
Disrupted functional connectivity patterns of the left inferior frontal gyrus subregions in benign childhood epilepsy with centrotemporal spikes
Introduction
Benign epilepsy with centrotemporal spikes (BECTS), also known as self-limited epilepsy with centrotemporal spikes or benign rolandic epilepsy (BRE), is the most common of the self-limited focal epilepsies (1). Although children with BECTS usually reach remission before puberty and have an excellent prognosis, recent studies have shown that the syndrome can lead to cognitive impairment affecting language, auditory–verbal memory, and visuospatial ability (2-4). Recent studies focusing on language development in children with BECTS have shown their language function to be significantly impaired by centrotemporal spikes (5-8), with this dysfunction of language becoming more severe with the early onset of the disease (9,10). Previous studies have also reported impaired language functions in semantic language processing (7), semantic verbal fluency, and sentence comprehension (11) in children with BECTS. However, the neural mechanism underlying the effect of BECTS on language dysfunction is unclear.
Abnormal cortical thickness, volume, gyrification, and sulcal depth in BECTS have been reported in previous studies. However, the study results are not consistent. Considering the influence of duration, antiepileptic drugs and the range of patients’ age, Li et al. showed extensive cortical thinning in bilateral frontal, temporal regions, and limbic system, increased cortical gyrification in the left hemisphere and partial right hemisphere, and the decreased cortical gyrification in the left hemisphere, and increased sulcal depth in the left fusiform gyrus in drug-naive BECTS patients (12). On the other hand, negative correlation between age of onset and cortical thickness in the right precentral gyrus, cortical gyrification in the left inferior parietal gyrus, and negative correlation between verbal intelligence quotient (VIQ) and cortical gyrification in the left supramarginal gyrus have also been revealed. Aberrant cortical thickness, cortical gyrification in left inferior frontal gyrus (LIFG) have been proposed. A language network study of BECTS carried out by McGinnity found decreased functional connectivity within a four-node subnetwork including the LIFG (13),
As a key brain area for language processing in the human brain, the LIFG plays an important role in the integration of different language domains including phonology, syntax, semantics, language comprehension, and language production (14,15). Damage to the LIFG can result in severe language problems (16). Structural and functional changes in the LIFG have been reported in the previous neuroimaging studies of BECTS (17-19). For instance, by analyzing functional magnetic resonance imaging (fMRI) data when language tasks were performed Besseling et al. found that activation mainly occurred in the left hemisphere (20). However, these studies analyzed the LIFG as a whole, which obscured the specificity change in each subregion of the LIFG. Given that the LIFG is a functionally heterogenous area and that different functional subregions are involved in different functions (21-23), delineating the specific functional changes in each LIFG subregion may clarify BECTS at the neural level.
With the development of resting-state fMRI, resting-state functional connectivity (RSFC) has been used to measure correlations in neural activity between different brain regions based on the blood-oxygen-level–dependent (BOLD) signal and thereby examine the intrinsic functional couplings (24,25). Furthermore, RSFC has the advantage of minimizing the effect of patients cooperation or specific tasks and can aid in the discovery of abnormalities in the brain network. RSFC has been widely used to explore the functional organization in healthy participants and to uncover the abnormal functional interactions in a diverse range of brain disorders (26-33).
To explore how BECTS affects language development, we analyzed specific functional connection changes in the LIFG subregions of children with BECTS by using the seed-based resting state functional connectivity method. The LIFG subregions were defined according to the Brainnetome Atlas. RSFC was then used to investigate the potentially abnormal connectivity patterns of the LIFG subregions in BECTS. We present the following article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-270/rc).
Methods
Participants
For this study, 98 right-handed participants, including 49 patients with BECTS (23 females, 26 males; mean age, 10.15±2.11 years) and 49 healthy controls (HCs; 29 females, 20 males; mean age, 10.21±2.21 years) were recruited (see Table 1). Patients were consecutively recruited from the Department of Pediatrics at the Affiliated Hospital of Zunyi Medical University, China. The Wechsler Intelligence Scale for Children (WISC; Chinese version) was conducted a day before MRI scans; the scale includes a performance intelligence quotient (PIQ), a VIQ, and a full-scale intelligence quotient (FSIQ). Interictal electroencephalogram (EEG) was performed within 1 week after the MRI scan.
