
Analysis of RAS gene mutations in adverse events during first induction chemotherapy in childhood acute lymphoblastic leukemia
Highlight box
Key findings
• Compared with negative RAS mutation group, the risk of hyperbilirubinemia was significantly reduced in RAS mutation group (P=0.018), while the risk of agranulocytosis was significantly increased (P=0.003) in RAS mutation group when the BMI exceeded the median value (BMI >15.38).
What is known and what is new?
• The mechanisms of RAS pathway in hematologic malignancies have been extensively studied. However, prognostic significance of RAS mutation is controversial in pediatric acute lymphoblastic leukemia (ALL).
• To investigate potential correlation between RAS mutations and adverse events during first induction chemotherapy in pediatric ALL, clinical characteristics, treatment, and outcomes of 93 newly diagnosed ALL children during first induction chemotherapy were reviewed. Of them, 26 (27.9%) were positive for RAS mutation.
What is the implication, and what should change now?
• RAS mutation may increase the risk of agranulocytosis duration during first induction chemotherapy in ALL children with lower BMI.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy originating from lymphoid precursors (1). If the infiltration of leukemic cells in the central nervous system and subsequent formation of brain tumors occur, the overall survival is still exceptionally low. The CNS involvement is more common in ALL than in adult acute myeloid leukemia (2). Long-term outcomes in childhood ALL have improved over the past half century, largely due to the greater precision in the risk stratification of patients based on the initial diagnostic risk factors and the early treatment response as assessed by minimal residual disease (MRD) (3-5). In addition, the identification of genetic abnormalities associated with prognosis and treatment, along with effective targeted therapies, also plays an important role (6). With the development of gene chip technology and next-generation gene sequencing, an increasing number of genes closely associated with the occurrence and development of childhood ALL are being identified (7).
Rat sarcoma virus (RAS) genes are important proto-oncogenes in cells and consist of 3 independent genes: N-RAS, K-RAS, and H-RAS. The RAS gene encodes RAS proteins, which belong to the small G protein family, regulate signal transduction by binding to a variety of cell membrane receptors, and play an important role in physiological processes such as cell proliferation, differentiation, and apoptosis (8). In many adult tumors, such as colon cancer, lung cancer, and melanoma, RAS gene activating mutations are considered to be important oncogenic events in tumor formation (9-11). However, the relationship between RAS and chemotherapy remains elusive. Jerchel et al. found RAS-related mutations (N-RAS, K-RAS, FLT3 and PTPN11) in 44.2% of 461 samples obtained from children with ALL at the time of initial diagnosis (12). The prognostic relationship between RAS genes and hematologic tumor diseases in children has recently attracted attention. In this study, we retrospectively analyzed the relationship between RAS gene mutations and the incidence of various adverse events during first induction chemotherapy in 93 children with newly diagnosed ALL and investigated the impact of RAS gene mutations on leukemia treatment and prognosis. We present the following article in accordance with the REMARK reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-683/rc).
Methods
Patient cohorts
A retrospective cohort analysis was conducted on 93 records of children who were diagnosed with ALL from January 2020 to July 2021 in the Department of Hematology and Oncology in Anhui Provincial Children’s and who met the following inclusion criteria: (I) age ≤14 years; (II) meeting the diagnostic criteria set by the recommendations on the Suggestion of diagnosis and treatment of acute lymphoblastic leukemia in childhood (the 4th revised version, Revised by Hematology Group, Pediatrics Branch of Chinese Medical Association) (11); and (III) diagnosed with ALL for the first time (did not receive any drug chemotherapy, including glucocorticoids, before the first induction chemotherapy). The exclusion criteria were the following: (I) children with unclear diagnosis; (II) children not diagnosed with ALL for the first time; (III) children who had received induction chemotherapy or nonstandard treatment in other hospitals; (IV) children who were transferred to the hospital during the first induction chemotherapy but did not complete the induction treatment; and (V) children who voluntarily abandoned treatment for non-disease-related reasons. Informed consent was obtained from patient’s parents. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocols were approved by the Ethics Committee of Anhui Provincial Children’s Hospital (No. CR20221210).
