
A newborn with a pathogenic variant in ASXL2 expanding the phenotype of SHAPNS: a case report and literature review
Highlight box
Key findings
• We presented a newborn diagnosing the Shashi-Pena syndrome (SHAPNS) by trio-WES, which is the earliest age of diagnosis.
• The application of octreotide for hypoglycemia, the small cerebellum and bilateral paving-stone-like white lesions of the retinas are described for the first time in an individual with ASXL2-related SHAPNS.
What is known and what is new?
• SHAPNS is a developmental disorder caused by mutations in additional sex combs-like protein 2 (ASXL2) and characterized by hypoglycemia, neonatal feeding difficulties, minor congenital heart disease, hypotonia, seizures, cerebral atrophy, developmental disabilities, macrocephaly and a typical facial appearance consisting of a glabellar nevus flammeus, hypertelorism, arched eyebrows and prominent eyes. Since 2016, only 12 cases from 10 families have been reported and the majority of related patients were discovered and diagnosed at an older age.
• This is the first time that octreotide was administered in patients with persistent hypoglycemia secondary to SHAPNS and successfully helped our patient achieve euglycemia without hypoglycemic brain injury. Unusual fundoscopy results of paving-stone like white-lesion and small cerebellum on the MRI were identified in patients with SHAPNS for the first time.
What is the implication, and what should change now?
• Further clinical reports of more neonatal individuals with damaging ASXL2 variants are necessary to verify the mechanism and optimal treatment of ASXL2-related hypoglycemia, neurological damage and optic impairment.
• Neurological, endocrinological, ophthalmological, and rehabilitative follow-ups of these patients are necessary and important.
Introduction
ASXL2-related Shashi-Pena syndrome (SHAPNS, OMIM #617190) was originally described in 2016 in six unrelated individuals who had de novo truncating variants in ASXL2 (NM_018263.6) and shared a recognizable clinical phenotype (1). SHAPNS is a rare autosomal dominant disorder characterized by hypoglycemia, neonatal feeding difficulties, minor congenital heart disease, hypotonia, seizures, cerebral atrophy, developmental disabilities, macrocephaly and a typical facial appearance consisting of a glabellar nevus flammeus, hypertelorism, arched eyebrows and prominent eyes (1).
The incidence of ASXL2-related SHAPNS is unknown. To date, only 12 individuals with ASXL2-related SHAPNS have been reported in the literature (1-5), three of whom came from one family (3). Clinical information for the early neonatal period was limited in all the case reports. Reports of individuals with damaging ASXL2 variants (NM_018263.6) and related clinical features are essential to reveal the full phenotype of this syndrome from birth to adulthood (1,3) and assist physicians in optimal clinical management, family consultation, and strategy determination for follow-ups.
Undoubtedly, in neonates admitted to the intensive care unit with nonspecific clinical features, a rapid genetic diagnosis is vital for prompt targeted treatments and improved clinical outcomes (6). However, early and timely diagnosis of rare congenital disease remains challenging in the neonatal period. In 2016, we launched the China Neonatal Genomes Project (CNGP), which led to the discovery of a number of new gene variants underlying congenital disorders, and a significant number of diagnosed neonatal patients have benefited from more focused medical management and long-term care (7).
In this study, we describe the clinical data of a neonate with a previously unreported ASXL2 variant (NM_018263.6) that was detected via trio exome sequencing carried out by the CNGP. In addition, we focus on the neonatal-onset manifestations, management and short-term prognosis of SHAPNS. Moreover, to assist physicians in optimal clinical management, family consultation and strategy determination for follow-ups, we summarize important information on the clinical genotype, phenotype, major clinical issues and prognosis of this syndrome found in a literature review, building on the data for ASXL2-related SHAPNS previously reported by Shashi et al. (1), Cuddapah et al. (2), Wang et al. (3), Alqaisi et al. (4) and Jiao et al. (5). We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-220-/rc).
Case presentation
A 2-day-old male neonate with feeding difficulty, recurrent hypoglycemia and specific facial features was admitted to the Department of Neonatology of Children’s Hospital of Fudan University. Trio-based WES was performed for this neonate and his parents according to the protocol of the CNGP (8). Clinical information was prospectively collected. Informed consent was acquired.
