Emerging surgical therapies in the treatment of pediatric epilepsy
Introduction
Pediatric epilepsy is a relatively common disorder afflicting 1 of every 100 children, and up to one third of these patients demonstrate drug-resistant epilepsy (DRE) (1,2). In a 2009 statement, the International League Against Epilepsy defined DRE as the “failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom” (1). DRE represents a major therapeutic challenge and can be associated with significant comorbidities, including cognitive impairment, depression, anxiety, developmental delay, and impaired activities of daily living (3,4). Although it was initially perceived as a therapeutic option only after all other options were exhausted, surgical treatment of properly selected patients can provide long-term seizure control rates of 50–70% (5). In fact, surgical intervention remains grossly underutilized, with approximately 100,000–500,000 surgical candidates in the U.S. but less than one percent of eligible patients undergoing epilepsy surgery each year (6). In light of improved seizure-control rates and reduced morbidity of modern epilepsy treatments, the American Academy of Neurology published a practice parameter recommending surgery as a treatment of choice for certain patients with DRE, as earlier referral can help avoid the adverse developmental and social effects of uncontrolled seizures (7). Despite this recommendation, several studies have shown that there has been no improvement in the delay from diagnosis to surgical referral for patients with DRE (8,9).
The reluctance to refer patients for evaluation at an epilepsy center is multifactorial, with reasons for delay including fear of surgical comorbidity, expense, and limited experience with modern, multidisciplinary treatment options (6). The recent emergence of novel surgical diagnostic and therapeutic strategies has the potential to increase the number of children who can benefit from surgical therapy, improve its efficacy, and decrease morbidity. This may turn the tide of misperception among families and community physicians regarding the surgical treatment of DRE in children. This review will discuss the application of several emerging diagnostic and therapeutic surgical techniques in pediatric epilepsy, including stereotactic electroencephalography (SEEG), MRI-guided laser interstitial thermal therapy (MRgLITT), and responsive neurostimulation (RNS).
Stereotactic electroencephalography (SEEG)
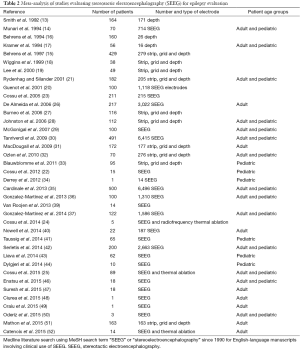
SEEG, the stereotactic placement of multiple depth electrodes for intracranial EEG recording, which was originally described more than 50 years ago by Talairach and Bancaud (10,11), has enjoyed a renaissance over the past decade in the context of improved stereotaxis, imaging, and seizure monitoring technologies (Figure 1). Diagnostic intracranial EEG evaluation of pediatric DRE is critical when seizure foci are poorly localized by noninvasive means and after interdisciplinary evaluation (12). SEEG particularly facilitates a three-dimensional spatiotemporal understanding of seizure onset and progression. Targets are individualized based on hypothesized ictal foci using clinical semiology, scalp video EEG monitoring, structural and metabolic imaging, and interdisciplinary evaluation. Patients for whom SEEG is commonly indicated are those children with DRE whose magnetic resonance imaging scans are normal and those with multifocal lesions, nonlateralized seizure onset on scalp video EEG, proximity of ictal onset to putative functionally eloquent cortex, or discordant noninvasive data (Table 1). In comparison to surface electrode monitoring with subdural electrodes, SEEG may be particularly useful in the evaluation of children with multiple lesions including tuberous sclerosis complex, as well as for sampling deep targets such as insular cortex, the cingulate gyrus, and mesial temporal structures. In addition, placement does not require craniotomy (67). Furthermore, SEEG can be useful during the investigation of epileptic networks, such as limbic circuitry (67). Robotic assistance in depth electrode placement has demonstrated benefits in planning safe trajectories and reducing operating time and is associated with excellent target accuracy of 1–3 mm (35,68).


Full table
SEEG successfully identified the ictal onset zone in 90% of children in one large series (69), and these results have been confirmed by multiple reports (Tables 1 and 2). The first consecutive study of SEEG in the pediatric population reported 15 children (mean age 34.1 months), with 14 undergoing subsequent surgical resection (22). Ten of 14 children had at least 12-month follow-up: six patients were seizure free (Engel I), 2 had rare seizures (Engel II), and 2 patients had no meaningful improvement (Engel IV) (70). Cortical dysplasia (10 children) was the most frequent underlying ictal substrate. One patient died after electrode placement because of severe cerebral edema in the setting of hyponatremia of unclear etiology. A large retrospective cohort study of 200 patients, including children and adults, with 2663 SEEG electrode implantations, reported successful identification of the ictal onset zone in 154 patients (77%) (67). In this cohort, 134 patients underwent subsequent resection and 61 patients (68%) were seizure-free at 12 months. Again, the most common underlying etiology was cortical dysplasia. Minimal morbidity occurred after SEEG, with an extremely low incidence of surgical site infection (0.08%), intracranial hemorrhage (0.08%), or new focal neurologic deficit (0.04%). Results demonstrating low morbidity and mortality with SEEG have been confirmed by multiple reports from different centers in both adult and pediatric cohorts. Some studies have reported a higher rate of permanent morbidity after evaluation with surface electrodes (3–6%) compared with the use of SEEG electrodes (0–3%) because of the need for craniotomy and invasive grid placement in both adult and pediatric patients (34-37,42); however, comparisons of surface electrodes and SEEG as well as complication rates have been limited because of differing study indications, methodology, and techniques.