Mutation analysis of RAS gene alterations
All genomic DNA (gDNA) samples were obtained from bone marrow mononuclear cells at the time of new diagnosis. Polymerase chain reaction (PCR) primers were used to amplify the target genome, which was enriched and sequenced on a NextSeq550 sequencer (Illumina NovaSeq6000, USA). The original data were analyzed and annotated with gene variation information through the optimized biomarker analysis process, and the pathogenic gene mutation sites were finally screened. This test covered point mutations in exonic regions and nearby intronic regions within ±10 bp as well as insertional and deletional mutations within 10 bp of common causative genes in ALL.
Classifications and treatments
All the enrolled participants with ALL were diagnosed according to the diagnostic criteria formulated by the recommendations on the Suggestion of diagnosis and treatment of acute lymphoblastic leukemia in childhood (the 4th revised version, Revised by Hematology Group, Pediatrics Branch of Chinese Medical Association) (13). The risk group assignment was based on age, white blood cell (WBC) count, immune typing, cytogenetic characteristics at diagnosis, and early treatment response and MRD level. All patients were then stratified into the standard risk group, medium risk group, and high-risk group.
The Chinese Children’s Leukemia Group-acute lymphoblastic leukemia 2018 (CCLG-ALL-2018) protocol was published in 2018 and is recommended as a clinical guideline for the treatment of pediatric ALL in China. The treatment regimen contains 5 phases, including induction, early reinforcement, consolidation, delayed reinforcement, and maintenance treatments.
All children were treated with risk-based regimens according to the CCLG-ALL-2018 protocol. The main drugs of the first induction chemotherapy regimen included prednisone, vincristine (VCR), daunorubicin (DNR), and L-asparaginase (L-ASP). The specific regimen is illustrated in Table 1.
Table 1
| Drug | LR | IR and HR |
|---|---|---|
| Pred | 60 mg/m2/d, d1–28 | 60 mg/(m2·d), d1 25% of total dose, d2 50% of total dose, d3 75% of total dose, d4 100% of total dose, d1–7 |
| VCR | 1.5 mg/m2, once a week, 4 times in total | 1.5 mg/m2, once a week, 4 times in total |
| DNR | 30 mg/m2, once a week, 2 times in total | 30 mg/m2, once a week, 4 times in total |
| L-ASP | 2,500 U/m2, d9, d23 | 2,500 U/m2, d9, d23 |
Pred, prednisone; VCR, vincristine; DNR, daunorubicin; L-ASP, L-asparaginase; LR, low risk; IR, intermediate risk; HR, high risk.
Adverse events
According to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, all children were classified and evaluated for adverse events during the first induction chemotherapy. The most common adverse events include fever, infection, sepsis, hypotension, hypertension, hypoxia, acute respiratory distress syndrome (ARDS), pancreatitis, hepatotoxicity [elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST)], hyperbilirubinemia, hyponatremia, and intestinal obstruction and constipation.
Statistical analysis
The software OriginPro 2021 (OriginLab Corporation, Northampton, MA, USA) was used to draw the heatmap of mutant genes. The χ2 test was used to compare the rates, and the t test was used to compare the continuous variables with normal distribution. The Mann-Whitney test was used for continuous variables that did not follow normal distribution, and the χ2 test was used for categorical variables. Univariate analysis variables with P<0.1 were substituted into a binary logistic regression model for multivariate analysis. The competitive risk model R 4.0.3 software (The R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis. Kaplan-Meier survival analysis, a Cox regression model, and multiple linear regression were used to explore the relationship between the RAS gene mutation and the risk and duration of some adverse events. Univariate analysis variables with P<0.1 were substituted into the Cox regression model for multivariate analysis. P<0.05 was considered statistically significant.
