Editor’s note:
Cancer has been called a metabolic disease for some time. To address the significance of metabolic alterations in cancer, this column on “Metabolism of Childhood Cancer” is launched with Dr. Dinesh Rakheja (Departments of Pathology and Pediatrics, University of Texas Southwestern Medical Center) serving as the column editor. More featured articles are expected to be published in the column.
Liver tumors in children with metabolic disorders
Introduction
Hepatic involvement in metabolic diseases is a natural corollary to the central and pivotal role of the liver in metabolism. The majority of childhood metabolic liver diseases are classical Mendelian single gene disorders affecting key enzymes and proteins in diverse metabolic pathways (1,2). Other disorders such as the metabolic syndrome and its hepatic manifestation, non-alcoholic fatty liver disease (NAFLD), are multifactorial (genetic dyslipidemias, insulin resistance, diet) (3-5) and increasingly prevalent in the pediatric age group in association with childhood obesity (6). Yet others (e.g., idiopathic copper toxicosis, Indian childhood cirrhosis) are postulated to be multifactorial, with contributions from as yet unresolved genetic and environmental risk factors (7-9).
Metabolic liver disease is often studied within the paradigm of ‘toxic metabolite’ accumulation and chronic injury. In this model of pathogenesis, metabolic disorders initially present with characteristic histologic and ultrastructural patterns on liver biopsy (10,11), but chronic injury over months or years leads to cirrhosis and/or hepatic neoplasia. Prototypes of such a model include the endoplasmic reticulum (ER) retention of α-1 antitrypsin (AAT) in AAT-deficiency, increased levels of succinylacetone (SA) in hereditary tyrosinemia type 1 (HT1), and increased glycogen in glycogen storage disorders (GSD). Accordingly, the most frequently diagnosed hepatic neoplasm in children with inherited metabolic disorders is hepatocellular carcinoma (HCC) (10,12), a tumor otherwise rare in the pediatric population (13) but the commonest to arise in a cirrhotic liver. Cholangiocarcinoma (CC), combined cholangio-HCC, hepatic adenoma (HA), and focal nodular hyperplasia are less frequently encountered (Table 1). In contrast, hepatoblastoma, the most common pediatric liver malignancy, is not usually associated with cirrhosis or metabolic disease but with other heritable defects (10,12).
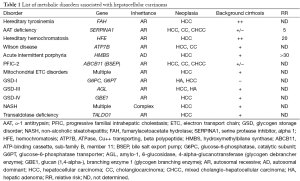
Full table
A few counterpoints deserve special mention. First, cirrhosis is not a prerequisite for developing HCC in metabolic disorders, the most prominent example being malignant transformation of HA in type I glycogenosis (14). De novo HCC in AAT deficiency (15) and hereditary hemochromatosis (HH) (16) have also been reported. Second, even in the presence of cirrhosis, HCC and other cancers develop in only a minority of patients with metabolic disease, underscoring the crucial role played by environmental and other genetic modifiers in tumorigenesis. Third, modifiers must also be involved in the latency of progression to cirrhosis and/or HCC. Even though the molecular defects are present since conception in AAT deficiency, HH, Wilson disease (WD), and acute hepatic porphyrias, tumors rarely occur before adulthood (1). In contrast, cirrhosis and hepatic carcinogenesis in untreated hereditary tyrosinemia occurs in early childhood (17).
A comprehensive review of all aspects of metabolic diseases is beyond the scope of this chapter and the reader is referred to several other exhaustive references (1,2,10). This chapter focuses on hepatic neoplasia as a complication of metabolic liver disease in the pediatric population with emphasis on recent advances in the molecular mechanisms of tumorigenesis in tyrosinemia, AAT deficiency, and GSD (see Table 1 for a list of disorders). Hepatic tumors not associated with metabolic disease (e.g., hepatoblastoma, mesenchymal hamartoma) (12) or arising in developmental disorders (e.g., Alagille syndrome, Caroli disease) (18,19) are therefore not included. Also excluded are the cancer predisposition syndromes (Fanconi anemia, ataxia telangiectasia, familial adenomatous polyposis, Li-Fraumeni syndrome, and Beckwith-Wiedemann syndrome) in which hepatic (and extrahepatic) tumorigenesis is driven by germline mutations and epimutations activating oncogenic pathways (20,21).
Hereditary tyrosinemia type 1 (HT1) (OMIM 276700)
HT1 is an autosomal recessive (AR) metabolic disorder caused by deficiency of fumarylacetoacetate hydrolase (FAH), the last enzyme of tyrosine degradation. FAH is present within the liver as well as in the kidney, lymphocytes, erythrocytes, fibroblasts, and chorionic villi (22). The disease is quite common in Quebec (1 in 16,786 live births) (17), and is especially prevalent in the French Canadian population of Saguenay-Lac-St-Jean Quebec due to a complex founder effect (23). Worldwide more than 40 mutations have been reported in the FAH gene, but the IVS12 + 5G > A allele is responsible for more than 90% of mutant alleles in Saguenay-Lac-St-Jean Quebec. The mutational spectrum in FAH in different ethnic groups has been reviewed elsewhere (22). An elevated SA level in serum or urine is confirmatory for the diagnosis (17).
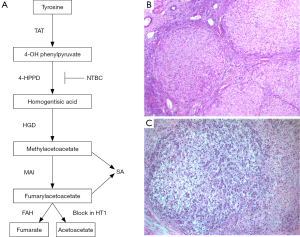
Prior to 1992, when Lindstedt et al. (24) published the first report of successful treatment of tyrosinemic children with 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3cyclohexanedione (NTBC or nitisinone), two major clinical presentations of HT1 were recognized: hepatic crises and neurologic crises. The hepatic forms consist of essentially two subsets including an acute form of hepatic decompensation with hepatomegaly, ascites, anasarca, and marked coagulopathy with greater than 80% affected children dying before the age of 2 years, and a chronic form consisting of chronic liver disease, renal tubular dysfunction, and hypophosphatemia with rickets (25). The neurologic crises (resulting from δ-aminolevulinic acid toxicity) consist of painful paresthesias and autonomic signs similar to those seen in acute porphyria (17). Liver biopsy of the acute stage typically shows a hepatitic pattern with portal and lobular necroinflammatory foci, varying degrees of steatosis, pseudoacinar formation with bile plugs, iron accumulation within Kupffer cells and zone 1 hepatocytes and occasional giant cell transformation (10). There may be micro-nodular cirrhosis with associated bile duct proliferation. Patients may undergo multiple acute crises during their lifetime. The chronic hepatic phase usually shows mixed micro- and macro-nodular cirrhosis with minimal ductular proliferation and mild lymphoplasmacytic infiltrates within the fibrous septa. Varying degrees of steatosis are also seen and may show variation within a nodule and/or between nodules. The most significant feature is the foci of dysplasia (large and small cell types) and/or HCC (26) (Figure 1).