Full table
Larger studies of SEEG monitoring in pediatric patients have reported that efficacy of seizure foci identification and safety are similar to those reported in studies of adults (22,41,43,44). A study of 15 patients (mean age seizure onset 6.4 months, SEEG placement 34.1 months) showed Engel I seizure freedom in 60% of patients (22). Liava et al. (43) evaluated 62 children (mean age epilepsy onset 3.2 years, surgery 7.9 years) with SEEG used in 39% of patients. The preoperative MRI demonstrated one or more structural lesions in over 90% of patients, including focal cortical dysplasia (50%), tumor (24%), and gliosis (14.5%). After surgery, 85.5% of patients achieved Engel I seizure freedom. In a study of ten children (mean age at epilepsy onset 1.3 years, SEEG placement 6.4 years, surgery 6.6 years), Dylgjeri et al. (44) reported Engel I seizure freedom in 70% of cases. No patient complications from SEEG placement were reported in this study. Taussig et al. (41) evaluated 65 pediatric patients (mean age at epilepsy onset 29.8 months, SEEG placement 98.6 months) who underwent SEEG placement. MRI results were normal or unremarkable in 20% of cases and 78% of patients underwent eventual surgery, with focal cortical dysplasia the most common diagnosis (51%). With a mean follow-up of 24 months, Engel I seizure freedom was achieved in 67% of patients. Overall Engel I seizure freedom outcomes are 60–80% in pediatric patients, a rate achieved with low morbidity and mortality from SEEG placement.
SEEG has enabled the development of quantitative algorithms to aid in seizure localization. A recent study used an algorithm-derived SEEG pattern to successfully identify the ictal onset zone using interictal fast activity (80–120 Hz) associated with slow transient polarizing shifts and voltage depression (71). A three-dimensional model of seizure activity was created from the position of the SEEG electrodes. This method was validated using a separate cohort of 14 patients, with complete correlation with clinician-identified seizure onset in 13 cases and partial correlation in 1 case. Single-pulse electrical stimulation (SPES) has also been used to improve detection of epileptic sites (72). Electrodes may act as either activators or bidirectional activator/detectors, and stimulation was able to generate three-dimensional reconstructed networks. Other studies have used various SEEG data measurements to model the epileptogenic zone, including absolute signal slope (e.g., change in signal strength/time), ordinal patterns of EEG activity, multivariate cross-correlation matrices of EEG activity, and multivariate model-free information theory (73). Other methods of EEG quantitation include the joint sign periodogram event characterization transformation algorithm (74), epilepticity index (75), and high-frequency oscillations (76). As quantitative algorithms analyzing specific interictal and ictal features of intracranial EEG recordings become more clinically informative, SEEG is playing a rapidly expanding role in modeling the epileptogenic zone in appropriately selected children.
MRI-guided laser interstitial thermal therapy (MRgLITT)
In parallel with the development of robot-assisted, minimally invasive intracranial EEG recordings and quantitative algorithms to model the epileptogenic zone, novel minimally invasive surgical treatment strategies have emerged. Specifically, MRgLITT has been an exciting new minimally invasive tool in the management of a variety of neurosurgical lesions, especially DRE in children (Tables 1 and 3). MRgLITT utilizes an optical fiber with a diffusing tip heated by a diode laser insulated in an outer cannula that cools the laser with either saline or carbon dioxide (78). The probe is delivered by frame-based stereotaxis, robotic stereotaxis, or MRI-based frameless stereotactic techniques to a defined target. After placement, heat diffused from the probe is monitored in real-time using MR thermography. Heating of tissue results in time- and temperature-dependent thermal denaturation of critical enzymes and eventually cell death.

