
The actual duration of preoperative fasting in pediatric patients, and its effects on hunger and thirst: a prospective observational study
Highlight box
Key findings
• Median preoperative fasting duration for food and clear liquid in pediatric surgical population was 11.1 and 10.0 h, respectively.
What is known and what is new?
• Preoperative fasting time for food and clear liquid was recommended as 2 and 6 h to prevent pulmonary aspiration.
• The actual duration of preoperative fasting was longer than recommended. A high hunger score was reported in 76.4% of participants. The younger age group and anesthesia start time in the afternoon were factors associated with high hunger scores.
What is the implication, and what should change now?
• Multidisciplinary team approach and quality improvement method should be implemented to minimize the unnecessary prolonged preoperative fasting duration.
Introduction
General anesthesia including intravenous sedation impaired protective airway reflex and put the patient at risk to pulmonary aspiration (1,2). Even though the incidence of pulmonary aspiration among children during anesthesia was low (0.007–0.18%) (3-9), preoperative fasting is mandatory for patient safety. According to the guideline from American Society of Anesthesiologists (ASA) published in 2017, the optimal preoperative fasting time prior to anesthesia was recommended at least 2, 4, 6, and 8 h after consuming clear liquid, breast milk, non-human formula milk, and fatty, fried foods or meats, respectively (10). The preoperative fasting guideline in children from European Society of Anaesthesiology and Intensive Care published in 2022 reduced preoperative fasting time to only 1 h for clear fluid and 3 h for breast milk to reduce patient discomfort (11).
The real-world fasting time reported in pediatric studies after 2010 was unnecessarily prolonged especially for clear liquid. The mean fasting times for food and clear liquid were 7.8–14.0 h (12-24) and 2.3–12.3 h, respectively (13-24). Multiple perioperative complications had been described after extensive fasting such as hypotension after anesthesia induction (25,26), ketosis (23), increased incidence of postoperative nausea and vomiting (27), and patient discomfort (16). Carbohydrate-rich drink was reported to reduce hunger, thirst and anxiety in adults (28,29) but not in pediatrics (30,31). However, dextrose-containing intravenous fluid administration has not been investigated to reduce hunger and thirst in children.
The primary objective of this study aimed to determine the actual duration of preoperative fasting in pediatric patients. Secondary objectives were to examine the relationship between actual fasting duration and patient discomfort reported by hunger and thirst score. We also explored other factors related to high hunger score. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-358/rc).
Methods
This prospective observational study was conducted at the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand during August 2019 to August 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Siriraj Institutional Review Board (No. Si434/2019). Written informed consent was taken from the parent(s) or legal guardian(s) of all included participants. In addition, written informed assent was obtained from participant who aged 7 years old and older.
This study included pediatric patients aged 0–15 years who were scheduled for elective surgery or for other investigational procedure to be performed under general anesthesia. Participants were divided by age into one of the following three groups: 0–2, 3–6, or 7–15 years. We excluded patients who had to be fasted due to a surgical or medical indication other than preoperative fasting, patients with delayed development or neurological impairment, children aged seven years or older who could not communicate, and parents of children aged younger than seven years who could not communicate.
Upon operating theatre arrival, all participants and their parents were asked for the last time that the participant ate food and/or drank clear liquid prior to surgery. For participants aged 7–15 years, hunger score and thirst score were self-rated using a 0–10 scale (0= feeling not hungry or thirsty, 10= feeling extremely hungry or thirsty). Among participants aged 0–6 years, the parents were asked to rate the child’s hunger score based on the child’s behavior using a 0–10 scale.
Primary outcome was actual fasting duration for food and clear liquid. Secondary outcomes were (I) severity of patient discomfort reported by hunger score and thirst score, and (II) factors associated with high hunger score such as fasting time, age, dextrose-containing fluid administration, and anesthesia start time. Demographic data were collected from the medical record. Dextrose-containing intravenous fluid administration and anesthesia start time were recorded. The volume of intravenous fluid was categorized as none, <10 mL/kg, or ≥10 mL/kg. Anesthesia start time was categorized into before 10:00 AM, 10:00–11:59 AM, or after 12:00 PM. Hunger score and thirst score were categorized as low (0–4) or high (5–10). Unacceptably prolonged fasting time was defined as a fasting period more than two times the recommended fasting duration. Therefore, the unacceptable fasting duration for food and clear liquid was more than 12 and 4 h, respectively (22).