In cirrhotic livers of individuals with HT1, a remarkable phenomenon of genetic ‘reversion’ has been reported (27,28), in which rare discrete regenerative nodules are found to be immunoreactive for FAH protein interspersed with the vast majority of FAH-negative nodules. DNA analyses of these ‘revertant nodules’ have shown heterozygous correction of the FAH gene sequence (25,28). These nodules are often large (macro-nodules) with no dysplasia or proliferating cell nuclear antigen staining observed and no steatosis is usually seen. The extent of replacement of the liver by these “revertant nodules” is inversely proportionate to the clinical severity, and it is reported that the incidence of hepatic dysplasia is lower in the chronic liver group (50%) than in the acute liver group (100%) and the average surface of reversion in the chronic group was much higher (36%) than in the acute group (1.6%) (25). More importantly, HCC was found to develop only within non-revertant nodules (25).
The risk of developing HCC in HT1 is considered the highest among all metabolic disorders (29). Earlier reports of a 37% incidence of HCC (26) may have been overestimated (17), but current estimates of a 13-17% risk (17,22,30) is still extremely high. In contrast to other metabolic disorders, tyrosinemic individuals are also at risk of developing HCC earlier, often before the age of 5 years (17,31), and as early as 1 year (32) (see below). Typically dysplastic changes within regenerative nodules with or without discrete foci of HCC (Figure 1) are evident. A recent retrospective study of 16 patients with liver transplantation for HT1 revealed cirrhotic nodules in all patients and well-differentiated HCC in 12 of 16 patients (33). Such findings promote regular screening for serum alphafetoprotein (AFP) elevation and ultrasonography and intervention via orthotopic liver transplantation in affected children. AFP levels do not reliably predict the development of HCC and may be markedly elevated following acute liver crises and decrease over time. HCC may also occur with normal or low AFP levels (22). The introduction of nitisinone has dramatically altered the non-surgical management and the short and medium-term outlook in these patients (see below), such that the indication for liver transplantation is now restricted to non-responders to NTBC, patients not treated with NTBC, or for patients with HCC (33).
Over 300 patients have now been enrolled in the International NTBC study (32) and the incidence of acute HT1 (liver failure, neurologic crises) has reduced to 10%, a remarkable outcome with very few adverse effects (22,32). Given that the vast majority of patients in the pre-NTBC era succumbed to acute symptoms, these results are hugely promising. Even more striking results were reported in a recent study from Quebec, where HT1 screening is included in the universal newborn screening program and NTBC treatment was initiated in a cohort of patients prior to 1 month of age before development of clinical symptoms. Compared to untreated HT1 patients (n=28), who had 184 hospitalizations during 1,312 months, no hospitalizations for acute complications of HT1 were reported in 50 patients who received NTBC therapy during 5,731 months of treatment (34). Furthermore, none of the 24 patients who received NTBC prior to 1 month of age developed any detectable liver disease or underwent liver transplantation at 5 years of follow-up; whereas 7 of 26 patients treated after 1 month of age and 20 of 28 untreated patients underwent liver transplantation, suggesting early institution of therapy prior to liver damage has a significant impact on the acute hepatic complications of HT1 (34).
NTBC has also been reported to decrease the short- and medium-term risk of HCC development when patients are started on therapy < age 2 years (32,35). In the smaller Quebec trial, only 1 of 35 patients (2.86%) on NTBC was transplanted for suspected cirrhosis and had hepatocellular dysplasia at 51 months of follow-up (22). In an interim report on the international NTBC study by Holme and Lindstedt (32), only 1 out of 80 patients (1.25%) placed on therapy < age 2 years was identified with proven HCC at 5-7 years follow-up. In a longer-term follow-up of French patients, none of 41 patients started on NTBC < age 2 years developed HCC (36). While these figures are encouraging, other statistics should give us pause; HCC developed in 8 out of 60 patients (13.3%) in the international study (32), and in 2 out of 5 patients in the French study (36) when NTBC treatment was started after 2 years of age. Furthermore, HCC has been reported in patients as late as 6 years after starting NTBC therapy (37) and 10 years (38) suggesting that the long-term risk assessment of HCC in patients on NTBC awaits further studies.
The molecular mechanisms of hepatic carcinogenesis in HT1 have been the focus of many studies. NTBC is a potent inhibitor of 4-hydroxyphenylpyruvate dioxygenase (4-HPPD) (39), an upstream enzyme in the tyrosine catabolism pathway (Figure 1). Several lines of evidence point toward a major role of fumarylacetoacetate (FAA), maleylacetoacetate (MAA), and SA (metabolites upstream of the enzymatic block in tyrosine degradation) in the development of HCC. First, NTBC treatment targets the 4-HPPD enzyme upstream of FAH (Figure 1) leading to a marked decrease in plasma SA levels in responsive patients (24). Second, while Fah knockout (Fah−/−) mice on NTBC may develop HCC by 10 months (40), Fah−/−Hpd −/− double knockout mice are protected from hepatic carcinogenesis and dysplastic lesions even at 18 months of age (41). Third, and perhaps most telling, is the observation that HCC develops only in FAH-negative nodules (see above) in livers with spontaneous reversion (25). Fourth, FAA has been shown to be a mutagen in cultured cell lines (42) inducing mitotic abnormalities and genomic instability and causing oxidative damage by reacting with glutathione and sulfhydryl groups (43). Due to the central role of Nrf2 (Nfe2l2) in cellular protection against oxidative stress, a Fah/Nrf2 double knockout mouse model showed increased mortality from acute HT1 as well as accelerated development of HCC, suggesting FAA induced oxidative damage as a key mechanism of carcinogenesis in HT1 (44). Fifth, SA may have a direct inhibitory effect on DNA repair mechanisms (45). Finally, the activation of the AKT (serine/threonine kainase) survival signaling pathway and inhibition of apoptosis in Fah−/− mice with discontinuation of NTBC may provide a mechanistic basis for the carcinogenesis in HT1 (46).