Full table
Two MRgLITT systems are currently approved by the Food and Drug Administration (FDA) for clinical use in the United States: Visualase (Medtronic, Minneapolis, MN) and NeuroBlate (Monteris Medical, Plymouth, NM). Although these systems are built around the same concept, there are differences between them, including computer-guided robotic catheter movement, directional manipulation of thermal energy, and low-temperature safety controls to protect critical structures from injury (78,79). Advantages of MRgLITT include the ability to target deep lesions (e.g., hypothalamic hamartomas, mesial temporal sclerosis) via a minimally invasive approach and monitoring of the treatment in real time. This contrasts with other minimally invasive or noninvasive treatment methods, including gamma knife radiosurgery or radiofrequency ablation (80-82). Further, MRgLITT can easily reach deep lesions close to the skull base, in contrast with focused ultrasound (83).
Multiple studies have shown an expanding role for MRgLITT in the treatment of pediatric DRE. A recent report evaluated MRgLITT in the management of 17 pediatric (mean age 15.3 years) patients with DRE and a variety of epileptogenic substrates, including focal cortical dysplasia (n=12), tuberous sclerosis (n=5), hypothalamic hamartoma (n=1), mesial temporal sclerosis (n=1), Rasmussen encephalitis (n=1), and tumor (n=1) (59). Seizure control rates were promising, with 41% of patients achieving seizure freedom and 59% achieving an Engel I or Engel II outcome. Four complications were reported, namely inaccurate probe placement (n=2), probe system failure (n=1), and postoperative dexamethasone-induced gastritis (n=1). The use of MRgLITT in the treatment of hypothalamic hamartomas was evaluated in a study of 14 pediatric patients (median age 8 years); postoperative Engel I seizure freedom was achieved in 86% of patients with a mean followup of 9 months (84). Another study evaluated the role of MRgLITT in five pediatric patients (age 5–15 years) for a combination of tuberous sclerosis, mesial temporal lobe epilepsy, cortical dysplasia, and hypothalamic hamartoma (53). Engel I seizure freedom was achieved in three patients with limited follow-up ranging from 2 to 13 months. MRgLITT has also been reportedly used for treatment of various other conditions including periventricular nodular heterotopia (54,77), insular epileptic foci (55), glial neoplasms (85), brain metastases (86), and teratoma (87).
To date, the most well-studied application of MRgLITT in DRE is the minimally invasive treatment of mesial temporal sclerosis (MTS). Although randomized trials have clearly demonstrated the efficacy of craniotomy and temporal lobectomy in patients with DRE and MTS (88,89), this procedure is frequently associated with a decline in verbal memory when performed in the dominant hemisphere (90,91). Minimally invasive approaches including radiofrequency ablation have shown good outcomes (81), suggesting a role for MRgLITT. A recent report of 20 children and adults (age range 0.9–50 years) undergoing MRgLITT for MTS demonstrated seizure control rates of 53% at 6 months, with 4 patients requiring anterior temporal lobectomy after MRgLITT for definitive seizure control (58). Unfortunately, although a median follow-up of 13.4 months (range, 1.3–38.5 months) was achieved, a large number of patients were lost to follow-up, limiting the available data on long-term seizure freedom. Another study of 13 adult patients from a single institution (9 with MTS) showed seizure control rates of 77% along with control of 67% in patients with MTS (n=6/9) (56). One prospective study of temporal lobe epilepsy compared 19 adult patients undergoing MRgLITT treatment with 39 adult patients undergoing standard temporal lobectomy for temporal lobe epilepsy in the dominant hemisphere. Patients undergoing craniotomy had a more significant decline in facial recognition and naming (P<0.0001) (57). Rates of seizure control were similar in both groups, with 57.9% of patients treated with MRgLITT and 61.5% who underwent craniotomy achieving seizure freedom. Although most of the data regarding MRgLITT in MTS treatment are from the adult literature, efficacy and safety in pediatric patients have been shown in several reports, suggesting an expanding role for MRgLITT in children in the future (58).
Responsive neurostimulation (RNS)
Initial evaluation of electrical stimulation in the study of epilepsy was performed by Penfield and Jasper in 1954 (92,93). Deep brain stimulation has been used successfully for treatment of epilepsy in small series targeting the cerebellum (94), centromedian thalamic nucleus (95,96), and anterior nucleus of the thalamus (97-99). Targeting of the hippocampus, subthalamic nucleus, and caudate have also been reported (100-108). These early studies of open-loop stimulation technology were important benchmarks in the use of neurostimulation to treat epilepsy and provided the foundation for RNS (109).
RNS is a novel treatment paradigm that is distinct from other types of neurostimulation because it employs continuous monitoring of a focal EEG signal via intracranial electrodes placed over an ictal onset zone, with cortical stimulation based on computer analysis of EEG signal input to abort seizure onset (Table 1). The first commercially available implantable system was introduced by NeuroPace, Inc. (Mountain View, CA, USA) and received FDA approval in the U.S. in 2013 for patients ≥18 years of age with medically refractory (appropriate selection and trial of ≥2 medications), partial-onset seizures, ≤2 epileptogenic foci, and an average of ≥3 seizures per 3-month average (Figure 2) (61,63). NeuroPace is compatible with both surface and depth electrodes that both detect epileptogenic activity and produce electrical stimulation to disrupt seizure onset. The device measures epileptic activity by the bandpass, line-length, and area algorithms to delivery stimulation ranging from 1 to 333 Hz, amplitudes of 0.5 to 12 mA, and pulse-widths from 40 to 1,000 µs, device settings familiar to most clinicians managing deep brain stimulation systems.