Sample size calculation
The sample size was calculated based on the clear liquid preoperative fasting time mean ± standard deviation (SD) of 6.3±4.5 h reported by Newton et al. (21). Assuming an SD of 4.5 h, and a mean error of 0.5 h at the 95% confidence level, a minimum sample size of 312 patients was required in this study.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics. Categorical data are reported as number and percentage, and non-normally distributed continuous data are given as median and interquartile range (IQR). Chi-square test was used to compare the proportion of high hunger and thirst scores between intravenous fluid status and the time to start of anesthesia groups. One-way analysis of variance (ANOVA) and independent-samples Kruskal-Wallis test were used to compare the fasting time for food, fasting time for clear fluid, and hunger score among three age groups. Spearman’s Rho correlation coefficient was used to measure the strength and direction of association between fasting time for food and hunger score, and between fasting time for clear liquid and thirst score. Pairwise comparisons were adjusted by Bonferroni correction. A 2-sided P value less than 0.05 was considered statistically significant for all tests.
Results
This study included 320 participants. Eleven participants aged 7–15 years who had delayed development were excluded, resulting in 309 participants being included in the final analysis. A flow diagram of participants is shown in Figure 1. The median age [IQR] was 3.0 years [IQR: 1.2, 6.0] (range, 8 days to 15 years). Seventy-seven percent of study subjects were aged younger than seven years. Most participants were scheduled for general surgery (56.3%), as described in Table 1. There was no missing data to be excluded from final analysis.
Table 1
| Characteristics | n (%) |
|---|---|
| Age, years | |
| 0–2 | 133 (43.0) |
| 3–6 | 106 (34.3) |
| 7–15 | 70 (22.7) |
| Gender (male) | 197 (63.8) |
| In-patient | 178 (57.6) |
| Type of procedure | |
| General surgery | 174 (56.3) |
| Ophthalmology | 69 (22.3) |
| Orthopedics | 58 (18.8) |
| Others | 8 (2.6) |
The median fasting time for food and clear liquid was 11.1 h [IQR: 8.0, 14.0] and 10.0 h [IQR: 7.2, 12.5], respectively. Fasting time for food and clear liquid by age groups were described in Table 2. There were significant differences in the fasting time for food and clear liquid between age groups. The 0–2 years group had a significantly shorter fasting time than the 2 older age groups (P<0.001). An unacceptably prolonged fasting time for food and clear liquid occurred in 106 (44.0%) and 295 (95.5%) participants, respectively.
Table 2
| Times and scores | Total (N=309) | 0–2 years (n=133) | 3–6 years (n=106) | 7–15 years (n=70) | P value |
|---|---|---|---|---|---|
| Fasting time for food (h) | 11.1 [8.0, 14.0] | 8.1 [6.9, 10.5] | 12.2 [8.9, 14.6] | 14.0 [12.2, 16.0] | <0.001* |
| Fasting time for clear liquid (h) | 10.0 [7.2, 12.5] | 8.0 [6.7, 10.3] | 11.3 [8.6, 13.9] | 11.8 [8.2, 13.5] | <0.001* |
| Hunger score (0–10) | 7.0 [5.0, 9.0] | 7.0 [5.0, 10.0] | 6.0 [4.9, 8.0] | 5.0 [1.4, 10.0] | 0.001* |
| Thirst score (0–10) | 4.5 [0, 7.3] | N/A | N/A | 4.5 [0, 7.3] | N/A |
Data presented as median and interquartile range. *, outcomes significantly different between children aged 0–2 years comparing to 3–6 years and 7–15 years. N/A, not applicable.