This male neonate was born to a 29-year-old woman (gravida 1, para 1) via spontaneous vaginal delivery on September 9th, 2021. No resuscitation was needed. He was born at 40+2 weeks’ gestational age with a birth weight of 3,030 g (10th–50th percentile), length of 48 cm (10th–50th percentile) and head circumference of 36 cm (90th percentile). The Apgar scores were 8, 10 and 10, respectively. At the age of two days (September 10th, 2021), he was transferred to the Department of Neonatology of Children’s Hospital of Fudan University due to three episodes of hypoglycemia (1.6–1.8 mmol/L) and two episodes of cyanosis while orally feeding.
Both mother and father were in good medical conditions. Antenatal care was unremarkable. There was no family history of inheritable disorders.
Upon admission, his physical examination revealed a large V-shaped glabellar nevus flammeus, proptosis, hypertelorism, a broad nasal tip, a broad forehead, low-set and posteriorly rotated ears, thick ear lobes, arched eyebrows, retrognathia, deep palmar creases (Figure 1A), overlapping second and fourth toes (Figure 1B), small nails and hirsutism on the back (Figure 1C).
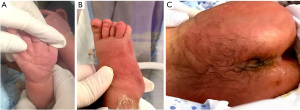
After admission, his feeding status was designated nil per os, and he received continuous intravenous parental nutrition. He required a maximum glucose infusion rate of 9 mg/kg/min to maintain normal glucose level. A series of investigations was performed to clarify the underlying cause of cyanosis. Echocardiography showed a patent ductus of arteriosus (3.7 mm) and an atrial septum defect (6.2 mm), both with left to right shunt. Barium radiography of the upper digestive tract showed evidence of gastroesophageal reflux disease (GERD). Laryngoscopy showed that he had laryngomalacia. On magnetic resonance imaging (MRI), the size of cerebellum was relatively small, with a diameter of 45.7 mm (Figure 2A). Fundoscopy showed bilateral paving-stone-like white lesions in the retinas (Figure 2B,2C) and no significant change at follow-ups when he was at the age of 6 weeks. The small cerebellum and bilateral paving-stone-like white lesions of the retinas were identified for the first time in an individual with ASXL2-related SHAPNS.

He underwent diagnostic fasting and glucagon tests, which indicated unreasonably high levels of insulin. When his plasma glucose level decreased to 2.1 mmol/L, the levels of insulin, low free fatty acids and beta hydroxybutyrate were 2.1 µU/mL, 0.34 and 0.06 mmol/L, respectively. Thirty minutes after glucagon administration, his plasma glucose level increased to 2.3 mmol/L.
He tolerated full enteral feeding orally without cyanosis at the age of 10 days. However, at the age of 19 days (September 27th, 2021) the volume of enteral feeding was decreased, and he required continuous intravenous infusion of dextrose at 9 mg/kg/min because of recurrent hypoglycemia. He was treated with glucagon at 12 µg/kg/hour (September 27th, 2021) which was gradually weaned off and switched to octreotide injection subcutaneously (started at 0.006 mg every 8 hours) at the age of 35 days (October 13th, 2021). Since then, he maintained euglycemia ranging from 3.0 to 5.8 mmol/L and was successfully weaned off intravenous dextrose when he was 41 days old (October 19th, 2021). At the age of 45 days (October 23rd, 2021), he was discharged home on full enteral feeding with additional 2.5 gram of enzymatic rice flour in every 70 mL of breast milk per 3 hour and subcutaneous octreotide injection (0.004 mg/kg every 8 hours). Figure 3 depicted a detailed timeline of the treatment for hypoglycemia.
He was followed-up in outpatient clinics and had no neurodevelopmental delay at the age of 4 months. The detailed clinical characteristics are summarized in Table S1. Based on the above history, we performed a genetic test.