A landmark study leading to FDA approval of NeuroPace was conducted by the RNS System in Epilepsy Study Group (61). This study involved 191 adults (mean age 34.9 years) with refractory epilepsy undergoing subdural or depth electrode placement at 1 or 2 specified epileptogenic foci. Double-blind randomization to either stimulation or sham stimulation was performed, followed by a three-month evaluation period. Thereafter, the sham group was also stimulated. An initial reduction in seizure frequency was seen, as in prior trials evaluating surgical treatment for epilepsy; however, a sustained reduction in mean seizure frequency was seen in 37.9% of stimulated patients vs. 17.3% of sham patients (P=0.012). Overall, 46% of patients showed a 50% reduction in mean seizure frequency as well as improvement in secondary outcomes, including quality-of-life assessment (63,66). Long-term reduction in median seizure frequency of 48–66% was observed over a mean follow-up of 5.4 years, with improvement in quality-of-life measures (110). No patient showed complete seizure freedom but 36.7% had 1 seizure-free period of >3 months, 23% had a seizure-free period of >6 months, and 12.9% had a seizure-free period of >12 months. Since follow-up, 11 deaths from the study group cohort were reported, with 7 due to sudden unexpected death in epilepsy (SUDEP), 2 from suicide in patients with histories of depression, 1 from status epilepticus, and 1 from lymphoma. Notably, rates of SUDEP were not decreased in patients treated via RNS compared with the overall epilepsy population. A follow-up secondary analysis of neuropsychological outcomes showed sustained improvements two years after implantation (65). Moreover, a 32% improvement in naming scores among patients (n=76) with focal neocortical epilepsy and 8.5% improvement in verbal memory among patients (n=86) with mesial temporal onset seizures were also observed. Evaluation of device activity after implantation as well as stimulation data show that the device can drastically alter the severity and character of patient seizures (62); however, the relationship between these electroencephalographic changes and clinical seizure behavior remains unclear.
While currently only FDA approved for adults, the NeuroPace system may have a role in the treatment of children with DRE who are not candidates for or who have failed other surgical treatments. To date, there have been no reports of the use of NeuroPace to treat patients younger than 18 years of age; however, a number of features of NeuroPace may appeal for use in a pediatric population. One study explored the possibility of using the device as an outpatient chronic ambulatory electrocorticography (ECoG) device using the original trial data (64). Among 191 patients in the study cohort, 82 had received bilateral mesial temporal lobe implants with the NeuroPace device because clear lateralization was not present on preoperative evaluation. After analysis of the captured electrographic data in this subgroup, bilateral ictal onset was confirmed in 84% after a mean of 41.6 days (range, 0–376 days) after implantation; however, unilateral localization was apparent in 16% of patients, suggesting the possibility of resection. Conversely, among 11 patients with presumed unilateral onset, 64% demonstrated bilateral electrographic seizures. Therefore, the potential of the NeuroPace device to serve as an ambulatory ECoG may make it a useful tool in a pediatric setting where patients may not tolerate the stress and morbidity of intracranial monitoring as well as adult patients do. Overall, RNS promises to be a useful tool in the arsenal of epilepsy treatment for patients with epileptogenic foci not amenable for surgical resection. Multiple questions remain regarding the use of RNS, including further elucidation of the mechanism of action to improve future trials and patient selection. Evaluation in pediatric patients remains to be explored.
Conclusions
The combination of novel seizure modifying approaches, such as RNS, and minimally invasive methods of intracranial monitoring with SEEG, quantitative algorithms for modeling the epileptogenic zone, and minimally invasive treatment paradigms such as laser ablation has the power to significantly change the surgical management of DRE in children in the future. The further development of these technologies in parallel may truly represent a disruptive innovation that significantly improves outcomes and decreases morbidity in children with disabling seizures.