Results
Clinical characteristics and outcomes
A total of 93 children with ALL were enrolled in this study, including 51 males and 42 females. All enrolled patients accepted vincristine + daunorubicin + L asparaginase + prednisone (VDLP) chemotherapy as the first induction chemotherapy. The mean age at consultation was 5.2±3.2 years. There were 11 cases (11.8%) of standard risk children, 62 cases (66.7%) of medium-risk children, and 15 cases (16.1%) of high-risk children. The patients with who turned MRD-negative on the 15th day during the first induction chemotherapy accounted for 37.6% of patients, while patients who turned MRD-negative on the 33rd day accounted for 78.5%. The bone marrow cytological CR rate on the 33rd day was 100%. Other general clinical data are shown in Table 2.
Table 2
| Characteristic | All (N=93) | RAS positive (n=26) | RAS negative (n=67) | P value |
|---|---|---|---|---|
| Age (years) | 5.2±3.2 | 4.8±3.1 | 5.3±3.35 | 0.515 |
| BMI (kg/m2) | 16.30±4.02 | 15.4±155.2 | 16.6±4.61 | 0.333 |
| Blood routine | ||||
| WBC (×109/L) | 66.55±134.97 | 85.89±22.74 | 59.05±126.75 | 0.404 |
| Hb (g/L) | 74.30±24.48 | 74.28±52.18 | 74.34±25.29 | 0.901 |
| PLT (×109/L) | 70.15±65.82 | 60.65±1.54 | 73.84±70.43 | 0.722 |
| Sex | 1.000 | |||
| Male | 51 (54.8%) | 14 (53.8%) | 37 (55.2%) | |
| Female | 42 (45.2%) | 12 (46.2%) | 30 (44.8%) | |
| Risk group | 0.929 | |||
| LR | 11 (11.8%) | 3 (11.5%) | 8 (11.9%) | |
| IR | 62 (66.7%) | 18 (69.2%) | 44 (65.7%) | |
| HR | 15 (16.1%) | 3 (11.5%) | 12 (17.9%) | |
| Immunophenotype | 0.281 | |||
| B-ALL | 82 (88.2%) | 21 (80.8%) | 61 (91.0%) | |
| T-ALL | 11 (11.8%) | 5 (19.2%) | 6 (9.0%) | |
| MRD status | ||||
| D15 MRD <10−4 | 35 (37.6%) | 11 (42.3%) | 24 (35.8%) | 0.604 |
| D33 MRD <10−4 | 73 (78.5%) | 13 (50%) | 54 (80.6%) | 0.502 |
| CNS status | 0.067 | |||
| CNSL1 | 82 (88.2%) | 20 (76.9%) | 62 (92.5%) | |
| CNSL2 | 11 (11.8%) | 6 (23.1%) | 5 (7.5%) | |
| CNSL3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Tumor lysis | 4 (4.3%) | 1 (3.8%) | 3 (4.5%) | 1.000 |
| Fusion gene abnormality | 32 (34.4%) | 2 (7.7%) | 30 (44.8%) | 0.002* |
| Chromosome abnormality | 38 (40.9%) | 15 (57.7%) | 23 (34.3%) | 0.059 |
| Induction failure death | 5 (5.4%) | 2 (7.7%) | 3 (4.5%) | 0.616 |
The data are shown as n (%) or mean ± standard deviation. *, compared with the RAS-negative group, P<0.05. RAS, rat sarcoma virus; BMI, body mass index; WBC, white blood cell; Hb, hemoglobin; PLT, platelets; LR, low risk; IR, intermediate risk; HR, high risk; B-ALL, Precursor B cell acute lymphoblastic leukemia; T-ALL, Precursor T cell acute lymphoblastic leukemia; MRD, minimal residual disease; CNSL, central nervous system leukemia.
Furthermore, 88 patients (94.6%) with ALL completed induction chemotherapy, and other 5 died during induction. One patient died of acute cerebral hemorrhage. One patient died of ARDS due to repeated hypoxemia during chemotherapy. The remaining 3 cases died of septic shock, including 1 case of multidrug-resistant bacteria infection.
