Surgical strategies for pediatric epilepsy
Introduction
Pediatric epilepsy is a debilitating condition that affects approximately 45 per 100,000 children per year (1). Drug-resistant epilepsy (DRE), defined by the International League Against Epilepsy as persistent seizures despite treatment with two appropriately selected first-line antiepileptic medications that are well tolerated, comprises up to 20–30% of childhood epilepsy cases (2) and can be especially devastating and difficult to manage. It can be associated with staggering social and economic costs for affected patients and their families. Trials of further medical therapies in these cases have extremely low success rates (3)—just 4% in one series for the third antiepileptic agent and beyond (4). As a result, surgical intervention for these patients is sometimes the only practical option, and in these patients, surgery can often offer significant therapeutic benefit, although it remains an underutilized resource (5).
At its most fundamental level, the goal of epilepsy surgery is the achievement of complete seizure freedom—a surgical cure (6). Although myriad benefits, including fewer medications and improvements in neurocognitive and behavioral measures (7), may be realized from any significant reduction in seizure frequency, the ultimate goal of surgical intervention is complete seizure remission whenever possible. Patients with DRE may be particularly vulnerable to the debilitating developmental effects of intractable seizures, especially those with an early age of onset (8), implying that early surgical evaluation may be associated with significant cognitive and developmental gains. The ability to achieve a surgical cure is influenced by a wide variety of factors, including demographic, pathologic, and surgical considerations (9). Knowledge of these variables is essential to understand and consider the efficacy of any surgical intervention and counsel families appropriately (10). The factor most consistently associated with increasing rates of seizure freedom regardless of the underlying cause of the seizures is the ability to completely remove the epileptogenic zone (11-13), defined as the theoretical area responsible for generation of focal seizures (14). Therefore, meticulous preoperative planning is critical to maximizing the possibility of surgical cure. The ability—or inability—to define a circumscribed, resectable seizure focus plays a significant role in the likelihood of postoperative seizure freedom and dictates the surgical strategy. Careful counseling of the patient and family regarding the goals of surgery with clarification that surgical procedures for epilepsy comprise a continuum from curative to palliative is also critical both before and after intervention (15).
In this review, we will discuss surgical strategies utilized in pediatric epilepsy. Our discussion will include preoperative planning, resective, ablative, and stimulation treatment paradigms, and therapeutic efficacy.
Preoperative planning
The initial step to any surgical epilepsy intervention is the careful characterization and localization of the seizures themselves. Prior to diagnostic studies, thorough assessment of seizure semiology through a careful family interview and medical history plays an important role in this process (16). Clinical seizure semiology often evolves over time because of the ongoing development of neural networks (17,18).
Despite recent advances in other diagnostic modalities, electroencephalographic testing (EEG) and structural magnetic resonance imaging (MRI) remain the cornerstone diagnostic tests in the preoperative evaluation for epilepsy surgery. Seizures that are localizable on EEG are significantly more likely to resolve with surgical resection (19,20). The utilization of intracranial electrode recording expands the diagnostic capabilities of EEG with improved spatial and temporal resolution of ictal recordings (21), especially in cases where scalp recordings are either nondiagnostic or incongruous with clinical and radiographic findings.
Similarly, the identification of a structural lesion on MRI is also associated with a higher likelihood of seizure freedom after surgery (20,22,23). With the utilization of high-field-strength (3T) MRI, multi-sequence techniques, and advanced postprocessing, subtle irregularities including lower-grade cortical dysplasia may become apparent in patients historically thought to have normal imaging, facilitating complete resection of the epileptogenic zone (22,24). Further refinement by utilizing combined techniques such as EEG—functional MRI (25) and high-resolution, voxel-based morphometry (26) may allow for the characterization of even more subtle irregularities in brain architecture. Magnetoencephalography (MEG) is also evolving into an important tool, especially in cases in which MRI fails to demonstrate a structural abnormality (27). Radionuclide imaging, including interictal positron emission tomography and peri-ictal single-photon emission computed tomography, may also be utilized to identify areas of hypometabolism in epileptogenic regions that are not identifiable on standard diagnostic testing (28,29).
Despite these advanced diagnostic techniques, a proportion of patients continue to have difficult-to-define epileptic foci, with a subsequent lower rate of seizure freedom postoperatively, highlighting the need for continued exploration and development of more precise noninvasive diagnostic modalities (30,31). More recent advancements in diagnostic survey procedures, including stereo-EEG, which has multiple bilateral depth electrodes allowing for three-dimensional characterization of ictal discharges (32), have shown promise in aiding in treatment of even the most challenging cases (33).
Resective surgery
Surgical resection of the epileptogenic focus is the preferred surgical approach when possible in children with DRE. The extent of resection may range from simple lesionectomy to single or multiple lobectomies and is tailored based on the individual patient’s seizure semiology, imaging findings, and ictal and functional mapping.
Anterior temporal lobectomy (ATL)
Temporal lobe pathology in the pediatric population is often distinct from that observed in adult patients. Whereas mesial temporal sclerosis is the most common pathologic basis of focal epilepsy in adults (34), children demonstrate this finding less commonly and are more likely to have neoplastic lesions (35) or congenital brain anomalies, such as cortical dysplasia, as the underlying substrate of refractory seizures (36). Because of this, the relative frequency of different surgical approaches and the extent of temporal lobe resection varies as well (37).
ATL is the most common brain resection performed for medically refractory epilepsy in the adult population (38) but constitutes a smaller proportion of childhood epilepsy procedures (39). The majority of ATL procedures performed today are based on the technique described by Spencer et al. (40) in 1984. From this common basis, variations are seen in elements such as the extent of temporal neocortical resection—most commonly <4 cm from the tip of the anterior temporal lobe on the dominant side and <6 cm on the nondominant side (41)—and the extent of the hippocampal resection. The specific technique should be tailored based on preoperative imaging and electrophysiological testing results.