Acknowledgements
We thank Kristin Kraus, MSc, for editorial assistance with the preparation of this paper.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069-77. [Crossref] [PubMed]
- Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics 2012;129:256-64. [Crossref] [PubMed]
- Berg AT, Zelko FA, Levy SR, et al. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology 2012;79:1384-91. [Crossref] [PubMed]
- Brooks-Kayal AR, Bath KG, Berg AT, et al. Issues related to symptomatic and disease-modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia 2013;54 Suppl 4:44-60. [Crossref] [PubMed]
- Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol 2014;13:1114-26. [Crossref] [PubMed]
- Engel J Jr. Why is there still doubt to cut it out? Epilepsy Curr 2013;13:198-204. [Crossref] [PubMed]
- Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003;60:538-47. [Crossref] [PubMed]
- Choi H, Carlino R, Heiman G, et al. Evaluation of duration of epilepsy prior to temporal lobe epilepsy surgery during the past two decades. Epilepsy Res 2009;86:224-7. [Crossref] [PubMed]
- Haneef Z, Stern J, Dewar S, et al. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology. 2010;75:699-704. [Crossref] [PubMed]
- Bancaud J, Dell MB. Technics and method of stereotaxic functional exploration of the brain structures in man (cortex, subcortex, central gray nuclei). Rev Neurol 1959;101:213-27. [PubMed]
- Talairach J, Bancaud J, Bonis A, et al. Functional stereotaxic investigations in epilepsy. Methodological remarks concerning a case. Rev Neurol (Paris) 1961;105:119-30. [PubMed]
- Jayakar P, Gaillard WD, Tripathi M, et al. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia 2014;55:507-18. [Crossref] [PubMed]
- Smith JR, Flanigin HF, King DW, et al. Relationship of depth electrode complications to implant trajectory. Epilepsy 1992;5:253-60. [Crossref]
- Munari C, Hoffmann D, Francione S, et al. Stereo-electroencephalography methodology: advantages and limits. Acta Neurol Scand Suppl 1994;152:56-67, discussion 68-9. [Crossref] [PubMed]
- Behrens E, Schramm J, Zentner J, et al. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery 1997;41:1-9; discussion 9-10. [Crossref] [PubMed]
- Behrens E, Zentner J, van Roost D, et al. Subdural and depth electrodes in the presurgical evaluation of epilepsy. Acta Neurochir (Wien) 1994;128:84-7. [Crossref] [PubMed]
- Kramer U, Riviello JJ Jr, Carmant L, et al. Morbidity of depth and subdural electrodes: Children and adolescents versus young adults. Epilepsy 1994;7:7-10. [Crossref]
- Wiggins GC, Elisevich K, Smith BJ. Morbidity and infection in combined subdural grid and strip electrode investigation for intractable epilepsy. Epilepsy Res 1999;37:73-80. [Crossref] [PubMed]
- Lee WS, Lee JK, Lee SA, et al. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol 2000;54:346-51. [Crossref] [PubMed]
- Guenot M, Isnard J, Ryvlin P, et al. Neurophysiological monitoring for epilepsy surgery: the Talairach SEEG method. StereoElectroEncephaloGraphy. Indications, results, complications and therapeutic applications in a series of 100 consecutive cases. Stereotact Funct Neurosurg 2001;77:29-32. [Crossref] [PubMed]
- Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990-1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery 2001;49:51-6; discussion 56-7. [PubMed]
- Cossu M, Schiariti M, Francione S, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy in infancy and early childhood. J Neurosurg Pediatr 2012;9:290-300. [Crossref] [PubMed]
- Cossu M, Cardinale F, Castana L, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery 2005;57:706-18. [Crossref] [PubMed]
- Cossu M, Fuschillo D, Cardinale F, et al. Stereo-EEG-guided radio-frequency thermocoagulations of epileptogenic grey-matter nodular heterotopy. J Neurol Neurosurg Psychiatry 2014;85:611-7. [Crossref] [PubMed]
- Cossu M, Fuschillo D, Casaceli G, et al. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: a retrospective study on 89 cases. J Neurosurg 2015;123:1358-67. [Crossref] [PubMed]
- De Almeida AN, Olivier A, Quesney F, et al. Efficacy of and morbidity associated with stereoelectroencephalography using computerized tomography--or magnetic resonance imaging-guided electrode implantation. J Neurosurg 2006;104:483-7. [Crossref] [PubMed]
- Burneo JG, Steven DA, McLachlan RS, et al. Morbidity associated with the use of intracranial electrodes for epilepsy surgery. Can J Neurol Sci 2006;33:223-7. [Crossref] [PubMed]
- Johnston JM Jr, Mangano FT, Ojemann JG, et al. Complications of invasive subdural electrode monitoring at St. Louis Children's Hospital, 1994-2005. J Neurosurg 2006;105:343-7. [PubMed]
- McGonigal A, Bartolomei F, Regis J, et al. Stereoelectroencephalography in presurgical assessment of MRI-negative epilepsy. Brain 2007;130:3169-83. [Crossref] [PubMed]
- Tanriverdi T, Ajlan A, Poulin N, et al. Morbidity in epilepsy surgery: an experience based on 2449 epilepsy surgery procedures from a single institution. J Neurosurg 2009;110:1111-23. [Crossref] [PubMed]
- MacDougall KW, Burneo JG, McLachlan RS, et al. Outcome of epilepsy surgery in patients investigated with subdural electrodes. Epilepsy Res 2009;85:235-42. [Crossref] [PubMed]
- Ozlen F, Asan Z, Tanriverdi T, et al. Surgical morbidity of invasive monitoring in epilepsy surgery: an experience from a single institution. Turk Neurosurg 2010;20:364-72. [PubMed]
- Blauwblomme T, Ternier J, Romero C, et al. Adverse events occurring during invasive electroencephalogram recordings in children. Neurosurgery 2011;69:ons169-75; discussion ons175.