The median hunger score among all patients was 7.0 [IQR: 5.0, 9.0]. The hunger score by age groups were presented in Table 2. High hunger score was reported in 236 (76.4%) participants. Among these three age groups, the 0–2 years hunger score was significantly higher than that in the two older groups (P=0.001), but no significant difference was found between the 3–6 and 7–15 years groups. There was no correlation between fasting time for food and hunger score (Rho: −0.150, P=0.008) or fasting time for clear liquid and thirst score (Rho: 0.007, P=0.955). There was also no correlation between fasting time for food and hunger score in participants who had anesthesia started in the morning or in the afternoon (Rho: −0.188, P=0.002, and Rho: −0.258, P=0.118), respectively.
One hundred and forty-seven (47.6%) participants received 5% dextrose intravenous fluid during the fasting period (Table 3). The participants who received 5% dextrose intravenous fluid preoperatively ≥10 mL/kg had a higher proportion of high hunger score (85.7%) compared to those who received no intravenous fluid (74.7%) or less than 10 mL/kg of intravenous fluid administration (63.3%) (P=0.008). Hunger score was not reduced by the administration of dextrose-containing intravenous fluid administration.
Table 3
| Parameters | Hunger score | Thirst score | |||||
|---|---|---|---|---|---|---|---|
| Total (N=309) | Low (0–4) | High (5–10) | Total (N=70) | Low (0–4) | High (5–10) | ||
| Dextrose-containing fluid administration | |||||||
| None | 162 (52.4) | 41 (25.3) | 121 (74.7) | 29 (41.4) | 17 (58.6) | 12 (41.4) | |
| <10 mL/kg | 49 (15.9) | 18 (36.7) | 31 (63.3) | 23 (32.9) | 13 (56.5) | 10 (43.5) | |
| >10 mL/kg | 98 (31.7) | 14 (14.3) | 84 (85.7)* | 18 (25.7) | 5 (27.8) | 13 (72.2) | |
| Anesthesia start time | |||||||
| Before 10:00 AM | 161 (52.1) | 46 (28.6) | 115 (71.4) | 34 (48.6) | 18 (52.9) | 16 (47.1) | |
| 10:00–11:59 AM | 110 (35.6) | 23 (20.9) | 87 (79.1) | 22 (31.4) | 12 (54.5) | 10 (45.5) | |
| After 12:00 PM | 38 (12.3) | 4 (10.5) | 34 (89.5)** | 14 (20.0) | 5 (35.7) | 9 (64.3) | |
Data presented as number (%). *, P=0.008 (chi-square test); **, P=0.044 (chi-square test).
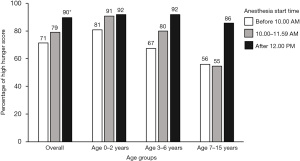
Most participants had anesthesia started in the morning (87.7%). Participants who had anesthesia start time after 12:00 PM had higher proportion (89.5%) of high hunger score compared with the other group (P=0.044) as described in Table 3. At the same anesthesia start time, the younger age group had a higher proportion of high hunger scores compared to the 2 older age groups (Figure 2). The 0–2 years group had a higher proportion (80–90%) of high hunger scores at all anesthesia start times. The 7–15 years group whose anesthesia started in the morning had a lower proportion (55%) of high hunger scores. Regarding anesthesia starting in the afternoon, at least 85% of participants in each age group had a high hunger score.
