
Intestinal prolapse and exposure after peritoneal dialysis in low-birth-weight preterm infants with acute renal failure: a case report
Introduction
The incidence and mortality rates of acute kidney injury (AKI) in low-birth-weight preterm infants are high (1). When conservative treatment cannot fully treat AKI and its complications, which include hyperkalemia, metabolic acidosis, and fluid overload, renal replacement therapy (RRT) must be promptly commenced to reduce the risk of death. Since the currently available hemodialysis equipment is generally too large for newborns, peritoneal dialysis (PD) has become the preferred treatment for neonatal RRT. A previous study found that PD effectively treated AKI and metabolic disorders in preterm infants (2). Therefore, a low-body-weight premature infant with AKI was treated using PD via the placement of a modified adult Tenckhoff two-cuff catheter. Despite the surgical complications, the resulting success of this case can add to the accumulating knowledge in this field. We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-238/rc).
Case presentation
The patient was the elder of twins, with a gestational age of 31 weeks and 2 days and a birth weight of 1,720 g. The infant presented bruising and was groaning and spitting saliva at birth. The physical examination results at birth were a body temperature of 36.1 ℃ and a blood pressure (BP) of 53/25 mmHg. The Apgar score was as follows: heart rate =145 beats/min (2 points); breathing rate =58 times/min; shallow and irregular breathing (1 point); relaxed muscle tone (0 points); weak laryngeal reflex (1 point); and pale skin color (0 points). The capillary filling time was less than 3 s, and the shock score was 3 points.
The infant was admitted to our hospital on September 8, 2021, 10 days after birth. On September 13, a chest X-ray revealed pulmonary hyaline membrane lesions, suggesting pulmonary hyaline membrane disease. The oxygen saturation (SpO2) of the patient without oxygenation was 74%. Invasive synchronized intermittent positive pressure ventilation (PPV) was used to assist ventilation, and 140 mg of bovine pulmonary surfactant was slowly injected into the trachea for 3 days. After the infant’s respiratory condition improved, he was moved onto noninvasive PPV for assisted ventilation.
At birth, the infant breathed 58 times/min with increased breathing, moaning, cyanosis, and three concave signs. To address the respiratory distress, invasive synchronized intermittent PPV was used to assist ventilation, and 140 mg of bovine pulmonary surfactant was slowly injected into the trachea for 3 days. In addition, caffeine was used to stimulate the respiratory center, and ambroxol was used to promote lung maturation alongside active prevention and treatment of apnea. The infant’s infection index was high; the mother had a history of premature membrane rupture. The diagnosis was an idiopathic perinatal infection. The infant was treated with meropenem, and his condition improved on the third day after birth. There were indications for weaning, and following the removal of the tracheal tube, the infant was switched to noninvasive PPV. Symptomatic treatments and nutritional supports, including atomization, rehydration, and myocardial protection, were applied. Vitamin C was used to nourish the myocardium, and vitamin K1 was used to prevent bleeding. Furthermore, the infant was given 1 to 2 mL of Aiershu milk every 3 h, and blood pressure, urine volume, blood gas, and other indicators were monitored alongside the ventilation and infection treatments.
The patient’s renal function increased progressively 10 days after birth. The blood urea nitrogen, creatinine, and potassium levels were 19.1 mmol/L, 191 µmol/L, and 8.76 mmol/L, respectively, and the patient did not urinate. Conservative treatment was ineffective, and PD was identified as a treatment option. An operation procedure was performed using the Seldinger technique; the percutaneous PD catheter placement was from the left side of the abdomen into the abdominal cavity. A modified adult Tenckhoff two-cuff PD catheter was used, with the inlet end shortened by 2 cm. The internal cuff was placed between the subcutaneous space and the peritoneum, and the external cuff was placed outside the skin. No subcutaneous tunnel was made (Figure 1). Meanwhile, the outlet end was connected to a titanium joint, an external short pipe, and an infusion tee joint. The first port was connected with an external catheter to transfer the PD solution into and out of the abdominal cavity. A syringe containing the PD solution was connected to the second port, and a syringe pump accurately controlled the dose and rate of injection into the abdominal cavity. The third port was connected to a graduated drainage bag for draining the waste liquid from the abdominal cavity.
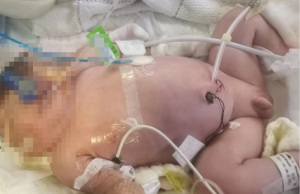
The PD treatment was commenced immediately after the operation. Baxter dialysate (glucose concentration, 2.5%; sodium ion, 132 mmol/L; calcium ion, 1.77 mmol/L; magnesium ion, 0.25 mmol/L; chloride ion, 96 mmol/L; and lactate, 40 mmol/L) was selected for the PD. Each injection containing 20 mL of PD solution was injected into the abdominal cavity over a 15-min period, with the PD solution retained for 30 min and then drained over a 15-min period. A total of eight cycles/day were given. After 3 days of dialysis, the blood potassium had decreased to 5.5 mmol/L, and the urine volume had gradually increased to 10 mL/day. Due to the patient’s skin edema, there was a minor leakage of PD solution around the incision catheter.