Table 1
| Characteristics | Mean ± SD | P value | |
|---|---|---|---|
| BECTS | HCs | ||
| Gender (female/male) | 49 (23/26) | 49 (29/20) | 0.461a |
| Age at scan (year) | 10.15±2.11 | 10.21±2.21 | 0.852b |
| Year of education | 3.65±1.94 | 3.94±2.16 | 0.291b |
| Epilepsy duration (month) | 25.46±22.87 | NA | NA |
| WISC | |||
| VIQ | 98.51±15.81 | NA | NA |
| PIQ | 95.76±14.45 | NA | NA |
| FSIQ | 97.39±14.49 | NA | NA |
| Abnormal discharge position of EEG (L/R/B/N) | 12/6/21/10 | NA | NA |
a, Chi-square test; b, two-sample t-test. BECTS, benign epilepsy with centrotemporal spikes; HCs, healthy controls; WISC, Wechsler Intelligence Scale for Children; VIQ, verbal intelligence quotient; PIQ, performance intelligence quotient; FSIQ, full scale intelligence quotient; EEG, electroencephalogram; L, left; R, right; B, bilateral; N, none; SD, standard deviation; NA, not available.
The inclusion criteria for BECTS patients were as follows: (I) BECTS diagnosed by an intermediate or above pediatrician according to the 2010 version of the Committee of the International League Against Epilepsy diagnostic criteria (34); (II) aged between 6 and 16 years old; (III) attending school regularly for education; (IV) FSIQ of >70; (V) no developmental disabilities; (VI) no history of addictions or other neurological diseases. Inclusion criteria for the HCs were the following: (I) no history of neurological or psychiatric disorders; (II) being age, gender and years of education matched to the BECTS group; (VI) no history of craniocerebral trauma, neuropsychiatric disease, or surgery. For all participants, exclusion criteria were (I) pathological focal brain lesions on T1-weighted or T2-weighted fluid-attenuated inversion-recovery magnetic resonance images; (II) falling asleep during the MRI session (assessed by means of self-reporting); (III) head motion of >3 mm in translation or 3° in rotation; (IV) any foreign implants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University (No. (2017) 1-047) and informed consent was taken from all individual participants’ parents.
Resting-state fMRI data collection
Resting-state fMRI data were acquired on a clinical 3.0T MRI scanner (Signa 3.0THDxt, GE Healthcare, Chicago, IL, USA) with a standard 8-channel head coil using a gradient echo-echo planar imaging (GRE-EPI) sequence. A total of 250 functional volumes were acquired under the following parameters: repetition time = 2,000 ms, echo time = 30 ms, thickness = 4.0 mm, interslice gap = 1.2 mm, field of view = 240 mm × 240 mm, matrix = 64 × 64, flip angle = 90°, and 33 transverse slices.
Resting-state fMRI preprocessing
Preprocessing of fMRI data was performed using DPARSFA 2.3 (Data Processing Assistant for Resting-State fMRI Advanced Edition; http://www.restfmri.net/forum/DPARSF). The preprocessing steps included the discarding of the first 10 volumes; head motion correction; normalizing to the echo planar images (EPI) template in Montreal Neurological Institute (MNI) space with a resolution of 3 mm3× 3 mm3 × 3 mm3; smoothing with a Gaussian kernel of 6 mm full-width at half maximum (FWHM); detrending; regressing out nuisance signals including Friston-24 head motion parameters, white matter, and cerebrospinal fluid and global mean signals; and temporal bandpass filtering (0.01–0.1 Hz). Participants who exhibited a maximum displacement in any of the cardinal directions (x, y, z) of >3 mm or a maximum spin (x, y, z) of >3° were excluded. Moreover, the fMRI data were scrubbed by censoring the volumes with the frame-wise displacement (FD) above 0.5.
Definition of LIFG subregions
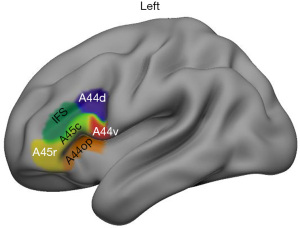
The LIFG subregions were defined using the Brainnetome Atlas (http://www.atlas.brainnetome.org) mapping with the anatomical connectivity-based parcellation approach (35). According to the atlas, 6 subregions of the LIFG were defined shown in Figure 1. These LIFG subregions included the dorsal Brodmann area (BA) 44 (A44d), the ventral BA 44 (A44v), the opercularis part of BA 44 (A44op), the rostral BA 45 (A45r), the caudal BA45 (A45c), and the dorsal inferior frontal sulcus (IFS).