Alpha-1 antitrypsin (AAT) deficiency (OMIM 613490)
AAT deficiency is inherited in an AR manner and is caused by mutations in the serine protease inhibitor, alpha 1 (SERPINA1) gene on chromosome 14. It primarily affects the lungs and the liver and is a relatively common condition affecting 1:1,500 to 1:3,500 live births. AAT deficiency liver disease is the most common inherited cause of liver disease and the most frequent genetic disease leading to liver transplantation in childhood. AAT is a glycoprotein synthesized and secreted by the liver and its principal physiologic role is to inhibit neutrophil elastase. While lung disease in AAT deficiency is the result of decreased levels of circulating AAT, liver disease (the focus of this section) is caused by toxicity due to the retention of the mutant protein within the ER of hepatocytes. Abnormal migration of mutant AAT proteins on serum isoelectric focusing constitutes the confirmatory test for diagnosing the disease.
The most common AAT variant associated with clinical liver disease [protease inhibitor Z (PiZZ)] is caused by a missense mutation (Glu342Lys) that produces a conformationally altered unstable protein prone to homomeric aggregation (protein polymers). Such aggregates [referred to as alpha-1 antitrypsin Z (ATZ)] are retained within the ER (hence the deficiency in the plasma) and may be identified on routine histological sections with periodic acid schiff (PAS) stains after diastase digestion (PASD). These lead to intracellular toxicity and cellular injury (mitochondrial dysfunction, autophagy, and caspase activation), which activates the nuclear factor-kappa β (NF-κB) pathway and is believed to underlie the molecular pathogenesis of liver disease. Perhaps the most interesting insight into the mechanisms of liver disease has come from the identification of ‘autophagy’ as a major pathway for disposal of insoluble ATZ polymers, and that a specific autophagy-enhancing drug, carbamazepine, can dramatically decrease the ATZ load in mouse models of AAT (47). Interestingly, even oral carbamazepine administered to PiZ mice was able to both decrease hepatic ATZ load as well as reverse hepatic fibrosis, a remarkable finding with obvious clinical implications that are now the basis for an ongoing phase II/III clinical trial to evaluate the efficacy of carbamazepine in AAT liver disease (48). These pre-clinical results have been further substantiated by screening drug libraries using a C. elegans model of AAT deficiency, in which several compounds, including phenothiazines, that dramatically reduced ATZ accumulation were found to be autophagy-enhancers (49,50), pointing to a decidedly critical role for this cellular pathway in influencing the severity of AAT liver disease.
Liver disease is primarily seen with homozygous PiZZ AAT deficiency. Long term follow-up studies of PiZZ newborns in Scandinavian populations indicate only 10-15% develop any biochemical evidence (without clinical signs) of liver dysfunction at 25 years age, suggesting AAT-deficiency liver disease to be a low penetrance disorder dependent on additional host or environmental risk factors. In this study it was also shown that the overall risk of life-threatening liver disease in childhood was about 5% and that 80% of patients who presented with neonatal cholestasis were healthy and free of chronic liver disease at 18 years of age (51). Approximately 50% of PiZZ children may show signs and symptoms of liver dysfunction but only about 5% show cirrhosis and life-threatening disease before 18 years of age (52). In skin fibroblasts from individuals with AAT deficiency liver disease, constitutively expressed PiZ protein was found to be degraded less efficiently than in PiZZ individuals without liver disease (53), confirming the presence of additional host factors in modifying disease phenotype. One such host factor involved in the degradation of ER-retained and misfolded glycoproteins is mannosidase I, the translation and protein levels of which are suppressed by a variant allele in its 3'untranslated region (3’UTR) that is more prevalent in symptomatic PiZZ infants, thus providing evidence for the role of genetic modifiers in AAT deficiency liver disease (54).
AAT deficiency should be considered in any newborn with liver dysfunction and biopsy showing neonatal cholestasis (lobular hepatitis, prominent steatosis, hepatocellular necrosis) with or without giant cells. Bile duct proliferation mimicking extrahepatic biliary atresia is a common finding. Uncommonly, a ductopenic pattern mimicking Alagille syndrome can be seen (10). Characteristic PASD-positive eosinophilic cytoplasmic globules of AAT may be seen in periportal hepatocytes, most prominent in older children, and can be diagnosed earlier with anti-AAT monoclonal antibodies. Chronic liver disease with hepatitis and/or cirrhosis usually occurs only in older children and adults.
Hepatic neoplasia arising in AAT-deficiency liver disease has been well documented. An autopsy study from Sweden first showed a strong association between AAT deficiency, cirrhosis and liver cancer, with the risk for liver cancer greater than explained by cirrhosis alone (55). The life-time relative risk (RR) of developing HCC in a PiZZ individual is increased (RR =5.0) (56,57) and with few exceptions (58), hepatic tumors develop in adults (15). Polymerized conformation of ATZ within the endoplasmic reticulin of the hepatocytes may lead to hepatocellular apoptosis and a compensatory proliferation in the liver possibly leading to cirrhosis and HCC (59). Unlike HT1, hepatic neoplasia in AAT deficiency often develops in non-cirrhotic livers, and CC or mixed cholangio-HCCs (CHCC) have also been reported (15). The development of HCC and CCs in PiMZ (heterozygous for Z) individuals has also been reported (60), although the incidence of liver disease in PiMZ individuals remains controversial (61).
Hepatic carcinogenesis in AAT-deficiency is incompletely understood but Perlmutter and colleagues have advanced an interesting cell non-autonomous model (62). Using a transgenic mouse model for AAT deficiency (PiZ mice), Rudnick et al. demonstrated two distinct hepatocyte populations: a globule-containing compartment with PASD-positive AAT aggregates and another globule-devoid compartment negative for PASD (63). Furthermore, using double labeling with the DNA proliferation marker BrdU, the proliferating hepatocytes were found to be within the globule-devoid compartment. These findings have led to a model whereby the globule-devoid hepatocytes are considered to have a selective proliferation advantage and respond to proliferation signals emanating from the globule-containing hepatocytes and explain why hepatic cancers appear to predominantly arise from the zones of globule-devoid hepatocytes (60,62). However, conclusive evidence for such a ‘trans’ signal awaits further studies. A recent study suggests that wild-type hepatocytes grafted in a mouse model of AAT liver disease have a much higher selective advantage compared to both globule-containing and globule-devoid compartments, and replace between 20-98% of mutant host hepatocytes even in the absence liver injury (64), suggesting that the mechanism of carcinogenesis remains to be fully understood. These findings also provide pre-clinical data that wild-type hepatocyte transplantation could be a therapeutic option in PiZZ individuals due to the potential of this approach to not only ameliorate liver disease but to lead to increased serum levels of the protein.