RAS mutations in children with ALL
Regarding gene mutations, 32 cases (34.4%) were gene mutation–positive, and 38 cases (40.9%) had bone marrow chromosome abnormalities at initial diagnosis. The most frequent type of fusion gene (n=16, 17.2%) was ETV6/RUNX1. The most common genetic alteration (n=26, 27.9%) was the RAS gene mutation, including 19 cases of N-RAS mutation, 8 cases of K-RAS mutation, and 1 case of double mutation, followed by the NOCTH1 gene mutation (n=8, 8.6%). NOTCH1 mutations were detected in 7 cases with Precursor T cell acute lymphoblastic leukemia (T-ALL) and only in 1 case with Precursor B cell acute lymphoblastic leukemia (B-ALL). The distribution of the remaining mutations is shown in the mutant gene heat map (Figure 1). It is worth noting that RAS-positive samples showed a lower detection rate of fusion genes than did the RAS-negative group (P=0.002; Table 1).
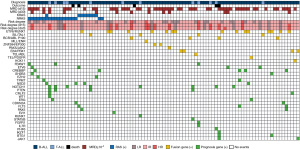
Statistics of various adverse events in first induction chemotherapy
The occurrence of 23 types of adverse events, including fever, infection, granulopenia, liver function impairment, and intensive care unit (ICU) admission during the first induction chemotherapy of children with ALL was counted in this study. The results showed that the most common adverse events during the first induction of ALL were coagulation dysfunction (n=76, 81.7%), fever (n=71, 81.7%), and elevated ALT (n=34, 36.6%). The risk of hyperbilirubinemia was significantly reduced in the RAS-positive group compared with the RAS- negative group (P=0.018), and there was no significant difference in the other types of adverse events (Table 3).
Table 3
| Adverse events | RAS mutation (n=26) | RAS negative (n=67) | OR (95%)1 | P value |
|---|---|---|---|---|
| Fever | 35 | 50 | 0.95(0.22, 4.96) | 0.947 |
| Infection | N/A | |||
| At diagnose | 12 | 29 | ||
| At induction | 16 | 39 | ||
| Sepsis | 5 | 17 | N/A | |
| Hypotension | 2 | 4 | 4.48(0.34, 77.76) | 0.253 |
| Hypertension | 0 | 1 | N/A | |
| Hypoxia | 2 | 3 | N/A | |
| ARDS | 2 | 3 | 4.48 (0.34, 77.76) | 0.253 |
| Pancreatitis | 0 | 0 | N/A | |
| ALT elevation | 13 | 19 | 0.80 (0.17, 3.75) | 0.774 |
| AST elevation | 11 | 16 | 0.64 (0.13, 2.97) | 0.57 |
| Hyperbilirubinemia | 0 | 7 | 0.03 (0, 0.39) | 0.018* |
| Constipation | 11 | 17 | 2.47 (0.88, 6.97) | 0.085 |
| ICU admission | 1 | 5 | 2.08 (0.37, 10.97)2 | 0.381 |
| Hyperglycemia | 0 | 1 | –2 | N/A |
| TE | 0 | 3 | – | N/A |
| Stroke | 0 | 0 | – | N/A |
| Neuropathy | 0 | 0 | – | N/A |
| Seizure | 0 | 0 | – | N/A |
| Anaphylaxis | 0 | 0 | – | N/A |
| Hyponatrem | 2 | 2 | – | N/A |
| Ileus | 1 | 1 | – | N/A |
| Agranulocytosis duration (day) | 18 | 24 | – | N/A |
| Death | 2 | 3 | – | N/A |
*, compared with the RAS-negative group, P<0.05; 1, model adjusted for age, sex, body mass index, infection status and antibiotic use; 2, the number of adverse outcomes during induction therapy was zero or too few to allow statistical analysis. ALL, acute lymphoblastic leukemia; ARDS, acute respiratory distress syndrome; ALT, alanine transaminase; AST, aspartate transaminase; TE, thromboembolism; ICU, intensive care unit; RAS, rat sarcoma virus; OR, odds ratio; N/A, not applicable.