- Derrey S, Lebas A, Parain D, et al. Delayed intracranial hematoma following stereoelectroencephalography for intractable epilepsy: case report. J Neurosurg Pediatr 2012;10:525-8. [Crossref] [PubMed]
- Cardinale F, Cossu M, Castana L, et al. Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery 2013;72:353-66. [Crossref] [PubMed]
- Gonzalez-Martinez J, Bulacio J, Alexopoulos A, et al. Stereoelectroencephalography in the "difficult to localize" refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia. 2013;54:323-30. [Crossref] [PubMed]
- Gonzalez-Martinez J, Mullin J, Vadera S, et al. Stereotactic placement of depth electrodes in medically intractable epilepsy. J Neurosurg 2014;120:639-44. [Crossref] [PubMed]
- Gonzalez-Martinez J, Mullin J, Bulacio J, et al. Stereoelectroencephalography in children and adolescents with difficult-to-localize refractory focal epilepsy. Neurosurgery 2014;75:258-68; discussion 267-8. [Crossref] [PubMed]
- van Rooijen BD, Backes WH, Schijns OE, et al. Brain imaging in chronic epilepsy patients after depth electrode (stereoelectroencephalography) implantation: magnetic resonance imaging or computed tomography? Neurosurgery 2013;73:543-9. [Crossref] [PubMed]
- Nowell M, Rodionov R, Diehl B, et al. A novel method for implementation of frameless StereoEEG in epilepsy surgery. Neurosurgery 2014;10 Suppl 4:525-33; discussion 533-4. [Crossref] [PubMed]
- Taussig D, Chipaux M, Lebas A, et al. Stereo-electroencephalography (SEEG) in 65 children: an effective and safe diagnostic method for pre-surgical diagnosis, independent of age. Epileptic Disord 2014;16:280-95. [PubMed]
- Serletis D, Bulacio J, Bingaman W, et al. The stereotactic approach for mapping epileptic networks: a prospective study of 200 patients. J Neurosurg 2014;121:1239-46. [Crossref] [PubMed]
- Liava A, Mai R, Tassi L, et al. Paediatric epilepsy surgery in the posterior cortex: a study of 62 cases. Epileptic Disord. 2014;16:141-64. [PubMed]
- Dylgjeri S, Taussig D, Chipaux M, et al. Insular and insulo-opercular epilepsy in childhood: an SEEG study. Seizure 2014;23:300-8. [Crossref] [PubMed]
- Dorfmüller G, Ferrand-Sorbets S, Fohlen M, et al. Outcome of surgery in children with focal cortical dysplasia younger than 5 years explored by stereo-electroencephalography. Childs Nerv Syst 2014;30:1875-83. [Crossref] [PubMed]
- Enatsu R, Gonzalez-Martinez J, Bulacio J, et al. Connectivity of the frontal and anterior insular network: a cortico-cortical evoked potential study. J Neurosurg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Suresh S, Sweet J, Fastenau PS, et al. Temporal lobe epilepsy in patients with nonlesional MRI and normal memory: an SEEG study. J Neurosurg 2015;123:1368-74. [Crossref] [PubMed]
- Ciurea A, Popa I, Maliia MD, et al. Successful epilepsy surgery in frontal lobe epilepsy with startle seizures: a SEEG study. Epileptic Disord 2015;17:363-71. [PubMed]
- Craiu D, Barborica A, Motoescu C, et al. Presurgical evaluation and epilepsy surgery in MRI negative resistant epilepsy of childhood with good outcome. Turk Neurosurg 2015;25:905-13. [PubMed]
- Oderiz CC, Aberastury M, Besocke AG, et al. Surgical treatment of focal symptomatic refractory status epilepticus with and without invasive EEG. Epilepsy Behav Case Rep 2015;4:96-8. [Crossref] [PubMed]
- Mathon B, Clemenceau S, Hasboun D, et al. Safety profile of intracranial electrode implantation for video-EEG recordings in drug-resistant focal epilepsy. J Neurol 2015;262:2699-712. [Crossref] [PubMed]
- Catenoix H, Mauguière F, Montavont A, et al. Seizures Outcome after stereoelectroencephalography-guided thermocoagulations in malformations of cortical development poorly accessible to surgical resection. Neurosurgery 2015;77:9-14; discussion 14-5. [Crossref] [PubMed]
- Curry DJ, Gowda A, McNichols RJ, et al. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav 2012;24:408-14. [Crossref] [PubMed]
- Esquenazi Y, Kalamangalam GP, Slater JD, et al. Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy Res 2014;108:547-54. [Crossref] [PubMed]
- Hawasli AH, Bandt SK, Hogan RE, et al. Laser ablation as treatment strategy for medically refractory dominant insular epilepsy: therapeutic and functional considerations. Stereotact Funct Neurosurg 2014;92:397-404. [Crossref] [PubMed]