Type I glycogen storage disorder (GSD-I) (OMIM 232200, 232220)
GSD-I, or von Gierke disease, is an AR disorder caused by mutations in the glucose-6-phosphatase complex. It is the most common of hepatic glycogenoses, representing ~25% of all cases (14). GSD-I has been further subdivided based on the molecular defect: type Ia (the classic form) is due to mutations in the catalytic subunit of glucose-6-phosphatase (G6PC), and type Ib due to mutations in the transporter (G6PT). More than 80 allelic variants in the G6PC gene have been reported in the literature (65) and sequence analysis of the gene is now offered commercially (www.genetests.org) and is especially useful for prenatal diagnosis (66). Stringent genotype-phenotype correlations do not exist however (65), and the gold standard for diagnosis is measurement of enzyme activity on fresh liver tissue (14).
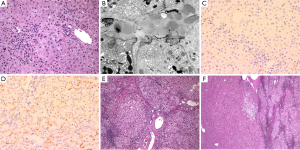
GSD-I is characterized by variable metabolic derangements (66), the chief being profound hypoglycemia and hyperlipidemia. The clinical manifestations and laboratory findings have been thoroughly reviewed (2,14,66). Patients can present in the newborn period but typically present within the first 6 months of life with hypoglycemia and hepatomegaly. Hepatic cirrhosis and liver failure are not seen and liver biopsy shows swollen, pale hepatocytes with cytoplasmic PAS positive storage material completely sensitive to diastase digestion (Figure 2). Microvesicular and macrovesicular steatosis are present by H&E and electron microscopy in the hepatocytes and the hepatocytes also have prominent cell membranes. Monoparticulate glycogen can be seen on electron microscopy.

Between 16-75% of GSD-I patients develop HA detectable by ultrasonography and radioisotopic scanning by age 15 years (67,68). In several series, the prevalence of adenomas was found to increase with age, developing first around puberty. The pathogenesis of adenomas in GSD-I is incompletely understood. The fact that maintenance of glycemic control through dietary therapy since childhood (with frequent meals, uncooked corn starch or continuous nocturnal nasogastric drip feeding) has reduced the incidence of adenomas (69) and that some adenomas have been reported to regress with dietary therapy (70) have led to the suggestion that increased glucagon levels are hepatotrophic (14). However, documented glucagon levels are usually normal (67). A recent study comparing genomic alterations in HA arising in GSD-I with adenomas in general population did not identify specific changes in any group (71). Further studies are necessary to resolve the mechanism of adenoma formation (and neoplasia in general) in this disorder; however, in light of the new proposed classification system for hepatocellular adenomas, a recent retrospective reanalysis of 38 nodules in seven transplanted livers in GSD-I patients with immunohistochemistry and molecular analysis revealed the majority to diffusely express serum amyloid-A (SAA) protein and have histological features of ‘inflammatory type’, although the authors did not find any IL6ST mutations in any of the nodules (72).
Ominously, adenomas have been reported to undergo malignant transformation to HCC or hepatocellular adenocarcinomas in several case series (73-75). GSD-I is probably unique among metabolic disorders to demonstrate the development of HCC in adenomas in the absence of concomitant cirrhosis. However, the pathogenesis of malignant transformation remains elusive. In a recent case series of 8 patients with GSD-I and HA, the age of diagnosis of HCC ranged from 19-49 years (73). Due to the risk of HCC in GSD-I patients, current recommendations propose imaging with regular monitoring of serum AFP levels. However, 6 of 8 patients with HCC had normal AFP levels (73), highlighting the need for other surveillance mechanisms in these patients.
Type III GSD (OMIM 232400)
Type III GSD (GSD-III or Cori disease) is caused by deficiency of the glycogen debranching enzyme (amylo-1, 6-glucosidase, 4-alpha-glucanotransferase). It is an AR disorder caused by mutations in the AGL gene. Affected patients typically present in infancy with hepatomegaly and hypoglycemia with or without myopathy. Liver biopsy at this stage depicts distended hepatocytes with PASD-positive cytoplasmic material that on ultrastructural examination reveal abnormal glycogen with short outer chains. Periportal fibrosis is common but progressive cirrhosis is a rare complication of long-term disease in these patients (76). Hepatic neoplasia is a rare complication of GSD-III in adults. Adenomas are seen in 5-25% of patients (76,77). There are only six reports of HCC in GSD-III, all arising in cirrhosis (76), the earliest at 32 years of age (78).
Type IV GSD (OMIM 232500)
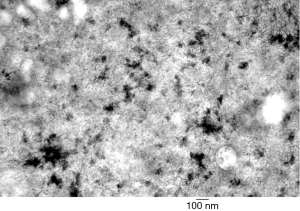
Type IV GSD (GSD-IV or Andersen disease) is an extremely rare (0.3% of glycogenoses) AR disorder caused by deficiency of the glycogen branching enzyme and leads to death by 3 to 5 years of age due to progressive liver cirrhosis (14). More than 15 alleles have been reported in the GBE1 gene to cause this clinically heterogeneous disorder that in the classic form presents with cirrhosis in infancy. The liver biopsy is remarkable for swollen hepatocytes with eccentrically located nuclei and discrete amphophilic cytoplasmic material that is PASD-positive and often has a halo effect. By electron microscopy, this cytoplasmic material consists of glycogen particles and amylopectin (Figure 3). The amylopectin-like material also stains with colloidal iron. Cirrhosis develops very early in GSD-IV patients, often at presentation. HA do occur but the development of HCC in GSD-IV has been rarely reported and this is most likely due to the young age of death of most patients afflicted with this disorder (79,80).
Hereditary hemochromatosis (HH) (OMIM 235200)
HH is an AR disorder of iron metabolism caused by mutations in the HFE gene in the vast majority of cases. Prior to the identification of the gene, a diagnosis of HH on liver biopsy was based on documentation of iron overload (grade 3 or 4 stainable iron, hepatic iron index >1.9, and iron content >4,500 µg/g dry liver weight). Current screening and confirmatory strategies for HH diagnosis include fasting transferrin saturation and ferritin levels, and if abnormal, genotyping for the C282Y and H63D alleles in HFE for confirmation (16). Homozygosity for the C282Y allele has a prevalence of 1 in 200-400 in Caucasian populations and accounts for 85-90% of all cases of classic HH. The molecular mechanisms of HFE-mediated regulation of iron metabolism have been reviewed elsewhere (16,81).