All procedures performed in this study were in accordance with the ethical standards of the Research Ethics Committee of the Children’s Hospital of Fudan University (CHFudanU_NNICU11) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Molecular studies
DNA samples were sequenced to identify the casual gene with whole-exome sequencing (WES). Genomic DNA samples were extracted from whole blood using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). DNA fragments were enriched for exome sequences using the Agilent SureSelectXT Human All Exon 50-Mb kit. Sequencing was performed on an Illumina HiSeq 2000/2500 platform. The average on-target sequencing depth was 200×. Clean reads were aligned to the reference human genome (UCSC hg19) by the Burrows-Wheeler Aligner (BWA; v.0.5.9-r16). For variant calling, GATK best practice (V.3.2) was employed for single-nucleotide variants (SNVs)/small indels. Variants were confirmed in the proband and the parents, if available, using Sanger sequencing (Figure 4), Primers were designed for polymerase chain reaction amplification using Primer Premier 5.0 software. Sequence analysis was performed with Mutation Surveyor (Soft Genetics, State College, PA).
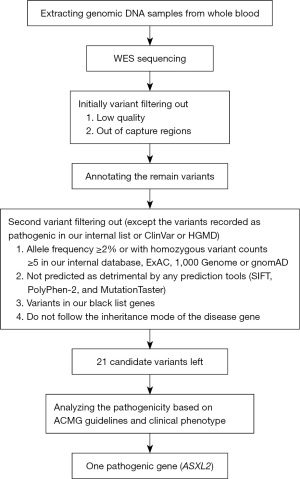
The variants with low quality and out of capture regions were initially filtered out. Then ANNOVAR (9) and VEP (10) software were applied to annotate the remain variants with gene information, variant impacts (splicing region, loss of function), scores of missense vairant (SIFT, PolyPhen-2, and MutationTaster), population frequencies (our internal database, ExAc, 1000 Genomes and gnomAD) and the classification of the variant in public database (ClinVar and HGMD). After annotation, a second variant filtering, except the variants recorded as pathogenic in our internal list or ClinVar or HGMD, was carried out based on the following criteria: (I) with allele frequency greater than or equal to 2% or with homozygous variant counts greater than or equal to 5 in our internal database, ExAC, 1000 Genome or gnomAD); (II) not predicted as detrimental by any prediction tools; (III) variants in our black list genes; (IV) do not follow the inheritance mode of the disease gene. Up to this point, a few dozen candidate variants for each sample need further clinical review. Finally, the pathogenicity of the candidate variants was analyzed according to the standards and guidelines recommended by the American College of Medical Genetics and Genomics (ACMG) (11-13). Based on the above strategy, after the first filtering, there were 21 candidate variants left in this patient that need further manual review (Table S2). After the analysis of the pathogenicity of the candidate variants based on ACMG guidelines, only one variant was discovered pathogenic and could be responsible for the phenotypes of the patient. This variant in ASXL2 is absent in ExAc, 1000 Genomes and gnomAD. A flow chart to illustrate the filtering strategy of variants is given below (Figure 5).
Results
The patient’s karyotyping result was normal. The results of trio-WES were returned at the age of 21 days and a heterozygous de novo truncating pathogenic c.1792C>T (p.Gln598*) variant in exon 11 of the ASXL2 gene was identified. This variant was classified as likely pathogenic based on the following evidence: PVS1 Strong [Clinical validity classification of gene is definitive and 13 pathogenic null variants were reported in ClinVar for this gene , across 2 different exons, of which 5 variants in this exon; and this nonsense variant predicted to undergo nonsense-mediated decay and exon is present in biologically-relevant transcript] + PS2_Moderate (de novo with confirmed parental relationships in a patient with phenotype consistent with gene but not highly specific) + PM2_Supporting (absent from gnomAD, 1000 Genomes and ExAC population databases) (12,13). Genetic counseling was performed, and he was diagnosed with ASXL2-related SHAPNS based on his phenotype and sequencing results.
Literature review and summary of 11 cases
A review of the literature in PubMed was performed using the following terms: “ASXL2” and “Shashi-Pena Syndrome”. For patients from one family, the medical records of the other patients from the proband’s family were incomplete, thus only the proband of this family was included in this review. The results were checked by two independent authors to exclude duplicate papers and to select those to read in extenso. Every author contributed to writing parts of the manuscript related to her or his specialty. Since 2016, only 12 cases from 10 families have been reported. Ten probands of the families were included in the review.