RSFC analysis
For each LIFG subregion, whole-brain functional connectivity, measured using Pearson correlation coefficients between the mean time series of each subregion and that of each voxel of the whole brain was calculated. Then, the whole-brain functional connectivity maps were converted to Z maps using a Fisher’s r-to-z transformation to improve normality. Finally, two-sample t-tests were performed (with age and education level as covariates) to determine brain areas with changed FCs to each left IFG subregion between the BECTS and healthy controls. The significance was determined using Gaussian random field correction at a cluster-level P<0.05 (voxel-level P<0.001)
Statistical analysis
Two-sample t-tests (P<0.05) were used in between-group comparison for age and education, and Chi-square tests (P<0.05) for sex. The brain areas with changed FCs to each left IFG subregions were compared between groups using two-sample t-tests with age and education level as covariates of no interest between the BECTS and healthy controls. The significance was determined using Gaussian random field correction at a cluster-level P<0.05 (voxel-level P<0.001). Correlation analyses were further applied to explore the relationship between clinical information (epilepsy duration, year of education, abnormal discharge position of EEG, VIQ, PIQ and FSIQ) and connectivity changes in children with BECTS.
Results
Demographics and clinical characteristics
The demographics and clinical characteristics of all the participants are shown in Table 1. The two groups exhibited no significant differences in sex (P=0.461), age (P=0.852), or years of education (P=0.291). The WISC scores were at normal IQ levels (normal level scores ranged from 90 to 110), including VIQ, PIQ, and FIQ score in the BECTS group. According to the electroencephalogram (EEG) results, at the time of inclusion in the study, EEG spike foci were bilateral sided in 21 patients (42.86%), left sided in 12 patients (24.49%), and right sided in 6 patients (12.24%).
Changed RSFCs of the left IFG subregions
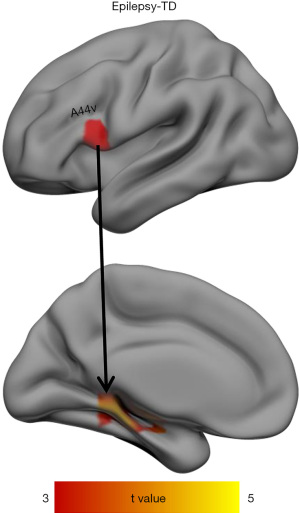
The RSFC of the LIFG subregions differed significantly between the patients with BECTS and the HCs. As shown in Figures 2,3, the BECTS patients had higher functional connectivity between the following: the IFS and right anterior cingulate cortex (ACC), and the A44v region and left hippocampus/parahippocampus. Also, a connectivity decrease was found between the IFS and the left inferior temporal gyrus (LITG). No other significant differences in functional connectivity were found in the other 4 functional subregions of the LIFG in the BECTS.
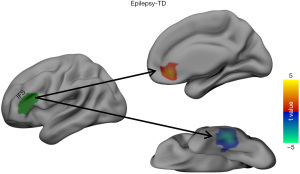
Association between connectivity changes and clinical characteristics
We further investigated whether connectivity changes were associated with clinical characteristics in BECTS, such as epilepsy duration, year of education, abnormal discharge position of EEG, VIQ, PIQ and FSIQ. There was no significant correlation between clinical features and connectivity changes in BECTS (P>0.05).
Discussion
The evidence of functional reorganization and connectivity changes in the LIFG subregions in BECTS is shown in our results. By analyzing the resting-state fMRI data, the current study found that there were altered functional connections between LIFG subregions and other brain regions in children with BECTS compared to HCs. There was higher functional connectivity between the IFS and right ACC in patients with BECTS compared to HCs, and interestingly, there was lower functional connectivity between the IFS and the LITG. In addition, there was higher functional connectivity between the A44v and left hippocampus/parahippocampus in BECTS patients compared to HCs. These findings revealed specific changes in LIFG subregions and provide greater insight into the pathomechanism of the BECTS.