HH is one of the most common inherited disorders in descendants of northern European ancestry. Histologically iron deposition is initially noted in zone 1 hepatocytes in a pericanalicular distribution with progressive extension into zones 2 and 3. Over time iron begins to accumulate in the biliary epithelium, Kupffer cells, and portal macrophages. Eventually cirrhosis may occur. In a comprehensive population-based study on 1847 Swedish individuals, Elmberg et al. (82) have reported a 20-fold increased risk of HCC in HH patients. With few exceptions, HCC arises in cirrhotic livers (81). HCC has also been shown to occur in iron free foci (Deugnier et al. 2007). Although the defect in iron metabolism may be detected in utero, clinical symptoms in HH rarely develop prior to adulthood. The long-term complications of HH (cirrhosis and HCC) are prime examples of the aforementioned hypothesis of cumulative ‘chronic injury’ through childhood. Therefore, children of affected parents should be screened annually with transferrin saturation and phlebotomy should be initiated with the first abnormal result.
The mechanism of ‘chronic injury’ is presumed to be related to iron overload. Excess iron has been shown to cause liver injury through the generation of free radicals and lipid peroxidation, leading to mitochondrial dysfunction and cell death (83). Furthermore, iron has been shown to activate hepatic stellate cells to promote fibrosis. Beyond the role of cirrhosis itself in predisposing to HCC, several observations support a direct role for iron in promoting hepatic carcinogenesis. First, there are several reports of HCC in non-cirrhotic livers of HH patients (81). Second, stainable iron can be found in >50% of patients with HCC without HH (81). Third, as reported by Lehmann et al. (84), epigenetic alterations commonly encountered in HCC are more frequently identified in liver biopsies of HH individuals without HCC. Finally, iron-mediated carcinogenesis is associated with increased frequency of mutations in TP53 gene (85,86).
Wilson disease (WD) (OMIM 277900)
WD is an AR disorder of copper metabolism caused by mutations in the ATP7B gene. Greater than 250 different mutations in the gene have been reported in WD patients (7). The gene encodes a copper-transporting ATPase protein in the trans-Golgi complex that is essential for copper secretion into bile. Deficiency of the protein leads to gradual copper accumulation in the liver, and then within extra-hepatic sites, leading to the typical clinical manifestations of the disease. Diagnosis of WD depends on a combination of several tests, including decreased serum ceruloplasmin levels (<20 mg/dL), increased hepatic copper content (>250 µg/g dry weight), increased 24-h urinary copper excretion post-penicillamine challenge (>25 µmol/24 h), ATP7B genotyping, and/or DNA haplotype analysis in affected families (7).
Liver biopsy in patients may reveal an acute phase characterized by hepatocyte swelling, steatosis, periportal glycogenated hepatocyte nuclei, mild cholestasis, and portal lymphocytic infiltrates. Chronic stages typically show hepatocyte ballooning, interface hepatitis (mimicking auto-immune hepatitis), and steatosis with periportal fibrosis or macronodular cirrhosis (10). Stainable copper is characteristic but not detectable in the younger patient or in regenerative nodules; hepatic copper content measurements are more informative. Ultrastructural examination typically shows abnormal and large mitochondria with dilated cristae and dense bodies as well as lipid deposition. Treatment consists of life-long copper chelation therapy and dietary zinc to compete for copper absorption from the diet, without which the disease is fatal (7).
The risk of hepatic neoplasia in WD patients has not been adequately determined but is likely to be low as there are very few reports. In the largest series, two HCC and three CCs were identified in 159 WD patients followed over 10 years (87). The pathophysiology of elevated copper has been studied in Atp7b−/− mice where it has been shown that liver damage is related to the duration of exposure to increased copper. In mice, elevated hepatocyte nuclear copper leads to increased DNA synthesis and older mice develop CCs (88). Gene expression profiling of livers from knockout mice has revealed selective down-regulation of genes involved in cholesterol biosynthesis (89), an interesting finding given the potential for altered cell-cell interactions.
Other metabolic conditions
A few other metabolic conditions are associated with a low incidence of hepatic neoplasia (Table 1). The mechanisms of carcinogenesis are similarly not understood in these disorders. Acute intermittent porphyria (OMIM 176000), an autosomal dominant (AD) disorder of heme biosynthesis, has been reported to have a >30 RR of developing HCC (56,90). Defective bile salt transport and resultant cytotoxicity in progressive familial intrahepatic cholestasis-2 (PFIC-2; OMIM 601847) may form the basis for the HCC and CCs seen even in children <10 years age (91,92) (Figure 4). A recent genome profiling study suggests that the progressive liver damage in PFIC-2 from chemical injury leads to massive amplification and copy-gain of several mitogen-activated protein kinases (MAPK) pathway genes, a mutational signature distinct from that seen in viral-associated HCC (93). Mutations in the tight junction protein 2 (TJP2) have been identified as leading to a new type of PFIC (94) presenting with severe early cholestasis requiring transplantation. Interestingly, case reports of young children (6 and 26 months old) presenting with complete TJP2 deficiency and HCC (95) suggest yet another potential association with chronic liver injury. Finally, in developed countries, with the growing prevalence of pediatric obesity, various forms of NAFLD, including non-alcoholic steatohepatitis (NASH) have become common causes of chronic liver disease in children. NAFLD, the histological manifestation of the metabolic syndrome, is characterized by insulin resistance and oxidative stress, and is often associated with ‘cryptogenic cirrhosis’ (96,97). A prospective cohort study in Japanese population has revealed a ~7% incidence of HCC in NASH patients (98). Studies on the molecular pathogenesis of cirrhosis and hepatic neoplasia in these disorders are essential, as this epidemic seems destined to replace hepatitis B viral infection as the leading antecedent of HCC.