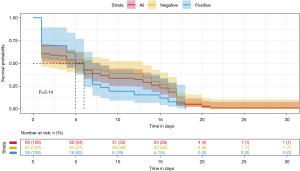
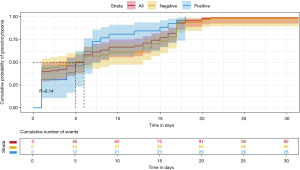
The median time of emergence of agranulocytosis was 7.57 days during the first induction chemotherapy. The RAS mutation-positive subgroup developed neutropenia earlier than RAS mutation-negative mutation subgroup (6.38 vs. 8.03 days), but there was no significant difference between the two groups (P=0.38). On other hand, as shown in Table 3, the duration of agranulocytosis in the RAS mutation-positive subgroup was shorter than that in the RAS mutation-negative subgroup (18 vs. 24 days); similarly, there was no significant difference between the two groups (P=0.14).
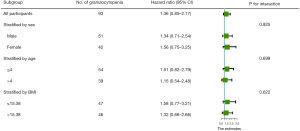
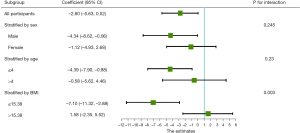
On the 33rd day of VDLP induction chemotherapy, routine blood examination revealed that all patients with ALL developed severe agranulocytosis (Figures 2,3). The Cox proportional hazards model and multiple linear regression analysis showed that in the RAS mutation-negative group, the risk of prolonged agranulocytosis was significantly increased when BMI exceeded the median value of the study population (BMI >15.38; P=0.003; Figures 4,5).
Discussion
The RAS gene contains 3 different mutant subtypes, which are mainly found in eukaryotes (8,14). N-RAS is located on chromosome 1 (1p22-p32), while H-RAS and K-RAS are located on chromosome 11 (11p15.1-p15.3) and chromosome 12 (12P1.1), respectively (15). Different subtypes of RAS genes are expressed differently in tumors (16). According to the Cancer Somatic Mutation Catalog database, K-RAS is the most common mutant subtype in all cancers. The main RAS genes of common mutations are located on codon 12, 13 and 61, and are somatic mutations (17,18).
As an important signal switch, RAS protein molecules participate in the binding and dissociation of guanosine triphosphate/diphosphate (GTP/GDP), which in turn activate downstream of the original activated protein kinase and phosphatidyl inositol-3-kinase/protein kinase β signaling pathway (19,20). This affects cell growth and other life processes, including proliferation, differentiation, and apoptosis, and promotes the occurrence and development of tumors. RAS is the most common mutated oncogene in human tumors, and is widely present in hematological tumors, colon cancer, thyroid cancer, gastric cancer, lung cancer, and other malignant tumors. In acute myeloid leukemia (AML), the RAS mutation is associated with hyperleukocytic leukemia, high lactate dehydrogenase (LDH) count and poor prognosis (21,22). Recent advances indicate that the RAS mutation also contributes to drug resistance in the treatment of AML (23). The relationship between RAS mutations and the prognosis of ALL remains unclear and controversial. This study analyzed the correlation between RAS gene mutations at initial diagnosis and adverse events during the first induction chemotherapy in 93 children with ALL and further evaluated new risk stratification and prognostic assessment indicators for pediatric ALL.
In this study, 19 cases of N-RAS mutation, 8 cases of K-RAS mutation, and 1 case of double mutation were detected in children with newly diagnosed ALL, with a detection rate of 26.9% (26/93). The RAS mutation rate in children with B-ALL was higher than that in children with T-ALL, which was basically consistent with the report by Wiemels et al. (24). Studies have reported that the RAS pathway can be used as a poor prognostic factor for B-ALL and that T-ALL with RAS pathway mutations are allergic to MEK inhibitor in vitro and in vivo (25). Huang et al. (26) analyzed bone marrow samples from 368 children newly diagnosed with Philadelphia chromosome-negative acute lymphoblastic leukemia (Ph-ALL), and found that RAS pathway mutation and IKZF1 deletion were independent predictors of poor prognosis. The 10-year event-free survival (EFS; 11.1%±10.5%) and 10-year overall survival rate (53.3%±17.6%) of those with IKZF1 deletion and RAS pathway mutation were the worst. In our study, only 2 children were detected with IKZF1 deletion and negative RAS mutation. Considering the low sample size, we refrained from performing correlation analyses. Notably, this child eventually died due to severe infection.