Unlike children in previous studies, the children with BECTS in our study had high IQs (the mean WISC FSIQ was 97.39). A meta-analysis by Smith et al., through showed there to be a relationship between IQ and language in children with BECTS (36) and found that language impairment was more likely to be found in children with BECTS with lower IQs. The mean age and age range of the children with BECTS included in our study (mean age 10.15 years, age range, 8–12 years) and that of Vannest et al. (18) (mean age 8.13 years, age range, 5–12 years) were lower than those in most studies included in the meta-analysis by Smith et al., and their IQs were higher than the mean IQ level. This suggests that the IQ level is more lower and language is more likely to be impaired as age at onset in children with BECT. In summary, it is very important for clinicians to assess the language of the BECTS as early as possible, as this can facilitate intervention programs early and shorten the gap between the language level of children with BECTS and the normal language level.
The current study found increased functional connectivity between A44v and the left hippocampal/parahippocampal gyrus in children BECTS. A review by Zaccarella and Friederici suggested that A44v might be involved in the most fundamental mechanisms regulating natural language syntax (37). It is well known that the hippocampus is a core part of the default-mode network (DMN) node, which has been widely reported to be associated with a variety of epilepsies (38). The DMN has been shown to contribute to the generation and propagation of epileptic activity (39). In addition, the role of the hippocampus in the brain is related to memory and recall. A study by Kim et al. reported memory deficits in children with BECTS (40). Previous studies showed that verbally loaded memory tasks are strongly correlated with left hippocampal volume (41,42). Moreover, other research has shown altered static amplitude of low frequency fluctuation (sALFF) and dynamic ALFF (dALFF) in the hippocampus of BECTS patients, implying improved cognitive function (43). Therefore, to some extent, the increased connectivity of A44v and the left hippocampal/parahippocampal gyrus might contribute to the strengthening of verbal contextual memory and cognitive improvement after language impairment in children with BECTS.
In the current study, the connectivity between the IFS region and right ACC was significantly higher in BECTS patients than in the HCs. The cortical thinning in the left inferior frontal cortex and cortical thickening in the posterior cingulate gyri has been shown in several studies about patients with BECTS and attention-deficit/hyperactivity disorder (ADHD) (44,45). The posterior cingulate gyri play an important role in emotional, cognition regulation and the attentional processes (46). A structural explanation is provided for the worse executive, speech production and attentional performance seen in patients with BECTS and ADHD in this study (46). Recent fMRI studies have suggested that the dorsal IFS is involved in the coordination of interference processes and the coordination of cognitive processes relating to the mapping of sensory information to corresponding motor responses (47). The ACC has been implicated in the cognitive processing of anxiety and fear as well as in conditioning circuits (48). Another study in rats demonstrated that the ACC carries a large number of essential signals related to regulating attention and tasks (49). In their study, ALFF was significantly increased in the left ACC in children with BECTS compared to controls, which may explain the attention deficit present in children with BECTS. It has also been shown that rats with ACC lesions have difficulty adjusting cognitive control (50). Furthermore, the study by Cerminara et al. found that BECTS patients had attention impairment (51). Collectively, these studies may indicate that the increased connectivity in these areas may be due to compensation of the cognitive impairment in the pathogenesis of BECTS, which may have an overall positive impact on the quality of life of children with BECTS.
A reduced functional connectivity between the dorsal IFS and LITG in the BECTS group compared HCs was found in our study. Previous fMRI studies have reported reduced activation or left lateralization specific to the inferior frontal regions in BECTS patients (2,52). Previous research also suggests that the ITG is involved in orthographic processing and has an important role in language, including in the semantic processing of spoken words (53). Another study found that the LITG is associated with abnormal neuronal activity in BECTS patients (54). Drug-receiving patients with BECTS showed additional abnormalities in the ITG (43). In addition, a greater functional connectivity was found between the LITG and the rolandic regions of interest (ROIs) in BECTS patients as compared to HCs, suggesting that abnormally high connectivity patterns may interrupt the normal language function and its network (55). However, a reduced functional connectivity between the dorsal IFS and LITG was found in our study. Based on this, we propose that normal language function is impaired while language function is being repaired. Interestingly, the decreased connectivity did not correspond to worse performance in children BECTS. This may be due to the fact that children with BECTS rely on alternative circuits to achieve language skills that are comparable to those exhibited by HCs (52,56).