Mortality after the ATL procedure is very low, with reported rates ranging from 0 to 0.5% in large series (42,43). Morbidity rates in a recent review ranged from 0 to 9.3% (42), with the most common complications being visual field disturbance, infection, and neuropsychological changes, most notably declines in verbal memory when the dominant hemisphere was resected. In children, neuropsychological outcomes may be better, with less decline in verbal memory compared with adults and improvement in functions ascribed to the contralateral hemisphere (44). Seizure outcomes after ATL are favorable in children. A recent systematic review by Englot et al. (20) demonstrated a 78% rate of seizure freedom after ATL. Rates of surgical cure vary based on the specific underlying pathology, with higher rates of cure in patients with neoplasms and mesial temporal sclerosis compared with those with cortical dysplasia (45). A history of secondary generalized seizures and the absence of structural pathology on MRI were risk factors for seizures postoperatively in this meta-analysis (20).
Selective amygdalohippocampectomy (SAH)
Although it is used less often than ATL, SAH may be used to spare temporal neocortex, especially in the dominant hemisphere. The underlying theory behind less radical resection is that preservation of these structures may lead to improved neuropsychological outcomes, although this theory has not necessarily been supported; various studies have shown equivocal results in postoperative neuropsychological function when comparing these SAH with standard ATL (46-48). Careful patient selection is especially critical prior to SAH, with key indications being MRI evidence of hippocampal sclerosis, appropriate seizure semiology, and concordant electrodiagnostic data. There are multiple approaches to SAH, including transsylvian (49), transcortical (50), and subtemporal (51) approaches, with the selection dependent on the patient and the surgeon.
Whether SAH provide better outcomes, specifically when compared with those after ATL, remains an area of active debate (46). Two large meta-analyses of studies comparing the two procedures head to head found higher rates of seizure freedom after ATL than after SAH (52,53). One study found no significant difference in intelligence quotient scores between patients receiving the two procedures, and one was unable to make significant conclusions regarding differences in neuropsychiatric outcomes between the two groups. The fact that seizure freedom after SAH may be lower in children than in adults (54), combined with the unique characteristics of seizures in the pediatric population—specifically the permanent cognitive disability related to persistent seizures during brain development (55)—makes the use of SAH infrequent in the pediatric population. New techniques, including MRI-guided laser interstitial thermal therapy, may offer minimally invasive alternatives to lobectomy procedures, although the data remain limited in the pediatric population (56).
Temporal lesionectomy
Among the pathological lesions in the temporal lobe that can lead to medically refractory epilepsy in children, the most common causes are neoplastic, mesial temporal sclerosis, cortical dysplasia, and vascular lesions (57,58). Neoplastic causes of epilepsy comprise up to 20% of surgical cases in the pediatric population (59). Most are low-grade lesions, with the high rates of epileptogenesis possibly related to the nature of some of these tumors (i.e., gangliogliomas, dysembryoplastic neuroepithelial tumors) as developmental lesions on the spectrum of focal cortical dysplasias (60). The unique challenge of temporal lesionectomy is to determine the appropriate extent of resection. Although the epileptogenic zone, the region of brain whose resection is necessary and sufficient for cessation of seizures, is the ideal target for removal, the theoretical nature of this area often defies precise anatomical definition with current noninvasive diagnostic studies (14). The epileptogenic lesion, the structural irregularity that causes seizures, often includes both the pathologic lesion itself and surrounding tissue (35). Defining the epileptogenic zone including a structural lesion frequently requires advanced imaging and invasive monitoring techniques. Resection of extra-lesional cortex based on these techniques has been shown in some series to improve rates of seizure freedom (61,62).
The rate of successful outcomes after temporal lesionectomy varies based on the causative lesion. Rates of seizure freedom after resection of neoplasms have, overall, been excellent, ranging from 72% to 92% in selected series (45,63,64). The most robust predictor of complete seizure freedom across available studies has been the extent of tumor resection (61,64). Tumor type may also play a role in rates of post resection seizure freedom. Patients with gangliogliomas have been shown in multiple studies to have very high rates of long-term seizure freedom after tumor removal, as have those with dysembryoplastic neuroepithelial tumors (65,66). The high rate of seizure freedom after surgery in patients with neoplasm-related epilepsy is especially meaningful given the long expected survival in children harboring low-grade brain tumors (67).
Lesionectomy of vascular lesions such as cavernous hemangiomas and arteriovenous malformations is also highly effective in controlling seizures, with 70–80% of patients achieving long-term seizure freedom in some series (68,69). For these lesions in particular, duration of seizures prior to intervention may play a critical role in the ability to achieve complete seizure freedom with surgery (69,70). Reduced success may be due to deposition of hemosiderin over time in the surrounding brain, leading to an epileptogenic tissue surrounding the lesion that also must be resected to achieve seizure freedom (71).
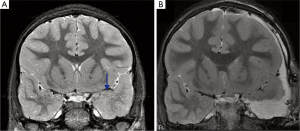
Control rates for mesial temporal sclerosis and cortical dysplasia are generally lower than those for other lesions, likely because the radiographic and intraoperative borders of these lesions are often less well-defined than those of tumors and vascular malformations (45). Temporal lesionectomy can be further complicated by the presence of dual pathology (Figure 1), which often involves hippocampal sclerosis in addition to a second pathology such as a neoplasm (72). Rates of dual pathology vary among studies, but generally rates of seizure freedom after removal of only one of the pathologies are low and are significantly improved by resection of both the sclerotic hippocampal region and the lesion (44). Many pediatric epilepsy surgeons favor resection of the nondominant amygdala and hippocampus in the setting of a temporal lesion adjacent to mesial structures and conversely favor lesionectomy alone when the epileptogenic zone is in the dominant hemisphere because of the risk of verbal memory decline.