All 93 children received induction chemotherapy under the CCLG-ALL 2018 protocol. Except for 5 children who died during induction period, all children completed induction chemotherapy. The 33-day bone marrow cell morphologically induced complete remission rate was 100%, and the 33-day MRD-negative conversion rate was 85.2% (75/88). The results show that the short-term efficacy of this regimen is excellent, which is similar to the clinical data of advanced pediatric hematology centers in Europe and America in recent years (27).
Studies have reported that hyperbilirubinemia during chemotherapy in ALL is mainly related to pegylated asparaginase (PEG-asparaginase) or asparaginase (28). Age >10 years was found to be a definitive risk factor for hyperbilirubinemia [odds ratio (OR) =3.83; 95% CI: 1.64–8.95] (29). In addition, asparaginase-related adverse reactions also include hypersensitivity reaction, venous thromboembolism, and pancreatitis. In an multicenter phase II trial (NCT01920737), investigators used pediatric ALL regimens to further assess the safety and efficacy of different doses of asparaginase in 39 adults aged 20 to 60 years (median 38 years) with newly diagnosed ALL or lymphoblastic lymphoma. The results showed that patients aged 40–60 years (n=18) had a significantly higher probability of developing grade 3–4 hyperbilirubinemia than did patients aged 18–39 years (n=21; 44% vs. 10%; P=0.025) (30). In the present clinical trial, according to different disease risk and prognostic factors, anti-infection and supportive therapeutic strategies are adjusted in a timely manner, aiming to lower the incidence of adverse events occurring during the first induction chemotherapy for childhood ALL. Due to the small sample size, our study failed to further explore the related mechanism between age and hyperbilirubinemia, and the occurrence of some specific CTCAE grade 2 or above adverse events with clinical significance in pediatric ALL was too low to be included in the statistical analysis. The sample size needs to be expanded in subsequent studies for further investigation.
In this study, multivariate analysis showed that, the risk of prolonged agranulocytosis was significantly increased in RAS mutation-positive group, when the BMI exceeded the median value of the study population (BMI >15.38; P=0.003). This may be due to the higher body fat percentage and lower systemic drug clearance rate in children with increased BMI. Chemotherapy drugs such as anthracyclines have a longer half-life, leading to prolonged agranulocytosis. According to the study by Sun et al. (31), compared with that in a normal BMI group, the MRD of children with abnormally elevated BMI on day 19 and day 46 was higher (P=0.04 and P=0.008), and there was a positive correlation (P=0.014). Leptin resistance should be considered in children with high BMI. Recent studies also reported obesity to be a risk factor for chemotherapy-related osteonecrosis in children with ALL (OR =2.10, 95% CI: 1.12–3.95; P=0.02) (32). However, in another cohort study of Hispanic children with ALL, researchers found there to be no statistically significant difference in the disease-free survival (DFS) and OS rates between the overweight and obese group and the normal-weight group (33). Although the risk of overweight or obese status in more common in ALL/LL survivors than in survivors of other tumor types (67% vs. 14%; P=0.037), a series of future studies are needed to determine whether abnormally increased BMI is clearly associated with the prognosis of children (34).
Conclusion
In conclusion, this study showed that children with newly diagnosed ALL and RAS gene mutations were less likely to have fusion gene expression. RAS gene mutation increases the risk of prolonged agranulocytosis during the first induction chemotherapy and reduces the risk of hyperbilirubinemia in children with ALL. Due to limitations in the sample size and follow-up time, the possible influence of the RAS gene on various adverse events and long-term prognosis during the first induction chemotherapy of ALL needs to be further studied with a greater number of cases.