There are several limitations to this study. First, a lack of neuropsychological evaluation of the HCs makes it difficult to draw accurate neuropsychological comparisons between the BECTS patients and the HCs. Second, this study was a cross-sectional study with a small number of participants. A study with a larger sample size and longitudinal tracking is needed to explain the dynamic in functional connections and their relationships with language damage in children with BECTS. In addition, the unbalanced and small sample size, which included first-episode and medication-treated BECTS patients, as well as possible individual differences, may limit the explanatory capacity of our findings. Second, the different types and densities of epileptiform discharges were not discussed and should thus be explored in future research.
Conclusions
These findings provided evidence for the BECTS-related effects in the functional connection patterns of the LIFG subregions and revealed that different subregions may be involved in different neural circuits associated with impairments in language function in children with BECTS.
Acknowledgments
The authors appreciate the academic support from the AME Radiology Collaborative Group, and thank professor Silvia Miano [Sleep Center, Neurocenter of Southern Switzerland, Civic Hospital of Lugano (EOC), via Tesserete 46, 6903, Lugano, Switzerland. silvia.miano@eoc.ch] for the critical comments and valuable advice on this study.
Funding: This work was supported by Young Outstanding Scientific and technological Talent of Guizhou Province (grant No. Qiankehepingtairencai[2021]5620).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-270/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-270/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-270/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University (No. (2017) 1-047) and informed consent was taken from all individual participants’ parents.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:512-21. [Crossref] [PubMed]
- Vannest J, Szaflarski JP, Eaton KP, et al. Functional magnetic resonance imaging reveals changes in language localization in children with benign childhood epilepsy with centrotemporal spikes. J Child Neurol 2013;28:435-45. [Crossref] [PubMed]
- Wickens S, Bowden SC, D'Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: A systematic review and meta-analysis. Epilepsia 2017;58:1673-85. [Crossref] [PubMed]
- Völkl-Kernstock S, Bauch-Prater S, Ponocny-Seliger E, et al. Speech and school performance in children with benign partial epilepsy with centro-temporal spikes (BCECTS). Seizure 2009;18:320-6. [Crossref] [PubMed]
- Garcia-Ramos C, Jackson DC, Lin JJ, et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia 2015;56:1615-22. [Crossref] [PubMed]
- Goldberg-Stern H, Gonen OM, Sadeh M, et al. Neuropsychological aspects of benign childhood epilepsy with centrotemporal spikes. Seizure 2010;19:12-6. [Crossref] [PubMed]
- Overvliet GM, Besseling RM, van der Kruijs SJ, et al. Clinical evaluation of language fundamentals in Rolandic epilepsy, an assessment with CELF-4. Eur J Paediatr Neurol 2013;17:390-6. [Crossref] [PubMed]
- Danielsson J, Petermann F. Cognitive deficits in children with benign rolandic epilepsy of childhood or rolandic discharges: a study of children between 4 and 7 years of age with and without seizures compared with healthy controls. Epilepsy Behav 2009;16:646-51. [Crossref] [PubMed]
- Jurkevičienė G, Endzinienė M, Laukienė I, et al. Association of language dysfunction and age of onset of benign epilepsy with centrotemporal spikes in children. Eur J Paediatr Neurol 2012;16:653-61. [Crossref] [PubMed]
- Ma Y, Chen G, Wang Y, et al. Language dysfunction is associated with age of onset of benign epilepsy with centrotemporal spikes in children. Eur Neurol 2015;73:179-83. [Crossref] [PubMed]
- Verly M, Gerrits R, Lagae L, et al. Evaluation of the language profile in children with rolandic epilepsy and developmental dysphasia: Evidence for distinct strengths and weaknesses. Brain Lang 2017;170:18-28. [Crossref] [PubMed]