Discussion
We found median fasting times for food and clear liquid of 11.1 and 10.0 h, respectively, which was extremely prolonged compared with the 6 and 2 h recommended by the practice guideline (10,32). We also reported a high incidence of unacceptably prolonged fasting times (44% for food, and 95.5% for clear liquid). Even though ASA had revised preoperative fasting guideline since 2011 to decrease fasting time for clear liquid from 6 to 2 h (32), recent studies published after 2020 still reported prolonged fasting times for food and clear liquid between 11–14 and 2–12 h (5,13-16,18,26), respectively. Pediatric studies regarding preoperative fasting were described in Table 4 (13-24). Our prolonged fasting time for clear liquid was in contrast to other studies that were successful in reducing the mean fasting time for clear liquid to less than 4 h (17,21). However, lengthy fasting times could be contributed by several factors. Although the fasting time for food is at least 6 h prior to operation, it is not practical to wake the patient up to eat during 2:00–3:00 AM on the morning of surgery. Therefore, the fasting time for food is at least as long as the duration of sleep. Decreasing the fasting time for clear liquid is much easier and more practical to achieve, but it has still not been widely implemented in routine practice. Most physicians usually order the start of fasting 6–8 h prior to operation regardless of solid or liquid status. In case there was a change in surgical schedule, the fasted patient would be ready to proceed in order to maximize the utilization time of operating theatres. To solve this problem, intensive communication and education among the anesthesiologists, surgeons, nurses, and parents are required.
Table 4
| Authors, year (reference) | Country | Participants | Age (years) | Food fasting time (h) | Clear liquid fasting time (h) |
|---|---|---|---|---|---|
| Assen et al., 2021 (13) | Ethiopia | 258 | 4.9±3.8 | 13.3±3.1 | 12.3±3.2 |
| Li et al., 2021 (14) | China | 211 | 4.4±3.7 | 12.9 | 9.3 |
| Özdemir et al., 2021 (15) | Turkey | 123 | 9.8±1.7 | 11.0±1.2 | N/A |
| Al-Robeye et al., 2020 (16) | UK | 48 | 8.3±4.1 | 11.7±4.4 | 6.9±5.0 |
| Beck et al., 2020 (17) | Germany | 12,093 | 5.2 [2.5, 9.9] | 14.0 [12.4, 15.7] | 2.3 [1.4, 4.8] |
| Hajian et al., 2020 (18) | Iran | 50 | 6.6±1.9 | 13.4±3.0 | 9.3±3.1 |
| Benouaz et al., 2018 (19) | Algeria | 1,003 | 5.2±3.7 | 6.9±4.2 | N/A |
| Dolgun et al., 2017 (20) | Turkey | 332 | N/A† | 10.51 | 6.27 |
| Newton et al., 2017 (21) | UK | 4,828 | N/A | N/A | Pre: 6.3±4.5 |
| Post: 3.1±2.3 | |||||
| Buller et al., 2016 (22) | Australia | 307 | 6.0±4.1 | 10.0±3.6 | 6.3±3.8 |
| Dennhardt et al., 2015 (23) | Germany | 100 | 9.9±9.5 months | 7.8±4.5 | – |
| Engelhardt et al., 2011 (24) | UK | 1,350 | 7.7 [2.0, 16.0] | 12:05 [00:45, 21:50] | 07:57 [00:05, 20:50] |
Data presented as mean plus/minus standard deviation or median and interquartile range. †, 65% of participants were 0–3 years old. N/A, not available.
In the present study, the median fasting time for food and clear liquid increased with age. However, the 0–2 years group still had the highest hunger score, and it was significantly higher than in the 2 older groups. Although the 0–2 years group was scheduled for surgery as early as possible, most of them expressed distress behavior that their parents rated as a high hunger score—even in the first case of the day. Al-Robeye et al. (16) studied preoperative fasting in children with a mean age of 8.3 years. They found that most children could cope well with preoperative fasting, and only a tiny proportion of patients rated extreme hunger. Our study also found high hunger scores to be lowest in the 7–15 years group regardless of anesthesia start time.
In contrast to Engelhardt et al. who reported a statistically significant correlation between the severity of hunger and the duration of fasting for solid food only in children who had no food intake after midnight for morning surgical schedule (r=0.92, P<0.001) (24), we were not able to demonstrate the same association (Rho: −0.188, P=0.002). However, this correlation was not found in children who had food after midnight for afternoon surgical schedule (r=0.57, P=0.32) (24). In addition, our study found that patients with an afternoon surgical schedule had a higher proportion of high hunger score (89.5%) compared to those with a morning surgical schedule in all age groups (P=0.044). This proportion was consistent with that reported by Engelhardt et al. who found severe hunger to be significantly higher in patients who had food after midnight for afternoon surgical schedule than in patients who had no food after midnight for morning surgical schedule (69.3% vs. 45.8%, P<0.001) (24). Therefore, the duration of fasting time that occurs while the patient is awake is the main influencing factor, not the total duration of fasting time.