Extratemporal resection
Epilepsy of extratemporal origin has historically had lower rates of cure compared with epilepsy of temporal lobe onset, although success can often be achieved in carefully selected patients (73,74). This difference is likely secondary to several unique characteristics of extratemporal epilepsy. The first is the unique pathologic substrate of extratemporal epilepsies, including poorly defined developmental abnormalities such as cortical dysplasia, and multifocal epilepsies, such as tuberous sclerosis complex and Sturge-Weber syndrome (75). Further, overlap of the epileptogenic region with areas of eloquent cortex may complicate surgical resection, resulting in lower rates of seizure freedom (76). Invasive monitoring with intracranial electrodes is often necessary to map the ictal onset zone and functionally eloquent cortex to guide surgical resection (77).
The often complex nature of these procedures and the invaluable information gained from careful preoperative evaluation with advanced electrodiagnostics, high-resolution/functional imaging modalities, and intraoperative mapping and monitoring mean that patients with these epilepsies are best managed at comprehensive epilepsy centers in a multidisciplinary setting. In instances where a distinct, radiographically evident lesion can be identified and is anatomically remote from eloquent cortex, a single, staged lesionectomy may be all that is required. This can be supplemented by intraoperative mapping utilizing electrocorticography to identify epileptogenic regions surrounding the border of the structural lesion. The diagnostic results of intraoperative mapping may be limited by both temporal factors (e.g., limited duration of monitoring during surgery) and physiologic factors (e.g., reduced electrical activity under anesthesia, reliance on interictal epileptiform discharges rather than capturing ictal events). To overcome these limitations, staged procedures with electrode implantation followed by awake long-term electrocorticography may be performed. This allows for long-term monitoring of both ictal and interictal activity, albeit at the cost of a second surgical intervention and associated complications such as infection (Figure 2) (78). For particularly challenging cases, some investigators (75) have also reported success with three-stage procedures, with implantation of intracranial electrodes, long-term seizure monitoring, electrode removal, surgical resection, and electrode reimplantation for post resection seizure monitoring, followed by further resection if necessary.
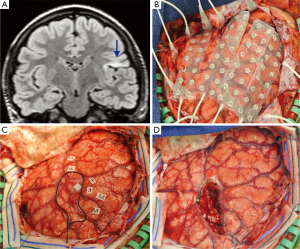
A recent meta-analysis of the efficacy of surgical resection for pediatric epilepsy reported a seizure freedom rate of 56% (23), with better results in children with a shorter duration of seizures, a lesion identified on structural MRI, localizing ictal electrodiagnostic studies, and no history of secondary generalized seizures. These results highlight the need for both early, aggressive intervention in this population and specialized, tertiary care with a team-based approach to provide the best chance of cure in these complex patients.
Hemispherectomy
Hemispherectomy has been utilized for decades in the treatment of hemispheric pathology (79). Today, several surgical techniques are used to remove or disconnect a cerebral hemisphere, including anatomical hemispherectomy, functional hemispherectomy, and hemidecortication (80). Each approach has its distinct risks and benefits, with higher rates of complications in complete anatomic hemispherectomy but lower rates of repeat surgery (81).
Children with diffuse seizure onset and injury throughout the affected hemisphere are candidates for hemispherectomy. These patients frequently have hemiparesis and hemianopia preoperatively (82). The causes of their seizure may be perinatal ischemia and trauma, congenital abnormalities such as Sturge-Weber syndrome, hemimegalencephaly, or inflammatory conditions such as Rasmussen encephalitis, a progressive, inflammatory condition associated with seizures and hemispheric atrophy (Figure 3) (83).
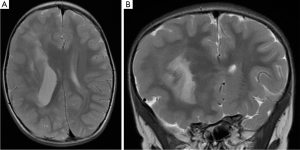
Because of the extensive nature of hemispherectomy, complications are not uncommon. Although rates of serious morbidity and mortality have decreased in modern case series, there remains the potential for significant blood loss relative to a small circulating blood volume in children, which may precipitate anemia and coagulopathy (84). Postoperative hydrocephalus is another common complication, with rates approaching 20–25% (85,86). The development of hydrocephalus may also be delayed in some patients; more than 25% of children who developed postoperative hydrocephalus after undergoing hemispherectomy in one study presented more than 3 months after surgery (86). The incidence of other adverse events varies by study, but a recent review showed an overall rate of surgical complications (e.g., hematoma formation, cerebral abscess) of 3.5% and a rate of medical complications (e.g., meningitis, ventriculitis, venous thrombosis) of 10.6% (87). Perioperative mortality was noted in the same review to occur at a rate of 2.2% (87).
Rates of seizure freedom after hemispherectomy procedures are high, with rates in the 70–80% range for most pathologies (87). Patients with epilepsy as a result of developmental abnormalities and those with seizure generalization, bilateral imaging irregularities, and non-lateralizing EEG have higher rates of seizure persistence after surgery (88).
Palliative surgery
Palliative surgical options may be used in patients in whom resective surgical treatment has failed to control their seizures or for those who are not candidates for procedures with curative intent. Palliative surgery typically includes neurostimulation and disconnected paradigms.
Neurostimulation
Currently, neurostimulation is the most commonly palliative surgical procedure. Vagal nerve stimulation (VNS) is the type of neurostimulation used most often, although therapies such as responsive neurostimulation and the application of deep brain stimulation (DBS) for epilepsy are emerging and evolving.