- Li Z, Zhang J, Wang F, et al. Surface-based morphometry study of the brain in benign childhood epilepsy with centrotemporal spikes. Ann Transl Med 2020;8:1150. [Crossref] [PubMed]
- McGinnity CJ, Smith AB, Yaakub SN, et al. Decreased functional connectivity within a language subnetwork in benign epilepsy with centrotemporal spikes. Epilepsia Open 2017;2:214-25. [Crossref] [PubMed]
- Ishkhanyan B, Michel Lange V, Boye K, et al. Anterior and Posterior Left Inferior Frontal Gyrus Contribute to the Implementation of Grammatical Determiners During Language Production. Front Psychol 2020;11:685. [Crossref] [PubMed]
- Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev 2011;91:1357-92. [Crossref] [PubMed]
- Friederici AD. The neural basis of language development and its impairment. Neuron 2006;52:941-52. [Crossref] [PubMed]
- Wu Y, Ji GJ, Zang YF, et al. Local Activity and Causal Connectivity in Children with Benign Epilepsy with Centrotemporal Spikes. PLoS One 2015;10:e0134361. [Crossref] [PubMed]
- Vannest J, Maloney TC, Tenney JR, et al. Changes in functional organization and functional connectivity during story listening in children with benign childhood epilepsy with centro-temporal spikes. Brain Lang 2019;193:10-7. [Crossref] [PubMed]
- Besseling RM, Jansen JF, Overvliet GM, et al. Reduced structural connectivity between sensorimotor and language areas in rolandic epilepsy. PLoS One 2013;8:e83568. [Crossref] [PubMed]
- Besseling RM, Overvliet GM, Jansen JF, et al. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res 2013;107:253-62. [Crossref] [PubMed]
- Wang J, Yang Y, Zhao X, et al. Evolutional and developmental anatomical architecture of the left inferior frontal gyrus. Neuroimage 2020;222:117268. [Crossref] [PubMed]
- Zhang Y, Fan L, Caspers S, et al. Cross-cultural consistency and diversity in intrinsic functional organization of Broca's Region. Neuroimage 2017;150:177-90. [Crossref] [PubMed]
- Gao Z, Guo X, Liu C, et al. Right inferior frontal gyrus: An integrative hub in tonal bilinguals. Hum Brain Mapp 2020;41:2152-9. [Crossref] [PubMed]
- Yu H, Li ML, Li YF, et al. Anterior cingulate cortex, insula and amygdala seed-based whole brain resting-state functional connectivity differentiates bipolar from unipolar depression. J Affect Disord 2020;274:38-47. [Crossref] [PubMed]
- Wang J, Becker B, Wang L, et al. Corresponding anatomical and coactivation architecture of the human precuneus showing similar connectivity patterns with macaques. Neuroimage 2019;200:562-74. [Crossref] [PubMed]
- Wang J, Wei Q, Bai T, et al. Electroconvulsive therapy selectively enhanced feedforward connectivity from fusiform face area to amygdala in major depressive disorder. Soc Cogn Affect Neurosci 2017;12:1983-92. [Crossref] [PubMed]
- Wang J, Wei Q, Yuan X, et al. Local functional connectivity density is closely associated with the response of electroconvulsive therapy in major depressive disorder. J Affect Disord 2018;225:658-64. [Crossref] [PubMed]
- Wang J, Xie S, Guo X, et al. Correspondent Functional Topography of the Human Left Inferior Parietal Lobule at Rest and Under Task Revealed Using Resting-State fMRI and Coactivation Based Parcellation. Hum Brain Mapp 2017;38:1659-75. [Crossref] [PubMed]
- Wang J, Yang Y, Fan L, et al. Convergent functional architecture of the superior parietal lobule unraveled with multimodal neuroimaging approaches. Hum Brain Mapp 2015;36:238-57. [Crossref] [PubMed]
- Wang C, Wu H, Chen F, et al. Disrupted functional connectivity patterns of the insula subregions in drug-free major depressive disorder. J Affect Disord 2018;234:297-304. [Crossref] [PubMed]
- Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007;62:429-37. [Crossref] [PubMed]
- Greicius MD, Srivastava G, Reiss AL, et al. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 2004;101:4637-42. [Crossref] [PubMed]
- Wu Y, Zhang Y, Liu Y, et al. Distinct Changes in Functional Connectivity in Posteromedial Cortex Subregions during the Progress of Alzheimer's Disease. Front Neuroanat 2016;10:41. [Crossref] [PubMed]