- Willie JT, Laxpati NG, Drane DL, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery 2014;74:569-84; discussion 584-5. [Crossref] [PubMed]
- Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia 2015;56:101-13. [Crossref] [PubMed]
- Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia 2016;57:325-34. [Crossref] [PubMed]
- Lewis EC, Weil AG, Duchowny M, et al. MR-guided laser interstitial thermal therapy for pediatric drug-resistant lesional epilepsy. Epilepsia 2015;56:1590-8. [Crossref] [PubMed]
- Waseem H, Osborn KE, Schoenberg MR, et al. Laser ablation therapy: An alternative treatment for medically resistant mesial temporal lobe epilepsy after age 50. Epilepsy Behav 2015;51:152-7. [Crossref] [PubMed]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295-304. [Crossref] [PubMed]
- Morrell MJ. In response: The RNS System multicenter randomized double-blinded controlled trial of responsive cortical stimulation for adjunctive treatment of intractable partial epilepsy: knowledge and insights gained. Epilepsia 2014;55:1470-1. [Crossref] [PubMed]
- Heck CN, King-Stephens D, Massey AD, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 2014;55:432-41. [Crossref] [PubMed]
- King-Stephens D, Mirro E, Weber PB, et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia 2015;56:959-67. [Crossref] [PubMed]
- Loring DW, Kapur R, Meador KJ, et al. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia 2015;56:1836-44. [Crossref] [PubMed]
- Meador KJ, Kapur R, Loring DW, et al. Quality of life and mood in patients with medically intractable epilepsy treated with targeted responsive neurostimulation. Epilepsy Behav 2015;45:242-7. [Crossref] [PubMed]
- Alomar S, Jones J, Maldonado A, et al. The Stereo-Electroencephalography Methodology. Neurosurg Clin N Am 2016;27:83-95. [Crossref] [PubMed]
- González-Martínez J, Bulacio J, Thompson S, et al. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery 2016;78:169-80. [Crossref] [PubMed]
- Cossu M, Cardinale F, Castana L, et al. Stereo-EEG in children. Childs Nerv Syst 2006;22:766-78. [Crossref] [PubMed]
- Engel J Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001;42:796-803. [Crossref] [PubMed]
- Gnatkovsky V, de Curtis M, Pastori C, et al. Biomarkers of epileptogenic zone defined by quantified stereo-EEG analysis. Epilepsia 2014;55:296-305. [Crossref] [PubMed]
- Boido D, Kapetis D, Gnatkovsky V, et al. Stimulus-evoked potentials contribute to map the epileptogenic zone during stereo-EEG presurgical monitoring. Hum Brain Mapp 2014;35:4267-81. [Crossref] [PubMed]
- Rummel C, Abela E, Andrzejak RG, et al. Resected brain tissue, seizure onset zone and quantitative EEG measures: towards prediction of post-surgical seizure control. PLoS One 2015;10:e0141023. [Crossref] [PubMed]
- Niederhauser JJ, Esteller R, Echauz J, et al. Detection of seizure precursors from depth-EEG using a sign periodogram transform. IEEE Trans Biomed Eng 2003;50:449-58. [Crossref] [PubMed]
- Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain 2008;131:1818-30. [Crossref] [PubMed]
- Jacobs J, Levan P, Chatillon CE, et al. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain 2009;132:1022-37. [Crossref] [PubMed]
- Gonzalez-Martinez J, Vadera S, Mullin J, et al. Robot-assisted stereotactic laser ablation in medically intractable epilepsy: operative technique. Neurosurgery 2014;10 Suppl 2:167-72; discussion 172-3. [Crossref] [PubMed]
- Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. Neurosurg Focus 2015;38:E13. [Crossref] [PubMed]
- Mohammadi AM, Schroeder JL. Laser interstitial thermal therapy in treatment of brain tumors--the NeuroBlate System. Expert Rev Med Devices 2014;11:109-19. [Crossref] [PubMed]
- Blume WT, Parrent AG, Kaibara M. Stereotactic amygdalohippocampotomy and mesial temporal spikes. Epilepsia 1997;38:930-6. [Crossref] [PubMed]
- Liscak R, Malikova H, Kalina M, et al. Stereotactic radiofrequency amygdalohippocampectomy in the treatment of mesial temporal lobe epilepsy. Acta Neurochir (Wien) 2010;152:1291-8. [Crossref] [PubMed]