The precise method through which VNS reduces seizure frequency remains unknown. A large proportion of the nerve is comprised of afferent fibers that project diffusely throughout the brain with a wide array of effects (89). A significant number of these fibers synapse in the solitary nucleus bilaterally and then project to a variety of deep and cortical structures and may play a particularly important part in epilepsy control with VNS (90). VNS is commonly utilized in cases where resective procedures are not possible, such as those involving drug-resistant generalized epilepsy or focal epilepsy overlapping with functional cortex, or in the setting of persistent seizures despite ictal focus resection (91). Rates of seizure freedom in adults with VNS are low (just 15–18%) (91,92), but one series showed a mean reduction in seizure frequency of nearly 58% in the pediatric population (91). The complications rate in this group was also low (6.1%), with only 3/141 patients developing an infection and only one of these requiring removal of the implanted device (91). Newer developments in heart rate–responsive stimulators may expand the utilization of VNS, although data in the pediatric population are extremely limited (93).
In 2010, the Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy (SANTE) investigators published the results of their DBS paradigm for epilepsy treatment (94). Their results showed that, at 2 years after implantation, 7.4% (6/81) of patients treated with DBS were seizure free, with a 56% median reduction in seizure frequency among the cohort. Patients with temporal lobe epilepsy received the highest degree of benefit. Although these outcomes are promising, the SANTE study excluded patients who were younger than 18 years of age. Thus, the lack of data with DBS and on anterior thalamic stimulation in children limits the applicability of these devices in the pediatric population at this time.
Responsive neurostimulation, a comparatively new set of devices that can detect electrocorticographic evidence of seizures and deliver targeted stimulation, are a third developing paradigm of stimulation therapy. As with the other techniques, however, this technique is limited by the paucity of data in the pediatric population, which, combined with the unique challenges associated with these devices (i.e., device size, lack of MRI compatibility, lack of standardized device settings), means further high-quality evidence is needed prior to widespread implementation (95).
Although stimulation remains primarily a palliative therapy, a minority of patients do achieve seizure freedom after these procedures, either independently of or in conjunction with other interventions. The evolution of neurostimulation as technology improves and our expanding knowledge of the mechanisms of epilepsy may provide significant advances in curative epilepsy treatment in the future.
Corpus callosotomy
Sectioning of the corpus callosum was first described in 1940 by van Wagenen and Herren (96), who developed the procedure after observing patients with brain tumors and epilepsy whose seizures remitted as the corpus callosum was infiltrated by the neoplasm. Since that time, refinements in surgical technique have reduced the initially steep morbidity and mortality of the procedure (97). As the major connective tract between the two cerebral hemispheres, the fibers of the corpus callosum play a major role in seizure generalization. In patients with severe, intractable epilepsy—especially atonic or drop seizures—who are not candidates for resective surgery, disconnection via transection of the corpus callosum is a viable option if they have persistent seizures despite undergoing lower morbidity palliative interventions such as VNS. Lennox-Gastaut syndrome (LGS)—an epileptic encephalopathy characterized by generalized slow spike wave activity on EEG, mental retardation, and multiple seizure types (98)—is one indication for callosotomy. Children with LGS frequently suffer from atonic drop attacks, which is the seizure type most responsive to callosotomy (99).
Callosotomy can be either partial or complete. In partial callosotomy, the anterior two thirds of the corpus callosum is sectioned with sparing of the splenium, while in complete callosotomy the entire structure is divided (100). Some studies suggest that a greater extent of resection is associated with improved seizure control (101), although others have not found this to be the case (102). Complete callosotomy is associated with a higher rate of complications, the most classic of which is a disconnection syndrome where communications between hemispheres are impaired, resulting in deficits such as the inability to name objects presented only to the nondominant visual field. Other complications include transient or permanent motor dysfunction, memory dysfunction, and language dysfunction. Mortality is reported, but rare (less than 2% in some reports) (100).
Patients rarely attain complete seizure freedom after corpus callosotomy. Complete seizure freedom rates range from 6 to 19%, with lower rates with longer-term follow-up (99,103,104). Rates of significant seizure improvement after corpus callosotomy are generally much higher, in keeping with its standard utilization as a palliative procedure (101).
Minimally invasive epilepsy treatment
Minimally invasive treatments for epilepsy have undergone significant development, with advances in both surgical technologies and imaging. Two of the most commonly utilized modalities are stereotactic radiosurgery and laser ablation therapy.
Stereotactic radiosurgery for the treatment of epilepsy has been the topic of investigation since the 1980s (105). Despite this, the precise mechanism through which radiation eliminates seizures remains unclear. Although injury to tissues at the site of treatment likely plays a role, studies have shown that actual necrotic changes are not necessarily seen at the treatment target (106). As with other treatment modalities, the challenge of radiosurgery is complete identification and treatment of the epileptogenic zone, which is further complicated by the fact that radiation dosing and drop off must be taken into account.
Magnetic resonance–guided laser-induced thermotherapy (MRgLITT), also known as laser ablation, is an emerging therapy that offers another option to achieve seizure freedom. In MRgLITT, thermal energy is delivered through a minimally invasive catheter under real-time MR thermography to the lesion. Although data in the pediatric population remain scarce (56), the efficacy and safety profile of the procedure is robust enough that multiple systems have received FDA approval. These procedures are especially useful in the treatment of deep lesions such as hypothalamic hamartomas, which frequently result in drug-resistant gelastic seizures and, because of their location, were previously difficult to access via other methods (107).
The major benefit of minimally invasive procedures is the reduction in the morbidity associated with open surgery. These techniques themselves, however, have unique complication profiles. With regard to radiosurgery, the possibility for significant post-treatment edema and radiation necrosis exists (108). The benefits of radiosurgery may also be delayed, and the interval between treatment and response can lead to continued seizure-related functional decline (109). Laser ablation can also lead to damage to surrounding structures and subsequent neurologic sequelae (110).