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 2010;51:676-85. [Crossref] [PubMed]
- Fan L, Li H, Zhuo J, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex 2016;26:3508-26. [Crossref] [PubMed]
- Smith AB, Bajomo O, Pal DK. A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol 2015;57:1019-26. [Crossref] [PubMed]
- Zaccarella E, Friederici AD. The neurobiological nature of syntactic hierarchies. Neurosci Biobehav Rev 2017;81:205-12. [Crossref] [PubMed]
- Li R, Ji GJ, Yu Y, et al. Epileptic Discharge Related Functional Connectivity Within and Between Networks in Benign Epilepsy with Centrotemporal Spikes. Int J Neural Syst 2017;27:1750018. [Crossref] [PubMed]
- Clemens B, Puskás S, Spisák T, et al. Increased resting-state EEG functional connectivity in benign childhood epilepsy with centro-temporal spikes. Seizure 2016;35:50-5. [Crossref] [PubMed]
- Kim EH, Yum MS, Kim HW, et al. Attention-deficit/hyperactivity disorder and attention impairment in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2014;37:54-8. [Crossref] [PubMed]
- Steffens DC, Payne ME, Greenberg DL, et al. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry 2002;10:62-71. [Crossref] [PubMed]
- Hardcastle C, O'Shea A, Kraft JN, et al. Contributions of Hippocampal Volume to Cognition in Healthy Older Adults. Front Aging Neurosci 2020;12:593833. [Crossref] [PubMed]
- Jiang S, Luo C, Huang Y, et al. Altered Static and Dynamic Spontaneous Neural Activity in Drug-Naïve and Drug-Receiving Benign Childhood Epilepsy With Centrotemporal Spikes. Front Hum Neurosci 2020;14:361. [Crossref] [PubMed]
- Lima EM, Rzezak P, Dos Santos B, et al. The relevance of attention deficit hyperactivity disorder in self-limited childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2018;82:164-9. [Crossref] [PubMed]
- Kim EH, Yum MS, Shim WH, et al. Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS). Seizure 2015;27:40-6. [Crossref] [PubMed]
- Aricò M, Arigliani E, Giannotti F, et al. ADHD and ADHD-related neural networks in benign epilepsy with centrotemporal spikes: A systematic review. Epilepsy Behav 2020;112:107448. [Crossref] [PubMed]
- Stelzel C, Schumacher EH, Schubert T, et al. The neural effect of stimulus-response modality compatibility on dual-task performance: an fMRI study. Psychol Res 2006;70:514-25. [Crossref] [PubMed]
- Linares IM, Jackowski AP, Trzesniak CM, et al. Cortical thinning of the right anterior cingulate cortex in spider phobia: a magnetic resonance imaging and spectroscopy study. Brain Res 2014;1576:35-42. [Crossref] [PubMed]
- Brockett AT, Roesch MR. Anterior cingulate cortex and adaptive control of brain and behavior. Int Rev Neurobiol 2021;158:283-309. [Crossref] [PubMed]
- Newman LA, Creer DJ, McGaughy JA. Cognitive control and the anterior cingulate cortex: how conflicting stimuli affect attentional control in the rat. J Physiol Paris 2015;109:95-103. [Crossref] [PubMed]
- Cerminara C, D'Agati E, Lange KW, et al. Benign childhood epilepsy with centrotemporal spikes and the multicomponent model of attention: a matched control study. Epilepsy Behav 2010;19:69-77. [Crossref] [PubMed]
- Datta AN, Oser N, Bauder F, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia 2013;54:487-94. [Crossref] [PubMed]
- Cao F, Bitan T, Chou TL, et al. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry 2006;47:1041-50. [Crossref] [PubMed]
- Besenyei M, Varga E, Fekete I, et al. EEG background activity is abnormal in the temporal and inferior parietal cortex in benign rolandic epilepsy of childhood: a LORETA study. Epilepsy Res 2012;98:44-9. [Crossref] [PubMed]
- Kim HJ, Lee JH, Park CH, et al. Role of Language-Related Functional Connectivity in Patients with Benign Childhood Epilepsy with Centrotemporal Spikes. J Clin Neurol 2018;14:48-57. [Crossref] [PubMed]
- Lillywhite LM, Saling MM, Harvey AS, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia 2009;50:2276-84. [Crossref] [PubMed]