Acknowledgments
Funding: This work was supported by Natural Science Foundation of Anhui Province (grant No. 2108085MH268), and the Scientific Research Foundation of Anhui Medical University (grant No. 2021xkj228).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist Available at https://tp.amegroups.com/article/view/10.21037/tp-22-683/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-683/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-683/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from patient’s parents. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocols were approved by the Ethics Committee of Anhui Provincial Children’s Hospital (No. CR20221210).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Deak D, Gorcea-Andronic N, Sas V, et al. A narrative review of central nervous system involvement in acute leukemias. Ann Transl Med 2021;9:68. [Crossref] [PubMed]
- Mai H, Li Q, Wang G, et al. Clinical application of next-generation sequencing-based monitoring of minimal residual disease in childhood acute lymphoblastic leukemia. J Cancer Res Clin Oncol 2022; Epub ahead of print. [Crossref]
- Arthur C, Rezayee F, Mogensen N, et al. Patient-Specific Assays Based on Whole-Genome Sequencing Data to Measure Residual Disease in Children With Acute Lymphoblastic Leukemia: A Proof of Concept Study. Front Oncol 2022;12:899325. [Crossref] [PubMed]
- Feng J, Cheng FWT, Chiang AKS, et al. Outcomes of adolescents with acute lymphoblastic leukaemia. Hong Kong Med J 2022;28:204-14. [Crossref] [PubMed]
- Severgnini M, D'Angiò M, Bungaro S, et al. Conjoined Genes as Common Events in Childhood Acute Lymphoblastic Leukemia. Cancers (Basel) 2022;14:3523. [Crossref] [PubMed]
- Liu Y, Nuriding H, Zhao L, et al. Genomic and Clinical Analysis of Children with Acute Lymphoblastic Leukemia. Comput Math Methods Med 2022;2022:7904293. [Crossref] [PubMed]
- Harvey JJ. An unidentified virus which causes the rapid production of tumours in mice. Nature 1964;204:1104-5. [Crossref] [PubMed]
- Matas J, Kohrn B, Fredrickson J, et al. Colorectal Cancer Is Associated with the Presence of Cancer Driver Mutations in Normal Colon. Cancer Res 2022;82:1492-502. [Crossref] [PubMed]
- Liu Z, Zhao M, Jiang X, et al. Upregulation of KLHL17 promotes the proliferation and migration of non-small cell lung cancer by activating the Ras/MAPK signaling pathway. Lab Invest 2022;102:1389-99. [Crossref] [PubMed]
- Krishnamurthy K, Urioste SN, Cusnir M, et al. Analysis of Genetic Alterations in Cutaneous Malignant Melanomas Unveils Unique Loco-Regional Variations and Novel Predictors of Metastatic Potential. Am J Dermatopathol 2021;43:e185-9. [Crossref] [PubMed]
- Jerchel IS, Hoogkamer AQ, Ariës IM, et al. RAS pathway mutations as a predictive biomarker for treatment adaptation in pediatric B-cell precursor acute lymphoblastic leukemia. Leukemia 2018;32:931-40. [Crossref] [PubMed]
- Subspecialty Group of Hematology Diseases, The Society of Pediatrics, Chinese Medical Association. Editorial Board, Chinese Journal of Pediatrics. Zhonghua Er Ke Za Zhi 2006;44:392-5.
- Kirsten WH, Mayer LA. Morphologic responses to a murine erythroblastosis virus. J Natl Cancer Inst 1967;39:311-35.