Outcomes following these interventions have generally been favorable. A prospective trial of radiosurgery for mesial temporal lobe epilepsy demonstrated seizure freedom in 67% of patients for a year at 36-month follow-up (111). Limited data exist for laser ablation, and long-term results are sorely lacking, but cure rates in early studies are also in the 60–70% range (56).
Conclusions
Epilepsy is a highly prevalent condition, impacting approximately 1% of the population (4). Among affected patients, as many as one third may have epilepsy that is drug resistant, leading to a massive burden on the healthcare system and on the individual patient and his or her family. Despite the high-quality evidence supporting the effectiveness of epilepsy surgery as a cure for this devastating condition, surgical intervention remains underutilized (8). Dissemination of knowledge regarding the range of surgical options along with interdisciplinary cooperation and continued innovation in the field of curative epilepsy surgery will be essential to maximizing our ability to treat patients suffering from this debilitating disease.
Acknowledgements
We thank Kristin Kraus, MSc, for editorial assistance with this paper.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wirrell EC, Grossardt BR, Wong-Kisiel LC, et al. Incidence and classification of new-onset epilepsy and epilepsy syndromes in children in Olmsted County, Minnesota from 1980 to 2004: a population-based study. Epilepsy Res 2011;95:110-8. [Crossref] [PubMed]
- Oldham MS, Horn PS, Tsevat J, et al. Costs and clinical outcomes of epilepsy surgery in children with drug-resistant epilepsy. Pediatr Neurol 2015;53:216-20. [Crossref] [PubMed]
- Joshi SM, Singh RK, Shellhaas RA. Advanced treatments for childhood epilepsy: beyond antiseizure medications. JAMA Pediatr 2013;167:76-83. [Crossref] [PubMed]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314-9. [Crossref] [PubMed]
- Ramey WL, Martirosyan NL, Lieu CM, et al. Current management and surgical outcomes of medically intractable epilepsy. Clin Neurol Neurosurg 2013;115:2411-8. [Crossref] [PubMed]
- Duchowny MS. Surgery for intractable epilepsy: issues and outcome. Pediatrics 1989;84:886-94. [PubMed]
- Perry MS, Duchowny M. Surgical management of intractable childhood epilepsy: curative and palliative procedures. Semin Pediatr Neurol 2011;18:195-202. [Crossref] [PubMed]
- Berg AT, Zelko FA, Levy SR, et al. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology 2012;79:1384-91. [Crossref] [PubMed]
- Cohen-Gadol AA, Wilhelmi BG, Collignon F, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg 2006;104:513-24. [Crossref] [PubMed]
- Englot DJ, Han SJ, Rolston JD, et al. Epilepsy surgery failure in children: a quantitative and qualitative analysis. J Neurosurg Pediatr 2014;14:386-95. [Crossref] [PubMed]
- Dorfmüller G, Ferrand-Sorbets S, Fohlen M, et al. Outcome of surgery in children with focal cortical dysplasia younger than 5 years explored by stereo-electroencephalography. Childs Nerv Syst 2014;30:1875-83. [Crossref] [PubMed]
- Bilginer B, Yalnizoglu D, Soylemezoglu F, et al. Surgery for epilepsy in children with dysembryoplastic neuroepithelial tumor: clinical spectrum, seizure outcome, neuroradiology, and pathology. Childs Nerv Syst 2009;25:485-91. [Crossref] [PubMed]
- Giulioni M, Rubboli G, Marucci G, et al. Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: lesionectomy compared with tailored resection. J Neurosurg 2009;111:1275-82. [Crossref] [PubMed]
- Obeid M, Wyllie E, Rahi AC, et al. Approach to pediatric epilepsy surgery: State of the art, Part I: General principles and presurgical workup. Eur J Paediatr Neurol 2009;13:102-14. [Crossref] [PubMed]
- McKhann GM 2nd, Bourgeois BF, Goodman RR. Epilepsy surgery: indications, approaches, and results. Semin Neurol 2002;22:269-78. [Crossref] [PubMed]
- Tufenkjian K, Luders HO. Seizure semiology: its value and limitations in localizing the epileptogenic zone. J Clin Neurol 2012;8:243-50. [Crossref] [PubMed]
- Cross JH, Jayakar P, Nordli D, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia 2006;47:952-9. [Crossref] [PubMed]
- Brockhaus A, Elger CE. Complex partial seizures of temporal lobe origin in children of different age groups. Epilepsia 1995;36:1173-81. [Crossref] [PubMed]
- Vossler DG, Wilkusa RJ, Ojemanna GA, et al. Preoperative EEG correlates of seizure outcome from epilepsy surgery in children. J Epilepsy 1995;8:236-45. [Crossref]