Demographic birth data of 11 patients with ASXL2-related SHAPNS
Among 8 male and 3 female patients, the median age at diagnosis of ASXL2-related SHAPNS was 4.6 years, ranging from the age of 21 days to 31 years. ASXL2 variants (NM_018263.6) are listed as follow: c.1792C>T, p.Gln598* (this paper); c.1225_1228delCCAA, p.Pro409Asnfs*13; c.1288G>T, p.Glu430*; c.2081dupG, p.Gly696Argfs*11; c.2424delC, p.Thr809Profs*32; c.2485C>T, p.Gln829*; c.2472delC, p.Ser825Valfs*16; c.2971_2974delGGAG, p.Gly991Argfs*3; c.4228T>G, p.Cys1410Gly; c.1217dup, p.Glu407* and t(2;11)(p23;q23). The majority of the patients (8/11) had de novo truncating mutations. Overall, the median (range) gestational age, birth weight, occipitofrontal circumference and length in this group were 39 weeks (32–42 weeks), 3,000 grams (2,071–4,860 grams), 35.5 cm (34–37 cm) and 49.3 cm (42–59.5 cm), respectively. The majority of the individuals were born at term, with birth parameters appropriate for gestational age. Only 2 patients had macrocephaly at birth. The detailed information is described in Table S1.
Clinical synopsis of 11 patients with ASXL2-related SHAPNS
Table 1 shows the common clinical features in 11 individuals with ASXL2-related SHAPNS. More than half of the patients shared recognizable facial features, including hypertelorism (11/11), broad nasal tip (10/11), arched eyebrows (9/11), a large V-shaped glabellar nevus flammeus on the forehead (9/11), low-set ears (8/11), posteriorly rotated ears (7/11), proptosis (6/11), and deep palmar creases (6/11). Less than half of them had a broad forehead (5/11), hirsutism (5/11), retrognathia (4/11), ptosis (4/11), capillary malformations (3/11), long faces (3/11) and overlapping toes (3/11).
Table 1
| Classification | Number (percentage) |
|---|---|
| Facial and skin features | |
| Hypertelorism | 11 (100.0) |
| Broad nasal tip | 10 (90.9) |
| Arched eyebrows | 9 (81.8) |
| V-shaped glabellar nevus flammeus on the forehead | 9 (81.8) |
| Low-set ears | 8 (72.7) |
| Posteriorly rotated ears | 7 (63.6) |
| Proptosis | 6 (54.5) |
| Broad forehead | 5 (45.5) |
| Hirsutism | 5 (45.5) |
| Retrognathia | 4 (36.4) |
| Ptosis | 4 (36.4) |
| Capillary malformations | 3 (27.3) |
| Long face | 3 (27.3) |
| Flat face | 1 (9.1) |
| Other abnormal eye findings | 4 (36.3) |
| Skeletal and/or extremity manifestations | |
| Deep palmar creases | 6 (54.5) |
| Overlapping toes | 3 (27.3) |
| Brain MRI findings | |
| Normal | 2 (18.2) |
| Enlarged extra-axial spaces | 2 (18.2) |
| White matter volume loss | 2 (18.2) |
| Ventriculomegaly | 2 (18.2) |
| Small cerebellum | 1 (9.1) |
| Choroid plexus papilloma | 1 (9.1) |
| Cardiovascular findings | |
| Normal | 1 (9.1) |
| Atrial septal defect | 4 (36.4) |
| Patent foramen ovale | 3 (27.3) |
| Patent ductus arteriosus* | 1 (9.1) |
| Mitral and tricuspid regurgitation | 1 (9.1) |
| Tricuspid insufficiency* | 1 (9.1) |
| Pericardial effusion* | 1 (9.1) |
| Ventricular ectopy bradycardia | 1 (9.1) |
*, one patient had evidence of patent ductus arteriosus, patent foramen ovale, mild tricuspid insufficiency, mild pericardial effusion and pulmonary hypertension at 2 months of age and died of heart disease at 16 months of age. SHAPNS, Shashi-Pena syndrome; MRI, magnetic resonance imaging.