- Parrent AG, Blume WT. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. Epilepsia 1999;40:1408-16. [Crossref] [PubMed]
- Monteith S, Sheehan J, Medel R, et al. Potential intracranial applications of magnetic resonance-guided focused ultrasound surgery. J Neurosurg 2013;118:215-21. [Crossref] [PubMed]
- Wilfong AA, Curry DJ. Hypothalamic hamartomas: optimal approach to clinical evaluation and diagnosis. Epilepsia 2013;54 Suppl 9:109-14. [Crossref] [PubMed]
- Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med 2014;3:971-9. [Crossref] [PubMed]
- Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med 2011;43:943-50. [Crossref] [PubMed]
- Alker G, Kelly PJ, Kall B, et al. Stereotaxic laser ablation of intracranial lesions. AJNR Am J Neuroradiol 1983;4:727-30. [PubMed]
- Engel J Jr, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012;307:922-30. [Crossref] [PubMed]
- Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311-8. [Crossref] [PubMed]
- Skirrow C, Cross JH, Harrison S, et al. Temporal lobe surgery in childhood and neuroanatomical predictors of long-term declarative memory outcome. Brain 2015;138:80-93. [Crossref] [PubMed]
- Lah S, Smith ML. Verbal memory and literacy outcomes one year after pediatric temporal lobectomy: a retrospective cohort study. Epilepsy Behav 2015;44:225-33. [Crossref] [PubMed]
- Jasper H. Electrocorticography. In: Penfield W, Jasper H. editors. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little Brown, 1954:692-738.
- Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics 2014;11:553-63. [Crossref] [PubMed]
- Velasco F, Carrillo-Ruiz JD, Brito F, et al. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia 2005;46:1071-81. [Crossref] [PubMed]
- Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia 1992;33:841-51. [Crossref] [PubMed]
- Velasco AL, Velasco F, Jimenez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia 2006;47:1203-12. [Crossref] [PubMed]
- Cooper IS, Upton AR, Amin I. Reversibility of chronic neurologic deficits. Some effects of electrical stimulation of the thalamus and internal capsule in man. Appl Neurophysiol 1980;43:244-58. [PubMed]
- Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010;51:899-908. [Crossref] [PubMed]
- Hodaie M, Wennberg RA, Dostrovsky JO, et al. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia 2002;43:603-8. [Crossref] [PubMed]
- Capecci M, Ricciuti RA, Ortenzi A, et al. Chronic bilateral subthalamic stimulation after anterior callosotomy in drug-resistant epilepsy: long-term clinical and functional outcome of two cases. Epilepsy Res 2012;98:135-9. [Crossref] [PubMed]
- Chabardès S, Kahane P, Minotti L, et al. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord 2002;4 Suppl 3:S83-93. [PubMed]
- Chkhenkeli SA, Chkhenkeli IS. Effects of therapeutic stimulation of nucleus caudatus on epileptic electrical activity of brain in patients with intractable epilepsy. Stereotact Funct Neurosurg 1997;69:221-4. [Crossref] [PubMed]
- Chkhenkeli SA, Sramka M, Lortkipanidze GS, et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg 2004;106:318-29. [Crossref] [PubMed]
- McLachlan RS, Pigott S, Tellez-Zenteno JF, et al. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: impact on seizures and memory. Epilepsia 2010;51:304-7. [Crossref] [PubMed]
- Tellez-Zenteno JF, McLachlan RS, Parrent A, et al. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology 2006;66:1490-4. [Crossref] [PubMed]
- Velasco M, Velasco F, Velasco AL, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia 2000;41:158-69. [Crossref] [PubMed]
- Vesper J, Klostermann F, Stockhammer F, et al. Results of chronic subthalamic nucleus stimulation for Parkinson's disease: a 1-year follow-up study. Surg Neurol 2002;57:306-11; discussion 311-3. [Crossref] [PubMed]
- Wille C, Steinhoff BJ, Altenmuller DM, et al. Chronic high-frequency deep-brain stimulation in progressive myoclonic epilepsy in adulthood--report of five cases. Epilepsia 2011;52:489-96. [Crossref] [PubMed]
- Fridley J, Thomas JG, Navarro JC, et al. Brain stimulation for the treatment of epilepsy. Neurosurg Focus 2012;32:E13. [Crossref] [PubMed]
- Bergey GK, Morrell MJ, Mizrahi EM, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 2015;84:810-7. [Crossref] [PubMed]