Discussion
Neoplasia in metabolic liver disease is a relatively rare complication of a group of individually rare disorders. For instance, homozygous PiZZ AAT deficiency, the most common genetic cause of liver disease in children, has an incidence of 1:1,500 to 1:3,500 newborns (51,99). In long-term follow-up studies by Sveger et al., only 10-15% had any biochemical evidence of liver disease at 25 years age (100,101). Even if all were to eventually develop HCC, this translates roughly to an incidence of 1:20,000 affected individuals. Nevertheless, the RR of developing HCC with the PiZZ genotype is significantly increased (57) (RR =5.0) (56), and warrants close surveillance of affected individuals. Similarly, the rate of children affected with HT1 in Quebec (17) is ~1:17,000, the highest in the world. By the estimated 15% incidence of HCC in tyrosinemic livers (17), the risk of HCC is approximately 1:190,000 births.
Our understanding of the molecular pathways involved in disease progression and carcinogenesis in metabolic disorders has benefited tremendously from studies in mouse models. Although the physiology of humans differs somewhat, several useful mouse knockouts (Fah−/−, Atp7b−/−, etc.) have provided significant insight into pathogenic mechanisms (40,102). One of the most significant advances in HT1 over the past two decades has been the introduction of NTBC, a triketone herbicide that was first tested in rats before introduction in humans (39). More recently, high-throughput approaches in genomics, transcriptomics, and metabolomics are currently making it possible to investigate these rare disorders with greater depth in humans. Such an unbiased ‘systems biology’ approach has the added advantage of uncovering hitherto unknown or ‘silent’ genetic modifiers that are likely to explain the variable penetrance seen in virtually all metabolic disorders.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd ed. New York: Cambridge University Press, 2007.
- Scriver CR, Sly WS, Childs B, et al. The Metabolic and Molecular Bases of Inherited Disease, 4 volume set. 8th ed. New York: McGraw-Hill Professional, 2001.
- Speliotes EK. Genetics of common obesity and nonalcoholic fatty liver disease. Gastroenterology 2009;136:1492-5. [PubMed]
- Schwimmer JB, Celedon MA, Lavine JE, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology 2009;136:1585-92. [PubMed]
- Malaguarnera M, Di Rosa M, Nicoletti F, et al. Molecular mechanisms involved in NAFLD progression. J Mol Med (Berl) 2009;87:679-95. [PubMed]
- Roberts EA. Pediatric nonalcoholic fatty liver disease (NAFLD): a "growing" problem? J Hepatol 2007;46:1133-42. [PubMed]
- O'Connor JA, Sokol RJ. Copper Metabolism and Copper Storage Disorders. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd ed. New York: Cambridge University Press, 2007:626-60.
- Müller T, Schäfer H, Rodeck B, et al. Familial clustering of infantile cirrhosis in Northern Germany: A clue to the etiology of idiopathic copper toxicosis. J Pediatr 1999;135:189-96. [PubMed]
- Muller T, Feichtinger H, Berger H, et al. Endemic Tyrolean infantile cirrhosis: an ecogenetic disorder. Lancet 1996;347:877-80. [PubMed]
- Jevon G, Dimmick J. Metabolic Disorders in Childhood. In: Russo P, Ruchelli ED, Piccoli DA, editors. Pathology of Pediatric Gastrointestinal and Liver Disease. 1st ed. New York: Springer-Verlag New York, 2004:270-99.
- Ishak KG. Inherited metabolic diseases of the liver. Clin Liver Dis 2002;6:455-79. viii. [PubMed]
- Finegold MJ. Hepatic Tumors in Childhood. In: Russo P, Ruchelli ED, Piccoli DA, editors. Pathology of Pediatric Gastrointestinal and Liver Disease. 1st ed. New York: Springer-Verlag New York, 2004:300-46.
- Emre S, McKenna GJ. Liver tumors in children. Pediatr Transplant 2004;8:632-8. [PubMed]
- Ghishan FK, Zawaideh M. Inborn Errors of Carbohydrate Metabolism. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd ed. New York: Cambridge University Press, 2007:595-625.
- Zhou H, Fischer HP. Liver carcinoma in PiZ alpha-1-antitrypsin deficiency. Am J Surg Pathol 1998;22:742-8. [PubMed]
- Knisely AS, Narkewicz MR. Iron Storage Disorders. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd ed. New York: Cambridge University Press, 2007:661-76.
- Mitchell G, Russo PA, Dubois J, et al. Tyrosinemia. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd ed. New York: Cambridge University Press, 2007:694-713.
- Kamath BM, Spinner NB, Piccoli DA. Alagille Syndrome. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd ed. New York: Cambridge University Press, 2007:326-45.
- Jonas MM, Perez-Atayde AR. Fibrocystic Liver Disease. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd ed. New York: Cambridge University Press, 2007:928-42.
- Litten JB, Tomlinson GE. Liver tumors in children. Oncologist 2008;13:812-20. [PubMed]
- Fearon ER. Human cancer syndromes: clues to the origin and nature of cancer. Science 1997;278:1043-50. [PubMed]
- Mitchell GA, Grompe M, Lambert M, et al. Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York, NY: McGraw-Hill, 2001:1777-806.