Dextrose-containing intravenous fluid administration was assumed to alleviate hunger and thirst during a prolonged fasting period. However, the present study found that those who received preoperative intravenous fluid ≥10 mL/kg had a significantly higher proportion of high hunger score when compared to the other 2 groups. This could be explained by the longer fasting duration the patient experienced, the higher amount of fluid the patient received. Furthermore, there were no significant differences in thirst scores between participants who received dextrose-containing intravenous fluid. This result indicated that dextrose-containing intravenous fluid did not reduce preoperative hunger and thirst in children.
Multiple strategies have been implemented to reduce fasting time and attenuate patient discomfort from prolonged fasting. A decrease in the clear fluid fasting time to be closer to 2 h before surgery would reduce child thirst and discomfort (33), and reduce the incidence of low blood pressure after anesthesia induction (25,26). A consensus statement from European pediatric anesthesiologist societies allows children to drink up to 3 mL/kg until 1 h before surgery (34). Changing routine practice is not easy, and it requires a multidisciplinary team approach via a continuous quality improvement method. Newton et al. (21) and Isserman et al. (35) increased the proportion of less than 4 h clear fluid fasting from 19% to 72% and 20% to 63%, respectively. Pediatric experience during fasting should be addressed and can be improved. Not only can the patient on the afternoon surgical list have breakfast, but providing clear fluid with adequate calories (33) combined with psychological distraction may reduce patient discomfort and anxiety. Many distraction methods were reported to be effective for reducing anxiety, including video games, playing with children during anesthesia induction (36,37). Swaddling (38), and 2% glucose (39) was shown to reduce stress during wound dressing and heel prick for blood collection procedures in neonates and infants.
Limitations
This study has some mentionable limitations. First, this study did not explore other perioperative complications such as ketosis and low blood pressure in relation to fasting time. Second, hunger and thirst scores were rated by different raters among age groups. Hunger score was self-reported, but hunger score of participants aged below 7 years was interpreted from behavioral observation, and then rated by the parents. The thirst score was self-rated only in the 7–15 years group. Hunger and thirst score were also subjective. Another limitation is that our study did not compare the physician’s order with the actual fasting time. Our fasting duration should be similar to national practice, however, in the institution that specifically describes fasting time for solid food and clear liquid might have shorter fasting time for clear liquid.
Conclusions
The actual duration of preoperative fasting was found to be longer than the recommendation for both food and liquid in pediatric surgical population. High hunger score was reported in 76.4% of participants. Younger age group and anesthesia start time in the afternoon were factors associated with high hunger score.
Acknowledgments
The authors gratefully acknowledge the children and their parents for agreeing to participate in this study. The authors thank Prof. Manee Raksakietisak, MD for her valuable comments on the manuscript. The authors thank Suthipol Udompunthurak and Orawan Supapueng, PhD for their assistance with statistical analysis, and Miss Arporn Pimtong for administrative support.
Funding: This work was supported by a grant from the Siriraj Research Development Fund of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand [grant No. (IO) R16235032].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-358/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-358/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-358/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-358/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Siriraj Institutional Review Board (No. Si 434/2019), and informed consent (and informed assent as appropriate) was taken from the parent(s) or legal guardian(s) of all included participants. In addition, written informed assent was obtained from participant who aged 7 years old and older.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Green SM, Mason KP, Krauss BS. Pulmonary aspiration during procedural sedation: a comprehensive systematic review. Br J Anaesth 2017;118:344-54. [Crossref] [PubMed]
- Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Continuing Education in Anaesthesia Critical Care & Pain 2014;14:171-5.