- Hall A, Marshall CJ, Spurr NK, et al. Identification of transforming gene in two human sarcoma cell lines as a new member of the ras gene family located on chromosome 1. Nature 1983;303:396-400. [Crossref] [PubMed]
- Gimple RC, Wang X. RAS: Striking at the Core of the Oncogenic Circuitry. Front Oncol 2019;9:965. [Crossref] [PubMed]
- Cazzanelli G, Pereira F, Alves S, et al. The Yeast Saccharomyces cerevisiae as a Model for Understanding RAS Proteins and their Role in Human Tumorigenesis. Cells 2018;7:14. [Crossref] [PubMed]
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res 2019;47:D941-7. [Crossref] [PubMed]
- Arrazola Sastre A, Luque Montoro M, Gálvez-Martín P, et al. Small GTPases of the Ras and Rho Families Switch on/off Signaling Pathways in Neurodegenerative Diseases. Int J Mol Sci 2020;21:6312. [Crossref] [PubMed]
- Harrell Stewart DR, Clark GJ. Pumping the brakes on RAS - negative regulators and death effectors of RAS. J Cell Sci 2020;133:jcs238865. [Crossref] [PubMed]
- Kaburagi T, Yamato G, Shiba N, et al. Clinical significance of RAS pathway alterations in pediatric acute myeloid leukemia. Haematologica 2022;107:583-92. [Crossref] [PubMed]
- Decroocq J, Birsen R, Montersino C, et al. RAS activation induces synthetic lethality of MEK inhibition with mitochondrial oxidative metabolism in acute myeloid leukemia. Leukemia 2022;36:1237-52. [Crossref] [PubMed]
- Zhang Q, Riley-Gillis B, Han L, et al. Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal Transduct Target Ther 2022;7:51. [Crossref] [PubMed]
- Wiemels JL, Zhang Y, Chang J, et al. RAS mutation is associated with hyperdiploidy and parental characteristics in pediatric acute lymphoblastic leukemia. Leukemia 2005;19:415-9. [Crossref] [PubMed]
- Zhu H, Dong B, Zhang Y, et al. Integrated genomic analyses identify high-risk factors and actionable targets in T-cell acute lymphoblastic leukemia. Blood Sci 2022;4:16-28. [Crossref] [PubMed]
- Huang YJ, Liu HC, Jaing TH, et al. RAS pathway mutation is an added-value biomarker in pediatric Philadelphia-negative B-cell acute lymphoblastic leukemia with IKZF1 deletions. Pediatr Blood Cancer 2021;68:e28899. [Crossref] [PubMed]
- Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia 2018;32:606-15. [Crossref] [PubMed]
- Vrooman LM, Blonquist TM, Stevenson KE, et al. Efficacy and Toxicity of Pegaspargase and Calaspargase Pegol in Childhood Acute Lymphoblastic Leukemia: Results of DFCI 11-001. J Clin Oncol 2021;39:3496-505. [Crossref] [PubMed]
- Dharia P, Swartz MD, Bernhardt MB, et al. Clinical and demographic factors contributing to asparaginase-associated toxicities in children with acute lymphoblastic leukemia. Leuk Lymphoma 2022;63:2948-54. [Crossref] [PubMed]
- Geyer MB, Ritchie EK, Rao AV, et al. Pediatric-inspired chemotherapy incorporating pegaspargase is safe and results in high rates of minimal residual disease negativity in adults up to age 60 with Philadelphia chromosome-negative acute lymphoblastic leukemia. Haematologica 2021;106:2086-94. [Crossref] [PubMed]
- Sun J, Zhang R, Tang J, et al. Prognostic Observational Analysis of BMI, Leptin, and Adiponectin in Children With Acute Lymphocytic Leukemia Undergoing Remission-Induction Chemotherapy. Front Pediatr 2022;10:797836. [Crossref] [PubMed]
- Thompson J, Fisher B, Sung L, et al. Musculoskeletal impairments in children receiving intensive therapy for acute leukemia or undergoing hematopoietic stem cell transplant: A report from the Children's Oncology Group. Pediatr Blood Cancer 2021;68:e29053. [Crossref] [PubMed]
- Jaime-Pérez JC, Turrubiates-Hernández GA, García-Salas G, et al. The Influence of Nutritional Status at Diagnosis of Childhood B-Cell Acute Lymphoblastic Leukemia on Survival Rates: Data from a Hispanic Cohort. Nutr Cancer 2022;74:889-95. [Crossref] [PubMed]
- van der Haak N, Edwards S, Perem M, et al. Nutritional Status at Diagnosis, During, and After Treatment in Adolescents and Young Adults with Cancer. J Adolesc Young Adult Oncol 2021;10:668-74. [Crossref] [PubMed]
(English Language Editor: J. Gray)