Four patients had remarkable eye findings, including retinal paving-stone-like white lesions since birth (1/11), pigmentary retinal dystrophy (1/11), mild left optic atrophy recognized at the age of 7 years, ovoid-shaped corneas (1/11) and bilateral presenile cataracts recognized at the age of 24 years (1/11).
Two patients (2/11) had normal MRI findings. The others had MRI findings of enlarged extra-axial spaces (2/11), white matter volume loss (2/11), ventriculomegaly (2/11), a small cerebellum (1/11), and choroid plexus papilloma (1/11).
Unremarkable cardiovascular findings were recorded for only 10 patients. The other had non-life-threatening heart problems, including an atrial septal defect (4/11), a patent foramen ovale (3/11), mild mitral and tricuspid regurgitation (1/11) and ventricular ectopy bradycardia (1/11). One patient had evidence of patent ductus arteriosus, patent foramen ovale, mild tricuspid insufficiency, mild pericardial effusion and pulmonary hypertension at the age of 2 months and died of heart disease at the age of 16 months. The detailed clinical characteristics are summarized in Table S1.
Major clinical issues and prognoses of 11 patients with ASXL2-related SHAPNS
Table 2 illustrates the major clinical issues and prognosis of the patients at the median follow-up age of 4.1 years, ranging from the age of 4 months to 31 years, one of whom died of heart disease at the age of 16 months.
Table 2
| Prognosis | Number (percentage) |
|---|---|
| Feeding difficulties | 10 (90.9) |
| Developmental delay | 10 (90.9) |
| Macrocephaly at follow-ups | 8 (72.7) |
| Skeletal and/or extremity manifestations | 8 (72.7) |
| Hypotonia | 8 (72.7) |
| Seizure activities | 6 (54.5) |
| Hypoglycemia | 6 (54.5) |
| Appendicular hypertonia | 2 (18.2) |
| Growth retardation | 2 (18.2) |
SHAPNS, Shashi-Pena syndrome.
Feeding difficulties were common in 10 patients after birth, the majority of which were transient (8/10), one of which caused by GERD, one of which caused by intestinal obstruction treated with Meckel’s diverticulectomy, six of which with unknown reasons, and only one patient required persistent total parental nutrition and life-long gastrojejunal feeding due to severe GERD.
Hypoglycemia was another major issue in 6 patients after birth. One patient with chromosomal balanced translocation had transient hypoglycemia after birth, which resolved quickly with 10% dextrose supplementation. The other neonatal patient of onset transient hypoglycemia with a heterozygous variant had episodic early morning hypoglycemia starting at the age of 2.5 years of unknown causes with inappropriate insulin level, treated with regular feeding to avoid fasting periods. The other four patients, including our patient, all had persistent hypoglycemia.
The underlying causes and therapies for persistent hypoglycemia included an inappropriate insulin level (2/4) treated with octreotide or continuous gastric tube feedings, ketotic hypoglycemia and central adrenal insufficiency (1/4) between the age of 12 months and 24 months treated with gastrojejunal feeding and glucocorticoid replacement, and an unknown etiology (1/4), where the patient received regular feeding to avoid fasting periods.
Only 2 patients had macrocephaly at birth, and this number increased to 8 at follow-ups. Axial hypotonia and appendicular hypertonia occurred in 8 and 2 patients in this group, respectively. Skeletal and/or extremity issues were common in this group, found in 8 patients, and included overlapping toes (3/11), osteoporosis (3/11), kyphosis (2/11), advanced bone age (2/11), fractures (2/11), scoliosis (1/11) and torticollis (1/11) with the youngest age of 4 months old.
Both febrile and nonfebrile seizure activity was recognized in 6 patients, with the earliest age at onset being 1.2 years. Among which 2 male had febrile seizure at 1.2 years and 8.5 years, 3 (2 male and 1 female) had non-febrile seizure activity at 4, 13 and 23 years and 1 female had both febrile and non-febrile seizure activity at 4 years. One patient had suspected seizure activity but not confirmed by EEG was not included. A total of 90.9% (10/11) of the patients had developmental delay at follow-ups, with the earliest age being 4 months. Two patients who had early normal MRI findings but developed nonfebrile seizures at the age of 13 years and 23 years also had development delay, which indicated the possibility of neurodevelopmental regression in this group.