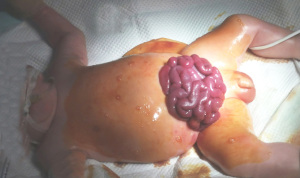
The patient’s crying caused the incision to tear and the small intestine to prolapse 19 days after birth (Figure 2). The patient immediately underwent abdominal wall debridement and small bowel reduction under endotracheal intubation and general anesthesia. After the original incision was sutured, a decompression rod was added to prevent the incision from tearing again. Due to oliguria and abnormal renal function, continued PD treatment was required. In addition, abdominal drainage was required after the small intestine was returned to its correct position. Therefore, an adult Tenckhoff two-cuff peritoneal diagnosis catheter reduced by 2 cm was placed into the abdominal cavity from the right abdomen, with the internal and external cuffs placed outside the skin. In subsequent PD cycles, 15 mL of 2.5% Baxter dialysate was introduced over a 20-min period, retained for 20 to 30 min, and removed over a 15-min period, with eight cycles/day. The glucose concentration and the abdominal retention time were adjusted according to the daily urine volume, and there was no leakage of PD solution at the incision point.
On the night of the small bowel reduction surgery, the patient suffered from a drop in BP and went into cardiac arrest on several occasions. Under the assistance of noninvasive PPV, the patient’s SpO2 was <70%. The patient was immediately administered cardiopulmonary resuscitation, volume expansion, and fluid replacement. Following treatment with norepinephrine, dopamine, and dobutamine, the patient’s vital signs gradually stabilized, and the heart rate recovered to 140 beats/min and the SpO2 to 99%.
The following day, the patient developed a severe lung infection, and the PD solution was turbid. A culture of the PD solution was positive for a Klebsiella pneumonia subspecies, and the patient was treated with antibiotics intravenously and intraperitoneally. After 2 days of treatment, the infection indexes and the number of leukocytes in the dialysate gradually decreased, the blood creatinine levels slowly returned to normal, and the urine volume gradually increased.
The PD treatment was stopped after 19 days, and the patient was extubated. At 47 days after birth, the patient weighed 2,050 g and was discharged from the hospital. The infant returned for re-examination 100 days after birth. Here, the Bailey Infant Development Scale was used to evaluate the infant’s intelligence, growth, and development. Computed tomography scan images of the brain were viewed at a children’s hospital, and no abnormalities were found. The infant has subsequently returned to the hospital every month for follow-ups, and no abnormalities have been identified. There is no patient perspective in this study.
All procedures performed in this study complied with the ethical standards of the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University, China (FEY-BG-39-2.0) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
AKI is an independent predictor of the incidence and mortality rates in neonatal intensive care units (1). When conservative treatment cannot alleviate AKI and its complications, it is imperative to commence dialysis treatment promptly. Currently, the hemodialysis equipment routinely used in hospitals is too large for most newborns. The PD method has many advantages over hemodialysis, including simple operation and stable hemodynamics, and has thus become the first choice for treating neonatal AKI (2,3).
Due to the sparse subcutaneous fat of newborns, catheter placement is different from that used in adults, including in terms of central venous, urinary, thoracic drainage, peripheral venous, and PD catheters (double or single cuff, straight, crimp, or gooseneck) (3-5). The patient in question presented a serious condition, and time was limited. Given that a PD catheter has greater peritoneal biocompatibility than other catheters and that the hospital had no special neonatal PD catheter, the decision was made to use an adult Tenckhoff two-cuff straight PD catheter. However, during the first PD catheter placement surgery, the internal cuff was placed under the skin, and the incision was relatively large, resulting in leakage of the PD solution. Furthermore, the patient experienced gastric retention, abdominal distension, and other symptoms following placement of the abdominal dialysis catheter. While the gastrointestinal tract had been decompressed, the abdominal pressure increased rapidly when the patient cried, resulting in complications, including incision tears and small intestine prolapse.
Bowel prolapse following abdominal surgery is rare in adults. Compared with adults, newborns have a thin abdominal wall, poor compliance, and more uncontrollable factors that could cause bowel prolapse. During the second PD catheter placement surgery, both the internal and external cuffs were placed outside the skin. The resulting incision was small, and the catheter could thus pass through without any PD solution leakage, which suggests that the incision size should be minimized during neonatal PD catheter placement. In addition, an appropriate dose of sedatives could be used for crying and restless infants to prevent a sudden increase in abdominal pressure.
No special PD catheter has been developed for newborns of different weights. Some medical centers (4-6) use a central venous catheter as a PD catheter for infants since it has a small diameter and is relatively soft, which positively improves the prognosis and reduces the mortality rate among infants and children. However, in these case reports, the surgical incisions resulted in different degrees of PD solution leakage. In the current case, the adult Tenckhoff catheter was cut short by 2 cm, making it suitable for infants, and the patient was successfully rescued.