- Laberge C. Hereditary tyrosinemia in a French Canadian isolate. Am J Hum Genet 1969;21:36-45. [PubMed]
- Lindstedt S, Holme E, Lock EA, et al. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 1992;340:813-7. [PubMed]
- Demers SI, Russo P, Lettre F, et al. Frequent mutation reversion inversely correlates with clinical severity in a genetic liver disease, hereditary tyrosinemia. Hum Pathol 2003;34:1313-20. [PubMed]
- Weinberg AG, Mize CE, Worthen HG. The occurrence of hepatoma in the chronic form of hereditary tyrosinemia. J Pediatr 1976;88:434-8. [PubMed]
- Kvittingen EA, Rootwelt H, Brandtzaeg P, et al. Hereditary tyrosinemia type I. Self-induced correction of the fumarylacetoacetase defect. J Clin Invest 1993;91:1816-21. [PubMed]
- Kvittingen EA, Rootwelt H, Berger R, et al. Self-induced correction of the genetic defect in tyrosinemia type I. J Clin Invest 1994;94:1657-61. [PubMed]
- Russo PA, Mitchell GA, Tanguay RM. Tyrosinemia: a review. Pediatr Dev Pathol 2001;4:212-21. [PubMed]
- van Spronsen FJ, Thomasse Y, Smit GP, et al. Hereditary tyrosinemia type I: a new clinical classification with difference in prognosis on dietary treatment. Hepatology 1994;20:1187-91. [PubMed]
- Mieles LA, Esquivel CO, Van Thiel DH, et al. Liver transplantation for tyrosinemia. A review of 10 cases from the University of Pittsburgh. Dig Dis Sci 1990;35:153-7. [PubMed]
- Holme E, Lindstedt S. Nontransplant treatment of tyrosinemia. Clin Liver Dis 2000;4:805-14. [PubMed]
- Seda Neto J, Leite KM, Porta A, et al. HCC prevalence and histopathological findings in liver explants of patients with hereditary tyrosinemia type 1. Pediatr Blood Cancer 2014;61:1584-9. [PubMed]
- Larochelle J, Alvarez F, Bussières JF, et al. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol Genet Metab 2012;107:49-54. [PubMed]
- Holme E, Lindstedt S. Tyrosinaemia type I and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione). J Inherit Metab Dis 1998;21:507-17. [PubMed]
- Masurel-Paulet A, Poggi-Bach J, Rolland MO, et al. NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis 2008;31:81-7. [PubMed]
- van Spronsen FJ, Bijleveld CM, van Maldegem BT, et al. Hepatocellular carcinoma in hereditary tyrosinemia type I despite 2-(2 nitro-4-3 trifluoro- methylbenzoyl)-1, 3-cyclohexanedione treatment. J Pediatr Gastroenterol Nutr 2005;40:90-3. [PubMed]
- Koelink CJ, van Hasselt P, van der Ploeg A, et al. Tyrosinemia type I treated by NTBC: how does AFP predict liver cancer? Mol Genet Metab 2006;89:310-5. [PubMed]
- Lock EA, Ellis MK, Gaskin P, et al. From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis 1998;21:498-506. [PubMed]
- Grompe M, Lindstedt S, al-Dhalimy M, et al. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet 1995;10:453-60. [PubMed]
- Endo F, Tanaka Y, Tomoeda K, et al. Animal models reveal pathophysiologies of tyrosinemias. J Nutr 2003;133:2063S-2067S. [PubMed]
- Jorquera R, Tanguay RM. Fumarylacetoacetate, the metabolite accumulating in hereditary tyrosinemia, activates the ERK pathway and induces mitotic abnormalities and genomic instability. Hum Mol Genet 2001;10:1741-52. [PubMed]
- Dieter MZ, Freshwater SL, Miller ML, et al. Pharmacological rescue of the 14CoS/14CoS mouse: hepatocyte apoptosis is likely caused by endogenous oxidative stress. Free Radic Biol Med 2003;35:351-67. [PubMed]
- Marhenke S, Lamlé J, Buitrago-Molina LE, et al. Activation of nuclear factor E2-related factor 2 in hereditary tyrosinemia type 1 and its role in survival and tumor development. Hepatology 2008;48:487-96. [PubMed]
- Prieto-Alamo MJ, Laval F. Deficient DNA-ligase activity in the metabolic disease tyrosinemia type I. Proc Natl Acad Sci U S A 1998;95:12614-8. [PubMed]
- Orejuela D, Jorquera R, Bergeron A, et al. Hepatic stress in hereditary tyrosinemia type 1 (HT1) activates the AKT survival pathway in the fah-/- knockout mice model. J Hepatol 2008;48:308-17. [PubMed]
- Hidvegi T, Ewing M, Hale P, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science 2010;329:229-32. [PubMed]
- Wang Y, Perlmutter DH. Targeting intracellular degradation pathways for treatment of liver disease caused by α1-antitrypsin deficiency. Pediatr Res 2014;75:133-9. [PubMed]
- Perlmutter DH, Silverman GA. Hepatic fibrosis and carcinogenesis in α1-antitrypsin deficiency: a prototype for chronic tissue damage in gain-of-function disorders. Cold Spring Harb Perspect Biol 2011.3. [PubMed]
- Gosai SJ, Kwak JH, Luke CJ, et al. Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin α1-antitrypsin Z. PLoS One 2010;5:e15460. [PubMed]
- Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med 1976;294:1316-21. [PubMed]
- Nelson DR, Teckman J, Di Bisceglie AM, et al. Diagnosis and management of patients with α1-antitrypsin (A1AT) deficiency. Clin Gastroenterol Hepatol 2012;10:575-80. [PubMed]
- Wu Y, Whitman I, Molmenti E, et al. A lag in intracellular degradation of mutant alpha 1-antitrypsin correlates with the liver disease phenotype in homozygous PiZZ alpha 1-antitrypsin deficiency. Proc Natl Acad Sci U S A 1994;91:9014-8. [PubMed]
- Pan S, Huang L, McPherson J, et al. Single nucleotide polymorphism-mediated translational suppression of endoplasmic reticulum mannosidase I modifies the onset of end-stage liver disease in alpha1-antitrypsin deficiency. Hepatology 2009;50:275-81. [PubMed]
- Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. N Engl J Med 1986;314:736-9. [PubMed]
- Dragani TA. Risk of HCC: genetic heterogeneity and complex genetics. J Hepatol 2010;52:252-7. [PubMed]
- Elzouki AN, Eriksson S. Risk of hepatobiliary disease in adults with severe alpha 1-antitrypsin deficiency (PiZZ): is chronic viral hepatitis B or C an additional risk factor for cirrhosis and hepatocellular carcinoma? Eur J Gastroenterol Hepatol 1996;8:989-94. [PubMed]
- Hadzic N, Quaglia A, Mieli-Vergani G. Hepatocellular carcinoma in a 12-year-old child with PiZZ alpha1-antitrypsin deficiency. Hepatology 2006;43:194. [PubMed]
- Teckman JH. Liver disease in alpha-1 antitrypsin deficiency: current understanding and future therapy. COPD 2013;10 Suppl 1:35-43. [PubMed]
- Zhou H, Ortiz-Pallardó ME, Ko Y, et al. Is heterozygous alpha-1-antitrypsin deficiency type PIZ a risk factor for primary liver carcinoma? Cancer 2000;88:2668-76. [PubMed]
- Perlmutter DH. α1-Antitrypsin Deficiency. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd ed. New York: Cambridge University Press, 2007:545-71.
- Perlmutter DH. Pathogenesis of chronic liver injury and hepatocellular carcinoma in alpha-1-antitrypsin deficiency. Pediatr Res 2006;60:233-8. [PubMed]
- Rudnick DA, Liao Y, An JK, et al. Analyses of hepatocellular proliferation in a mouse model of alpha-1-antitrypsin deficiency. Hepatology 2004;39:1048-55. [PubMed]
- Ding J, Yannam GR, Roy-Chowdhury N, et al. Spontaneous hepatic repopulation in transgenic mice expressing mutant human α1-antitrypsin by wild-type donor hepatocytes. J Clin Invest 2011;121:1930-4. [PubMed]
- Chou JY, Mansfield BC. Mutations in the glucose-6-phosphatase-alpha (G6PC) gene that cause type Ia glycogen storage disease. Hum Mutat 2008;29:921-30. [PubMed]
- Kishnani PS, Koeberl D, Chen YT. Glycogen Storage Diseases. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York, NY: McGraw-Hill, 2001:1521-51.