Discussion
This study also expands the detailed neonatal phenotype spectrum of ASXL2-related SHAPNS, provides further confirmation of the previously described phenotypes attributed to pathogenic variants in ASXL2 and outlines treatments for persistent hypoglycemia. Through the literature review, we identified an additional 10 individuals from 10 different families who had pathogenic variants in this gene, providing a clearer picture of the spectrum of clinical features and prognoses and a better understanding of ASXL2-related SHAPNS.
ASXL2 is one of the human homologs of the Drosophila additional sex combs (ASX) gene, which is an enhancer of the trithorax and polycomb (ETP) gene and is likely to act as a histone methyltransferase in complexes with other proteins (14). ASXL2 is located on chromosome 2p23 and is associated with lipogenesis, heart morphogenesis, skeletal destruction, glucose homeostasis and leukemia (15-19).
As depicted in Table 1, individuals with pathogenic variants in ASXL2 share several features, including hypoglycemia, neonatal feeding difficulties, skeletal and/or extremity manifestations, minor congenital heart disease, axial hypotonia, febrile and nonfebrile seizure activity, developmental delay as early as the age of four months, macrocephaly and a typical facial appearance consisting of arched eyebrows, hypertelorism, a broad nasal tip, a large V-shaped glabellar nevus flammeus on the forehead, low-set ears, posteriorly rotated ears and proptosis. The clinical features of the individuals from one family with a complex chromosomal balanced translocation affecting the ASXL2 gene were not significant, and the mental developmental prognosis was better than that of individuals with classic de novo truncating mutations in ASXL2.
Special attention should be given to ASXL2-related persistent hypoglycemia. Neonatal recurrent or prolonged hypoglycemia can lead to cerebral injury and poor neurodevelopmental outcomes (20). In an animal study, ASXL2-deficient mice were found to be insulin resistant and glucose intolerant, indicating that ASXL2 is a master regulator of glucose homeostasis (18). Thus, tight control of glucose levels is of utmost importance in high-risk neonates, including those with ASXL2-related SHAPNS. In previous studies, the etiology of hypoglycemia was not clear. Inappropriate insulin levels, ketotic hypoglycemia and central adrenal insufficiency might be the causes.
Our patient underwent diagnostic fasting and glucagon tests, which indicated inappropriately high levels of insulin. ASXL2 acts as an epigenetic regulator and regulates gene networks in various types of cancers, such as breast cancer, hepatocellular carcinoma and acute myeloid leukemia (14,19,21,22). To date, no patients with SHAPNS have been reported to develop cancer at follow-ups. Octreotide is a long-acting somatostatin analog and is usually prescribed in patients with hypoglycemia due to inappropriate insulin levels (23). Our patient quickly achieved stable glucose levels after octreotide therapy and was successfully weaned off intravenous dextrose. Further studies and close follow-ups to verify the efficacy and advantages of octreotide in the treatment of persistent hypoglycemia are needed.
Patients with ASXL2 mutations might have optic impairment. In previously reported cases, the patients were diagnosed with ASXL2-related SHAPNS beyond the neonatal period; thus, little is known about eye-related manifestations in early life. Only three previously reported cases included descriptions of eye examinations. A 7-year-old male patient who experienced persistent hypoglycemia was noticed to have pigmentary retinal dystrophy and mild left optic atrophy (2). An 8-year-old male child had ovoid-shaped corneas, and another female patient had bilateral presenile cataracts diagnosed at 24 years old, both of whom had transient hypoglycemia (1). Our patient had prolonged hypoglycemia, underwent several fundoscopy investigations and was noticed to have persistent bilateral paving-stone-like white lesions of the retinas.
