
- Pignatti C, Tantini B, Stefanelli C, et al. Signal transduction pathways linking polyamines to apoptosis. Amino Acids 2004;27:359-65. [Crossref] [PubMed]
- Smirnova OA, Isaguliants MG, Hyvonen MT, et al. Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N1-acetyltransferase in human hepatoma HUH7 cells. Biochimie 2012;94:1876-83. [Crossref] [PubMed]
- Tong F, Liu S, Yan B, et al. Endogenous ornithine decarboxylase/polyamine system mediated the antagonist role of insulin/PEG-CMCS preconditioning against heart ischemia/reperfusion injury in diabetes mellitus. Int J Nanomedicine 2018;13:2507-20. [Crossref] [PubMed]
- Kaczmarek L, Kaminska B, Messina L, et al. Inhibitors of polyamine biosynthesis block tumor necrosis factor-induced activation of macrophages. Cancer Res 1992;52:1891-4.
(English Language Editor: L. Huleatt)