- Andersson H, Zarén B, Frykholm P. Low incidence of pulmonary aspiration in children allowed intake of clear fluids until called to the operating suite. Paediatr Anaesth 2015;25:770-7. [Crossref] [PubMed]
- Beach ML, Cohen DM, Gallagher SM, et al. Major Adverse Events and Relationship to Nil per Os Status in Pediatric Sedation/Anesthesia Outside the Operating Room: A Report of the Pediatric Sedation Research Consortium. Anesthesiology 2016;124:80-8. [Crossref] [PubMed]
- Beck CE, Rudolp D, Becke-Jakob K, et al. Real fasting times and incidence of pulmonary aspiration in children: Results of a German prospective multicenter observational study. Paediatr Anaesth 2019;29:1040-5. [Crossref] [PubMed]
- Borland LM, Sereika SM, Woelfel SK, et al. Pulmonary aspiration in pediatric patients during general anesthesia: incidence and outcome. J Clin Anesth 1998;10:95-102. [Crossref] [PubMed]
- Tan Z, Lee SY. Pulmonary aspiration under GA: a 13-year audit in a tertiary pediatric unit. Paediatr Anaesth 2016;26:547-52. [Crossref] [PubMed]
- Walker RW. Pulmonary aspiration in pediatric anesthetic practice in the UK: a prospective survey of specialist pediatric centers over a one-year period. Paediatr Anaesth 2013;23:702-11. [Crossref] [PubMed]
- Warner MA, Warner ME, Warner DO, et al. Perioperative pulmonary aspiration in infants and children. Anesthesiology 1999;90:66-71. [Crossref] [PubMed]
- Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology 2017;126:376-93. [Crossref] [PubMed]
- Frykholm P, Disma N, Andersson H, et al. Pre-operative fasting in children: A guideline from the European Society of Anaesthesiology and Intensive Care. Eur J Anaesthesiol 2022;39:4-25. [Crossref] [PubMed]
- Cantellow S, Lightfoot J, Bould H, et al. Parents' understanding of and compliance with fasting instruction for pediatric day case surgery. Paediatr Anaesth 2012;22:897-900. [Crossref] [PubMed]
- Assen HE, Hassen AM, Abate A, et al. Preoperative Fasting Time and Its Association with Hypoglycemia during Anesthesia in Pediatric Patients Undergoing Elective Procedures at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Biomed Res Int 2021;2021:9166603. [Crossref] [PubMed]
- Li Y, Lu Q, Wang B, et al. Preoperative Fasting Times for Patients Undergoing Elective Surgery at a Pediatric Hospital in Shanghai: The Big Evidence-Practice Gap. J Perianesth Nurs 2021;36:559-63. [Crossref] [PubMed]
- Özdemir Ş, Dolgun E. The effect of preoperative fasting to postoperative agitation, nausea, and vomiting in children with tonsillectomy. Medical Science and Discovery 2021;8:394-400.
- Al-Robeye AM, Barnard AN, Bew S. Thirsty work: Exploring children's experiences of preoperative fasting. Paediatr Anaesth 2020;30:43-9. [Crossref] [PubMed]
- Beck CE, Rudolph D, Mahn C, et al. Impact of clear fluid fasting on pulmonary aspiration in children undergoing general anesthesia: Results of the German prospective multicenter observational (NiKs) study. Paediatr Anaesth 2020;30:892-9. [Crossref] [PubMed]
- Hajian P, Shabani M, Khanlarzadeh E, et al. The Impact of Preoperative Fasting Duration on Blood Glucose and Hemodynamics in Children. J Diabetes Res 2020;2020:6725152. [Crossref] [PubMed]
- Benouaz S, Batouche DD, Benatta FN, et al. Improvement in the duration of preoperative fasting in children. Anaesthesiol Clin Sci Res 2018;2:6-11.