ASXL2 is highly expressed in eyes and plays an important role in genetic pathways that regulate optic development (24,25). A complex phenotype with bilateral chorioretinal, iris colobomas and neurological structural changes was found to be associated with a fused protein partially encoded by the ASXL2 gene (26), indicating that ASXL2 might be associated with some optic developmental disorder. Moreover, patients with persistent hypoglycemia were found to have visual impairment (20,27,28). Therefore, long-term follow-ups of the eyes and further clinical reports of individuals regarding eye investigations are necessary and important.
ASXL2 mutations play an important role in neurological damage. A few patients had MRI findings of enlarged extra-axial spaces (2/11), white matter volume loss (2/11) and ventriculomegaly (2/11). In our patient, MRI at the age of six days revealed a small cerebellum. Additional clinical reports of individuals regarding the structure of the cerebellum are helpful. Here, we found that in addition to these core features, patients with ASXL2 pathogenic variants tended to have a higher likelihood of having progressive macrocephaly (2/11 at birth and 8/11 at follow-ups) and a possibility of neurodevelopmental regression in patients (2/11) with normal early MRI findings but later development of nonfebrile seizures. These findings suggested that early and regular follow-ups in the neurology and rehabilitation department might be necessary.
Strengths and limitations
This is a rare disease that the numbers of the patients were limited, and the majority of related patients were discovered and diagnosed at an older age. Our patient had the diagnosis at the age of 21 days, leading to the timeliest interventions and treatment for relevant early onset medical issues. This is the first time that octreotide was administered in patients with persistent hypoglycemia secondary to SHAPNS and successfully helped our patient achieve euglycemia without hypoglycemic brain injury. So far, the long-term prognosis of this patient was lacked. Unusual fundoscopy results of paving-stone like white-lesion and small cerebellum on the MRI were identified for the first time which required future follow-ups.
Conclusions
Further clinical reports of more neonatal individuals with damaging ASXL2 variants are necessary to verify the mechanism and optimal treatment of ASXL2-related hypoglycemia, neurological damage and optic impairment. With the advent of advanced genomic screening, such as CNGP, we are likely to identify younger patients. Neurological, endocrinological, ophthalmological, and rehabilitative long-term follow-ups of these patients is necessary and important.
Acknowledgments
We would like to thank all the nursing staffs, physicians and residents taking care of the baby. We would also like to express our gratitude to parents’ cooperation and consent.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-220/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-220/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-220/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Research Ethics Committee of the Children’s Hospital of Fudan University (CHFudanU_NNICU11) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shashi V, Pena LD, Kim K, et al. De Novo Truncating Variants in ASXL2 Are Associated with a Unique and Recognizable Clinical Phenotype. Am J Hum Genet 2016;99:991-9. Correction in Am J Hum Genet 2017;100:179. [Crossref] [PubMed]
- Cuddapah VA, Dubbs HA, Adang L, et al. Understanding the phenotypic spectrum of ASXL-related disease: Ten cases and a review of the literature. Am J Med Genet A 2021;185:1700-11. [Crossref] [PubMed]
- Wang Y, Tan J, Wang Y, et al. Diagnosis of Shashi-Pena Syndrome Caused by Chromosomal Rearrangement Using Nanopore Sequencing. Neurol Genet 2021;7:e635. [Crossref] [PubMed]
- Alqaisi D, Hassona Y. Oral findings and healthcare management in Shashi-Pena syndrome. Spec Care Dentist 2022;42:432-6. [Crossref] [PubMed]
- Jiao Z, Zhao X, Wang Y, et al. A de novo and novel nonsense variants in ASXL2 gene is associated with Shashi-Pena syndrome. Eur J Med Genet 2022;65:104454. [Crossref] [PubMed]
- Wu B, Kang W, Wang Y, et al. Application of Full-Spectrum Rapid Clinical Genome Sequencing Improves Diagnostic Rate and Clinical Outcomes in Critically Ill Infants in the China Neonatal Genomes Project. Crit Care Med 2021;49:1674-83. [Crossref] [PubMed]
- Yang L, Wei Z, Chen X, et al. Use of medical exome sequencing for identification of underlying genetic defects in NICU: Experience in a cohort of 2303 neonates in China. Clin Genet 2022;101:101-9. [Crossref] [PubMed]
- Xiao F, Yan K, Wang H, et al. Protocol of the China Neonatal Genomes Project: an observational study about genetic testing on 100,000 neonates. Pediatr Med 2021;4:28.