- Englot DJ, Rolston JD, Wang DD, et al. Seizure outcomes after temporal lobectomy in pediatric patients. J Neurosurg Pediatr 2013;12:134-41. [Crossref] [PubMed]
- Pondal-Sordo M, Diosy D, Tellez-Zenteno JF, et al. Usefulness of intracranial EEG in the decision process for epilepsy surgery. Epilepsy Res 2007;74:176-82. [Crossref] [PubMed]
- Knake S, Triantafyllou C, Wald LL, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology 2005;65:1026-31. [Crossref] [PubMed]
- Englot DJ, Breshears JD, Sun PP, et al. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr 2013;12:126-33. [Crossref] [PubMed]
- Wang ZI, Jones SE, Jaisani Z, et al. Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann Neurol 2015;77:1060-75. [Crossref] [PubMed]
- Moeller F, Stephani U, Siniatchkin M. Simultaneous EEG and fMRI recordings (EEG-fMRI) in children with epilepsy. Epilepsia 2013;54:971-82. [Crossref] [PubMed]
- Riney CJ, Chong WK, Clark CA, et al. Voxel based morphometry of FLAIR MRI in children with intractable focal epilepsy: implications for surgical intervention. Eur J Radiol 2012;81:1299-305. [Crossref] [PubMed]
- Rheims S, Jung J, Ryvlin P. Combination of PET and magnetoencephalography in the presurgical assessment of MRI-negative epilepsy. Front Neurol 2013;4:188. [Crossref] [PubMed]
- Kumar A, Juhasz C, Asano E, et al. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med 2010;51:1901-7. [Crossref] [PubMed]
- Perissinotti A, Setoain X, Aparicio J, et al. Clinical role of subtraction ictal SPECT coregistered to MR imaging and (18)F-FDG PET in pediatric epilepsy. J Nucl Med 2014;55:1099-105. [Crossref] [PubMed]
- Burkholder DB, Sulc V, Hoffman EM, et al. Interictal scalp electroencephalography and intraoperative electrocorticography in magnetic resonance imaging-negative temporal lobe epilepsy surgery. JAMA Neurol 2014;71:702-9. [Crossref] [PubMed]
- Jayakar P, Dunoyer C, Dean P, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia 2008;49:758-64. [Crossref] [PubMed]
- Alomar S, Jones J, Maldonado A, et al. The stereo-electroencephalography methodology. Neurosurg Clin N Am 2016;27:83-95. [Crossref] [PubMed]
- Cossu M, Schiariti M, Francione S, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy in infancy and early childhood. J Neurosurg Pediatr 2012;9:290-300. [Crossref] [PubMed]
- Asadi-Pooya AA, Sperling MR. Age at onset in patients with medically refractory temporal lobe epilepsy and mesial temporal sclerosis: impact on clinical manifestations and postsurgical outcome. Seizure 2015;30:42-5. [Crossref] [PubMed]
- Cataltepe O, Turanli G, Yalnizoglu D, et al. Surgical management of temporal lobe tumor-related epilepsy in children. J Neurosurg 2005;102:280-7. [PubMed]
- Marin-Valencia I, Guerrini R, Gleeson JG. Pathogenetic mechanisms of focal cortical dysplasia. Epilepsia 2014;55:970-8. [Crossref] [PubMed]
- Hindi-Ling H, Kipervasser S, Neufeld MY, et al. Epilepsy surgery in children compared to adults. Pediatr Neurosurg 2011;47:180-5. [Crossref] [PubMed]
- Schaller K, Cabrilo I. Anterior temporal lobectomy. Acta Neurochir (Wien) 2016;158:161-6. [Crossref] [PubMed]
- Sinclair DB, Aronyk K, Snyder T, et al. Pediatric temporal lobectomy for epilepsy. Pediatr Neurosurg 2003;38:195-205. [Crossref] [PubMed]
- Spencer DD, Spencer SS, Mattson RH, et al. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery 1984;15:667-71. [Crossref] [PubMed]
- Joo EY, Han HJ, Lee EK, et al. Resection extent versus postoperative outcomes of seizure and memory in mesial temporal lobe epilepsy. Seizure 2005;14:541-51. [Crossref] [PubMed]
- Georgiadis I, Kapsalaki EZ, Fountas KN. Temporal lobe resective surgery for medically intractable epilepsy: a review of complications and side effects. Epilepsy Res Treat 2013;2013:752195.
- Adelson PD. Temporal lobectomy in children with intractable seizures. Pediatr Neurosurg 2001;34:268-77. [Crossref] [PubMed]
- Li LM, Cendes F, Andermann F, et al. Surgical outcome in patients with epilepsy and dual pathology. Brain 1999;122:799-805. [Crossref] [PubMed]
- Benifla M, Otsubo H, Ochi A, et al. Temporal lobe surgery for intractable epilepsy in children: an analysis of outcomes in 126 children. Neurosurgery 2006;59:1203-13; discussion 13-4. [Crossref] [PubMed]
- Mansouri A, Fallah A, McAndrews MP, et al. Neurocognitive and seizure outcomes of selective amygdalohippocampectomy versus anterior temporal lobectomy for mesial temporal lobe epilepsy. Epilepsy Res Treat 2014;2014:306382.