- Dolgun E, Yavuz M, Eroğlu B, et al. Investigation of Preoperative Fasting Times in Children. J Perianesth Nurs 2017;32:121-4. [Crossref] [PubMed]
- Newton RJG, Stuart GM, Willdridge DJ, et al. Using quality improvement methods to reduce clear fluid fasting times in children on a preoperative ward. Paediatr Anaesth 2017;27:793-800. [Crossref] [PubMed]
- Buller Y, Sims C. Prolonged fasting of children before anaesthesia is common in private practice. Anaesth Intensive Care 2016;44:107-10. [Crossref] [PubMed]
- Dennhardt N, Beck C, Huber D, et al. Impact of preoperative fasting times on blood glucose concentration, ketone bodies and acid-base balance in children younger than 36 months: A prospective observational study. Eur J Anaesthesiol 2015;32:857-61. [Crossref] [PubMed]
- Engelhardt T, Wilson G, Horne L, et al. Are you hungry? Are you thirsty?--fasting times in elective outpatient pediatric patients. Paediatr Anaesth 2011;21:964-8. [Crossref] [PubMed]
- Dennhardt N, Beck C, Huber D, et al. Optimized preoperative fasting times decrease ketone body concentration and stabilize mean arterial blood pressure during induction of anesthesia in children younger than 36 months: a prospective observational cohort study. Paediatr Anaesth 2016;26:838-43. [Crossref] [PubMed]
- Simpao AF, Wu L, Nelson O, et al. Preoperative Fluid Fasting Times and Postinduction Low Blood Pressure in Children: A Retrospective Analysis. Anesthesiology 2020;133:523-33. [Crossref] [PubMed]
- Huang Y, Tai J, Nan Y. Effect of fasting time before anesthesia on postoperative complications in children undergoing adenotonsillectomy. Ear Nose Throat J 2022; Epub ahead of print. [Crossref]
- Zhang Z, Wang RK, Duan B, et al. Effects of a Preoperative Carbohydrate-Rich Drink Before Ambulatory Surgery: A Randomized Controlled, Double-Blinded Study. Med Sci Monit 2020;26:e922837. [Crossref] [PubMed]
- Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg 2001;93:1344-50. [Crossref] [PubMed]
- Tudor-Drobjewski BA, Marhofer P, Kimberger O, et al. Randomised controlled trial comparing preoperative carbohydrate loading with standard fasting in paediatric anaesthesia. Br J Anaesth 2018;121:656-61. [Crossref] [PubMed]
- Zhang YL, Li H, Zeng H, et al. Ultrasonographic evaluation of gastric emptying after ingesting carbohydrate-rich drink in young children: A randomized crossover study. Paediatr Anaesth 2020;30:599-606. [Crossref] [PubMed]
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology 2011;114:495-511. [Crossref] [PubMed]
- Castillo-Zamora C, Castillo-Peralta LA, Nava-Ocampo AA. Randomized trial comparing overnight preoperative fasting period Vs oral administration of apple juice at 06:00-06:30 am in pediatric orthopedic surgical patients. Paediatr Anaesth 2005;15:638-42. [Crossref] [PubMed]
- Thomas M, Morrison C, Newton R, et al. Consensus statement on clear fluids fasting for elective pediatric general anesthesia. Paediatr Anaesth 2018;28:411-4. [Crossref] [PubMed]
- Isserman R, Elliott E, Subramanyam R, et al. Quality improvement project to reduce pediatric clear liquid fasting times prior to anesthesia. Paediatr Anaesth 2019;29:698-704. [Crossref] [PubMed]
- Manyande A, Cyna AM, Yip P, et al. Non-pharmacological interventions for assisting the induction of anaesthesia in children. Cochrane Database Syst Rev 2015;2015:CD006447. [Crossref] [PubMed]
- Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev 2015;2015:CD006275. [Crossref] [PubMed]
- Hall RW, Anand KJ. Pain management in newborns. Clin Perinatol 2014;41:895-924. [Crossref] [PubMed]
- Stevens B, Yamada J, Ohlsson A, et al. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2016;7:CD001069. [Crossref] [PubMed]