- Lee PJ. Glycogen storage disease type I: pathophysiology of liver adenomas. Eur J Pediatr 2002;161 Suppl 1:S46-9. [PubMed]
- Rake JP, Visser G, Labrune P, et al. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr 2002;161 Suppl 1:S20-34. [PubMed]
- Matern D, Starzl TE, Arnaout W, et al. Liver transplantation for glycogen storage disease types I, III, and IV. Eur J Pediatr 1999;158 Suppl 2:S43-8. [PubMed]
- Parker P, Burr I, Slonim A, et al. Regression of hepatic adenomas in type Ia glycogen storage disease with dietary therapy. Gastroenterology 1981;81:534-6. [PubMed]
- Kishnani PS, Chuang TP, Bali D, et al. Chromosomal and genetic alterations in human hepatocellular adenomas associated with type Ia glycogen storage disease. Hum Mol Genet 2009;18:4781-90. [PubMed]
- Sakellariou S, Al-Hussaini H, Scalori A, et al. Hepatocellular adenoma in glycogen storage disorder type I: a clinicopathological and molecular study. Histopathology 2012;60:E58-65. [PubMed]
- Franco LM, Krishnamurthy V, Bali D, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis 2005;28:153-62. [PubMed]
- Nakamura T, Ozawa T, Kawasaki T, et al. Case report: Hepatocellular carcinoma in type 1a glycogen storage disease with identification of a glucose-6-phosphatase gene mutation in one family. J Gastroenterol Hepatol 1999;14:553-8. [PubMed]
- Bianchi L. Glycogen storage disease I and hepatocellular tumours. Eur J Pediatr 1993;152 Suppl 1:S63-70. [PubMed]
- Demo E, Frush D, Gottfried M, et al. Glycogen storage disease type III-hepatocellular carcinoma a long-term complication? J Hepatol 2007;46:492-8. [PubMed]
- Labrune P, Trioche P, Duvaltier I, et al. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr 1997;24:276-9. [PubMed]
- Cosme A, Montalvo I, Sánchez J, et al. Type III glycogen storage disease associated with hepatocellular carcinoma. Gastroenterol Hepatol 2005;28:622-5. [PubMed]
- de Moor RA, Schweizer JJ, van Hoek B, et al. Hepatocellular carcinoma in glycogen storage disease type IV. Arch Dis Child 2000;82:479-80. [PubMed]
- Onal IK, Turhan N, Oztas E, et al. Hepatocellular carcinoma in an adult patient with type IV glycogen storage disease. Acta Gastroenterol Belg 2009;72:377-8. [PubMed]
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004;127:S79-86. [PubMed]
- Elmberg M, Hultcrantz R, Ekbom A, et al. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology 2003;125:1733-41. [PubMed]
- Wallace DF, Subramaniam VN. Co-factors in liver disease: the role of HFE-related hereditary hemochromatosis and iron. Biochim Biophys Acta 2009;1790:663-70.
- Lehmann U, Wingen LU, Brakensiek K, et al. Epigenetic defects of hepatocellular carcinoma are already found in non-neoplastic liver cells from patients with hereditary haemochromatosis. Hum Mol Genet 2007;16:1335-42. [PubMed]
- Vautier G, Bomford AB, Portmann BC, et al. p53 mutations in british patients with hepatocellular carcinoma: clustering in genetic hemochromatosis. Gastroenterology 1999;117:154-60. [PubMed]
- Marrogi AJ, Khan MA, van Gijssel HE, et al. Oxidative stress and p53 mutations in the carcinogenesis of iron overload-associated hepatocellular carcinoma. J Natl Cancer Inst 2001;93:1652-5. [PubMed]
- Walshe JM, Waldenström E, Sams V, et al. Abdominal malignancies in patients with Wilson's disease. QJM 2003;96:657-62. [PubMed]
- Huster D, Finegold MJ, Morgan CT, et al. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am J Pathol 2006;168:423-34. [PubMed]
- Huster D, Purnat TD, Burkhead JL, et al. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem 2007;282:8343-55. [PubMed]
- Andant C, Puy H, Bogard C, et al. Hepatocellular carcinoma in patients with acute hepatic porphyria: frequency of occurrence and related factors. J Hepatol 2000;32:933-9. [PubMed]
- Scheimann AO, Strautnieks SS, Knisely AS, et al. Mutations in bile salt export pump (ABCB11) in two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma. J Pediatr 2007;150:556-9. [PubMed]
- Knisely AS, Strautnieks SS, Meier Y, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 2006;44:478-86. [PubMed]
- Iannelli F, Collino A, Sinha S, et al. Massive gene amplification drives paediatric hepatocellular carcinoma caused by bile salt export pump deficiency. Nat Commun 2014;5:3850. [PubMed]
- Sambrotta M, Strautnieks S, Papouli E, et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet 2014;46:326-8. [PubMed]
- Zhou S, Hertel PM, Finegold MJ, et al. Hepatocellular carcinoma associated with tight-junction protein 2 deficiency. Hepatology 2015. [Epub ahead of print]. [PubMed]
- Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci 2010;55:560-78. [PubMed]
- Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer 2009;115:5651-61. [PubMed]
- Hashimoto E, Yatsuji S, Tobari M, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol 2009;44 Suppl 19:89-95. [PubMed]
- Perlmutter DH. Alpha-1-antitrypsin deficiency. Semin Liver Dis 1998;18:217-25. [PubMed]
- Sveger T, Eriksson S. The liver in adolescents with alpha 1-antitrypsin deficiency. Hepatology 1995;22:514-7. [PubMed]
- Piitulainen E, Carlson J, Ohlsson K, et al. Alpha1-antitrypsin deficiency in 26-year-old subjects: lung, liver, and protease/protease inhibitor studies. Chest 2005;128:2076-81. [PubMed]
- Lutsenko S. Atp7b-/- mice as a model for studies of Wilson's disease. Biochem Soc Trans 2008;36:1233-8. [PubMed]