- Boucher O, Dagenais E, Bouthillier A, et al. Different effects of anterior temporal lobectomy and selective amygdalohippocampectomy on verbal memory performance of patients with epilepsy. Epilepsy Behav 2015;52:230-5. [Crossref] [PubMed]
- Suriadi MM, Usui K, Tottori T, et al. Preservation of absolute pitch after right amygdalohippocampectomy for a pianist with TLE. Epilepsy Behav 2015;42:14-7. [Crossref] [PubMed]
- Wheatley BM. Selective amygdalohippocampectomy: the trans-middle temporal gyrus approach. Neurosurg Focus 2008;25:E4. [Crossref] [PubMed]
- Adada B. Selective amygdalohippocampectomy via the transsylvian approach. Neurosurg Focus 2008;25:E5. [Crossref] [PubMed]
- Sajko T, Skoro I, Rotim K. How I do it - selective amygdalohippocampectomy via subtemporal approach. Acta Neurochir (Wien) 2013;155:2381-7. [Crossref] [PubMed]
- Hu WH, Zhang C, Zhang K, et al. Selective amygdalohippocampectomy versus anterior temporal lobectomy in the management of mesial temporal lobe epilepsy: a meta-analysis of comparative studies. J Neurosurg 2013;119:1089-97. [Crossref] [PubMed]
- Josephson CB, Dykeman J, Fiest KM, et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology 2013;80:1669-76. [Crossref] [PubMed]
- Datta A, Sinclair DB, Wheatley M, et al. Selective amygdalohippocampectomy: surgical outcome in children versus adults. Can J Neurol Sci 2009;36:187-91. [PubMed]
- Robinson S, Park TS, Blackburn LB, et al. Transparahippocampal selective amygdalohippocampectomy in children and adolescents: efficacy of the procedure and cognitive morbidity in patients. J Neurosurg 2000;93:402-9. [Crossref] [PubMed]
- Buckley R, Estronza-Ojeda S, Ojemann JG. Laser ablation in pediatric epilepsy. Neurosurg Clin N Am 2016;27:69-78. [Crossref] [PubMed]
- Mittal S, Montes JL, Farmer JP, et al. Long-term outcome after surgical treatment of temporal lobe epilepsy in children. J Neurosurg 2005;103:401-12. [PubMed]
- Kan P, Van Orman C, Kestle JR. Outcomes after surgery for focal epilepsy in children. Childs Nerv Syst 2008;24:587-91. [Crossref] [PubMed]
- Harvey AS, Cross JH, Shinnar S, et al. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008;49:146-55. [Crossref] [PubMed]
- Aronica E, Crino PB. Epilepsy related to developmental tumors and malformations of cortical development. Neurotherapeutics 2014;11:251-68. [Crossref] [PubMed]
- Khajavi K, Comair YG, Wyllie E, et al. Surgical management of pediatric tumor-associated epilepsy. J Child Neurol 1999;14:15-25. [Crossref] [PubMed]
- Jooma R, Yeh HS, Privitera MD, et al. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg 1995;83:231-6. [Crossref] [PubMed]
- Hamiwka L, Jayakar P, Resnick T, et al. Surgery for epilepsy due to cortical malformations: ten-year follow-up. Epilepsia 2005;46:556-60. [Crossref] [PubMed]
- Choi JY, Chang JW, Park YG, et al. A retrospective study of the clinical outcomes and significant variables in the surgical treatment of temporal lobe tumor associated with intractable seizures. Stereotact Funct Neurosurg 2004;82:35-42. [Crossref] [PubMed]
- Clusmann H, Kral T, Fackeldey E, et al. Lesional mesial temporal lobe epilepsy and limited resections: prognostic factors and outcome. J Neurol Neurosurg Psychiatry 2004;75:1589-96. [Crossref] [PubMed]
- Fallah A, Weil AG, Sur S, et al. Epilepsy surgery related to pediatric brain tumors: Miami Children's Hospital experience. J Neurosurg Pediatr 2015;16:675-80. [Crossref] [PubMed]
- Ranger A, Diosy D. Seizures in children with dysembryoplastic neuroepithelial tumors of the brain--A review of surgical outcomes across several studies. Childs Nerv Syst 2015;31:847-55. [Crossref] [PubMed]
- Moran NF, Fish DR, Kitchen N, et al. Supratentorial cavernous haemangiomas and epilepsy: a review of the literature and case series. J Neurol Neurosurg Psychiatry 1999;66:561-8. [Crossref] [PubMed]
- Hoh BL, Chapman PH, Loeffler JS, et al. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizure incidence and seizure outcomes. Neurosurgery 2002;51:303-9; discussion 309-11. [PubMed]
- von der Brelie C, Kuczaty S, von Lehe M. Surgical management and long-term outcome of pediatric patients with different subtypes of epilepsy associated with cerebral cavernous malformations. J Neurosurg Pediatr 2014;13:699-705. [Crossref] [PubMed]
- Baumann CR, Schuknecht B, Lo Russo G, et al. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia 2006;47:563-6. [Crossref] [PubMed]
- Moosa AN, Wyllie E. Focal epileptogenic lesions. Handb Clin Neurol. 2013;111:493-510. [Crossref] [PubMed]
- Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311-8. [Crossref] [PubMed]
- Liava A, Francione S, Tassi L, et al. Individually tailored extratemporal epilepsy surgery in children: anatomo-electro-clinical features and outcome predictors in a population of 53 cases. Epilepsy Behav 2012;25:68-80. [Crossref] [PubMed]
- Anderer E, Bollo R, Weiner H. Extratemporal resection. In: Çataltepe O, Jallo G, editors. Pediatric Epilepsy Surgery: Preoperative Assessment and Surgical Treatment. New York: Thieme;2010:174-84.