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010;38:e164. [Crossref] [PubMed]
- McLaren W, Pritchard B, Rios D, et al. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 2010;26:2069-70. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Abou Tayoun AN, Pesaran T, DiStefano MT, et al. Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat 2018;39:1517-24. [Crossref] [PubMed]
- Harrison SM, Biesecker LG, Rehm HL. Overview of Specifications to the ACMG/AMP Variant Interpretation Guidelines. Curr Protoc Hum Genet 2019;103:e93. [Crossref] [PubMed]
- Katoh M. Functional proteomics of the epigenetic regulators ASXL1, ASXL2 and ASXL3: a convergence of proteomics and epigenetics for translational medicine. Expert Rev Proteomics 2015;12:317-328. [Crossref] [PubMed]
- Park UH, Seong MR, Kim EJ, et al. Reciprocal regulation of LXRα activity by ASXL1 and ASXL2 in lipogenesis. Biochem Biophys Res Commun 2014;443:489-94. [Crossref] [PubMed]
- Lai HL, Grachoff M, McGinley AL, et al. Maintenance of adult cardiac function requires the chromatin factor Asxl2. J Mol Cell Cardiol 2012;53:734-41. [Crossref] [PubMed]
- Khan FF, Li Y, Balyan A, et al. WTIP interacts with ASXL2 and blocks ASXL2-mediated activation of retinoic acid signaling. Biochem Biophys Res Commun 2014;451:101-6. [Crossref] [PubMed]
- Izawa T, Rohatgi N, Fukunaga T, et al. ASXL2 Regulates Glucose, Lipid, and Skeletal Homeostasis. Cell Rep 2015;11:1625-37. [Crossref] [PubMed]
- Micol JB, Pastore A, Inoue D, et al. ASXL2 is essential for haematopoiesis and acts as a haploinsufficient tumour suppressor in leukemia. Nat Commun 2017;8:15429. [Crossref] [PubMed]
- Burns CM, Rutherford MA, Boardman JP, et al. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 2008;122:65-74. [Crossref] [PubMed]
- Abdel-Wahab O, Dey A. The ASXL-BAP1 axis: new factors in myelopoiesis, cancer and epigenetics. Leukemia 2013;27:10-5. [Crossref] [PubMed]
- Li J, He F, Zhang P, et al. Loss of Asxl2 leads to myeloid malignancies in mice. Nat Commun 2017;8:15456. [Crossref] [PubMed]
- Kostopoulou E, Shah P. Hyperinsulinaemic hypoglycaemia-an overview of a complex clinical condition. Eur J Pediatr 2019;178:1151-60. [Crossref] [PubMed]
- Fisher CL, Randazzo F, Humphries RK, et al. Characterization of Asxl1, a murine homolog of Additional sex combs, and analysis of the Asx-like gene family. Gene 2006;369:109-18. [Crossref] [PubMed]
- An MJ, Kim CH, Nam GY, et al. Transcriptome analysis for UVB-induced phototoxicity in mouse retina. Environ Toxicol 2018;33:52-62. [Crossref] [PubMed]
- Ramocki MB, Dowling J, Grinberg I, et al. Reciprocal fusion transcripts of two novel Zn-finger genes in a female with absence of the corpus callosum, ocular colobomas and a balanced translocation between chromosomes 2p24 and 9q32. Eur J Hum Genet 2003;11:527-34. [Crossref] [PubMed]
- Tam EW, Widjaja E, Blaser SI, et al. Occipital lobe injury and cortical visual outcomes after neonatal hypoglycemia. Pediatrics 2008;122:507-12. [Crossref] [PubMed]
- Hu L, Gu Q, Zhu Z, et al. Flash visual evoked potentials are not specific enough to identify parieto-occipital lobe involvement in term neonates after significant hypoglycaemia. Acta Paediatr 2014;103:e329-33. [Crossref] [PubMed]