Twin newborns are prone to many clinical problems, such as premature delivery, asphyxia, infection, and twin transfusion syndrome (7). Compared with singleton newborns, twin newborns have a higher risk of perinatal death. Neonatal death occurs mainly in pregnant women at 34 weeks of gestation (8). Respiratory distress syndrome is a common complication for premature twin infants and a common cause of premature infant death (7,8). The incidence of premature membrane rupture in the mother is also higher in twin pregnancies than in singleton pregnancies (9). This complex mechanism may be related to the increase in intrauterine pressure and is a serious complication that may lead to premature delivery.
The younger the gestational age and the lower the birth weight of premature twin infants, the more complications occur and the higher the mortality rate. The current case involved a premature, low-birth-weight twin. The mother had a history of premature membrane rupture. The elder of the twins had a birth weight of 1,720 g, and developed respiratory distress syndrome at birth, followed by AKI.
Following PD catheterization, the patient had incision exudation, a small bowel prolapse, and a sharp decrease in heart rate and BP for a short time. Symptomatic treatments, such as cardiopulmonary resuscitation, 186 volume expansion, and rehydration were performed, but the patient developed pneumonia and peritonitis. Anti-inflammatory treatment was then administered, and the patient recovered well and was discharged following a re-examination once the infection had been treated, and the liver and kidney function indexes were normal.
The younger twin had a birth weight of 1,500 g and also had neonatal respiratory distress syndrome and hypoxemia after birth, but after continuous oxygen inhalation, his renal function remained normal. By 28 days after birth, his body weight was more than 2,000 g, and he was thus discharged from the hospital following oxygen inhalation.
Conclusions
The organs of low-birth-weight premature newborns are underdeveloped, which makes them prone to a series of clinical problems and a high chance of mortality. In addition, since their organs are underdeveloped, children often suffer from respiratory distress syndrome, water and electrolyte disorders, anemia, infection, and other issues, all of which present significant challenges for treatment. At present, the literature on the use of PD in treating children with AKI is extensive, but few reports describe the treatment of low-birth-weight premature infants with AKI.
Peritoneal fluid leakage at the surgical incision is a common complication of PD surgery. The outcomes of the two operations in this case report suggest that the surgical incision should be as small as possible and that only the catheter should pass through the incision. If the catheter cuffs are placed under the skin, the incision becomes larger, which increases the possibility of peritoneal fluid leakage. The use of pediatric PD tubing may also reduce the risk of peritoneal fluid leakage. We believe that this case report can provide some guidance for the treatment of neonatal AKI.
Acknowledgments
This article was edited by a native English speaker Kim Huggens.
Funding: This work was supported by Famous Doctor Project of Xingdian Talents Support Program in Yunnan Province (No. YNWR-MY-2019-075).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-238/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-238/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arcinue R, Kantak A, Elkhwad M. Acute kidney injury in ELBW infants (< 750 grams) and its associated risk factors. J Neonatal Perinatal Med 2015;8:349-57. [Crossref] [PubMed]
- Vidal E, van Stralen KJ, Chesnaye NC, et al. Infants Requiring Maintenance Dialysis: Outcomes of Hemodialysis and Peritoneal Dialysis. Am J Kidney Dis 2017;69:617-25. [Crossref] [PubMed]
- Yildiz N, Erguven M, Yildiz M, et al. Acute peritoneal dialysis in neonates with acute kidney injury and hypernatremic dehydration. Perit Dial Int 2013;33:290-6. [Crossref] [PubMed]
- Burgmaier K, Hackl A, Ehren R, et al. Peritoneal dialysis in extremely and very low-birth-weight infants. Perit Dial Int 2020;40:233-6. [Crossref] [PubMed]
- Chen YJ, Hung HH, Li CY, et al. A central venous catheter as an alternative peritoneal dialysis tube in an extremely low birth weight infant: A practical life-saving method for medical-resource-limited institutions. J Formos Med Assoc 2021;120:1928-9. [Crossref] [PubMed]
- Noh J, Kim CY, Jung E, et al. Challenges of acute peritoneal dialysis in extremely-low-birth-weight infants: a retrospective cohort study. BMC Nephrol 2020;21:437. [Crossref] [PubMed]
- Guillén-Sacoto MA, Barquiel B, Hillman N, et al. Gestational diabetes mellitus: glycemic control during pregnancy and neonatal outcomes of twin and singleton pregnancies. Endocrinol Diabetes Nutr 2018;65:319-27. (Engl Ed). [Crossref] [PubMed]
- Mori AT, Kampata L, Musonda P, et al. Cost-benefit and extended cost-effectiveness analysis of a comprehensive adolescent pregnancy prevention program in Zambia: study protocol for a cluster randomized controlled trial. Trials 2017;18:604. [Crossref] [PubMed]
- Mackie FL, Morris RK, Kilby MD. The prediction, diagnosis and management of complications in monochorionic twin pregnancies: the OMMIT (Optimal Management of Monochorionic Twins) study. BMC Pregnancy Childbirth 2017;17:153. [Crossref] [PubMed]