- Cossu M, Lo Russo G, Francione S, et al. Epilepsy surgery in children: results and predictors of outcome on seizures. Epilepsia 2008;49:65-72. [Crossref] [PubMed]
- Hong SJ, Kim H, Schrader D, et al. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology 2014;83:48-55. [Crossref] [PubMed]
- Roth J, Carlson C, Devinsky O, et al. Safety of staged epilepsy surgery in children. Neurosurgery 2014;74:154-62. [Crossref] [PubMed]
- Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia. Preliminary report. JAMA 1928;90:823–5. JAMA 1928;90:823-5. [Crossref]
- Schramm J, Behrens E, Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery 1995;36:509-15; discussion 515-16. [Crossref] [PubMed]
- Pinto AL, Lohani S, Bergin AM, et al. Surgery for intractable epilepsy due to unilateral brain disease: a retrospective study comparing hemispherectomy techniques. Pediatr Neurol 2014;51:336-43. [Crossref] [PubMed]
- Marras CE, Granata T, Franzini A, et al. Hemispherotomy and functional hemispherectomy: indications and outcome. Epilepsy Res 2010;89:104-12. [Crossref] [PubMed]
- Granata T, Matricardi S, Ragona F, et al. Hemispherotomy in Rasmussen encephalitis: long-term outcome in an Italian series of 16 patients. Epilepsy Res 2014;108:1106-19. [Crossref] [PubMed]
- Dorfer C, Ochi A, Snead OC 3rd, et al. Functional hemispherectomy for catastrophic epilepsy in very young infants: technical considerations and complication avoidance. Childs Nerv Syst 2015;31:2103-9. [Crossref] [PubMed]
- Kestle J, Connolly M, Cochrane D. Pediatric peri-insular hemispherotomy. Pediatr Neurosurg 2000;32:44-7. [Crossref] [PubMed]
- Lew SM, Matthews AE, Hartman AL, et al. Posthemispherectomy hydrocephalus: results of a comprehensive, multiinstitutional review. Epilepsia 2013;54:383-9. [Crossref] [PubMed]
- Griessenauer CJ, Salam S, Hendrix P, et al. Hemispherectomy for treatment of refractory epilepsy in the pediatric age group: a systematic review. J Neurosurg Pediatr 2015;15:34-44. [Crossref] [PubMed]
- Hu WH, Zhang C, Zhang K, et al. Hemispheric surgery for refractory epilepsy: a systematic review and meta-analysis with emphasis on seizure predictors and outcomes. J Neurosurg 2016;124:952-61. [Crossref] [PubMed]
- Klinkenberg S, van den Borne CJ, Aalbers MW, et al. The effects of vagus nerve stimulation on tryptophan metabolites in children with intractable epilepsy. Epilepsy Behav 2014;37:133-8. [Crossref] [PubMed]
- Krahl SE, Clark KB. Vagus nerve stimulation for epilepsy: A review of central mechanisms. Surg Neurol Int 2012;3:S255-9. [Crossref] [PubMed]
- Elliott RE, Rodgers SD, Bassani L, et al. Vagus nerve stimulation for children with treatment-resistant epilepsy: a consecutive series of 141 cases. J Neurosurg Pediatr 2011;7:491-500. [PubMed]
- Elliott RE, Carlson C, Kalhorn SP, et al. Refractory epilepsy in tuberous sclerosis: vagus nerve stimulation with or without subsequent resective surgery. Epilepsy Behav 2009;16:454-60. [Crossref] [PubMed]
- Boon P, Vonck K, van Rijckevorsel K, et al. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure 2015;32:52-61. [Crossref] [PubMed]
- Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010;51:899-908. [Crossref] [PubMed]
- Cox JH, Seri S, Cavanna AE. Clinical utility of implantable neurostimulation devices as adjunctive treatment of uncontrolled seizures. Neuropsychiatr Dis Treat 2014;10:2191-200. [PubMed]
- Van Wagenen VP, Herren RY. Surgical division of commissural pathways in the corpus callosum relation to spread of an epileptic attack. Arch NeurPsych 1940;44:740-59. [Crossref]
- Pendl G, Eder HG, Schroettner O, et al. Corpus callosotomy with radiosurgery. Neurosurgery 1999;45:303-7; discussion 307-8. [Crossref] [PubMed]
- Markand ON. Lennox-Gastaut syndrome (childhood epileptic encephalopathy). J Clin Neurophysiol 2003;20:426-41. [Crossref] [PubMed]
- Stigsdotter-Broman L, Olsson I, Flink R, et al. Long-term follow-up after callosotomy--a prospective, population based, observational study. Epilepsia 2014;55:316-21. [Crossref] [PubMed]
- Asadi-Pooya AA, Sharan A, Nei M, et al. Corpus callosotomy. Epilepsy Behav 2008;13:271-8. [Crossref] [PubMed]
- Kasasbeh AS, Smyth MD, Steger-May K, et al. Outcomes after anterior or complete corpus callosotomy in children. Neurosurgery 2014;74:17-28. [Crossref] [PubMed]
- Sorenson JM, Wheless JW, Baumgartner JE, et al. Corpus callosotomy for medically intractable seizures. Pediatr Neurosurg 1997;27:260-7. [Crossref] [PubMed]
- Fuiks KS, Wyler AR, Hermann BP, et al. Seizure outcome from anterior and complete corpus callosotomy. J Neurosurg 1991;74:573-8. [Crossref] [PubMed]
- Kwan SY, Wong TT, Chang KP, et al. Seizure outcome after corpus callosotomy: the Taiwan experience. Childs Nerv Syst 2000;16:87-92. [Crossref] [PubMed]
- Elomaa E. Focal irradiation of the brain: an alternative to temporal lobe resection in intractable focal epilepsy? Med Hypotheses 1980;6:501-3. [Crossref] [PubMed]
- Régis J, Kerkerian-Legoff L, Rey M, et al. First biochemical evidence of differential functional effects following Gamma Knife surgery. Stereotact Funct Neurosurg 1996;66 Suppl 1:29-38. [Crossref] [PubMed]
- Wilfong AA, Curry DJ. Hypothalamic hamartomas: optimal approach to clinical evaluation and diagnosis. Epilepsia 2013;54 Suppl 9:109-14. [Crossref] [PubMed]
- Chen N, Du SQ, Yan N, et al. Delayed complications after Gamma Knife surgery for intractable epilepsy. J Clin Neurosci 2014;21:1525-8. [Crossref] [PubMed]
- Kawamura T, Onishi H, Kohda Y, et al. Serious adverse effects of gamma knife radiosurgery for mesial temporal lobe epilepsy. Neurol Med Chir (Tokyo) 2012;52:892-8. [Crossref] [PubMed]
- Hawasli AH, Bagade S, Shimony JS, et al. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery 2013;73:1007-17. [Crossref] [PubMed]
- Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol 2009;65:167-75. [Crossref] [PubMed]


