Continuous electroencephalogram detection of non-convulsive seizures in the pediatric intensive care unit: review of the utility and impact on management and outcomes
Introduction
Non-convulsive seizures (NCS) and non-convulsive status epilepticus (NCSE) are common in critically ill children, occurring in up to 47% of pediatric patients with acute encephalopathy, and as knowledge in this field advances, so do resources for identifying and treating them (1-3). Continuous electroencephalogram (CEEG) monitoring, a non-invasive and widely used technology, remains essential to diagnosing NCS and NCSE in the acutely ill patient, and its use in the pediatric intensive care unit (PICU) is increasing (1,4-6). However, given the high resource burden of CEEG, including trained personnel and unique equipment, it is important to understand the utility of this test, how best to allocate it amongst patients, and whether it makes a difference in patient care and outcomes.
Seizure has been defined in the reviewed studies as >10 seconds of clinical and/or electrographic seizure activity. If electrographic only, it qualifies as NCS. More recently, status epilepticus has been defined as continued seizure activity greater than 5 minutes in duration (4,6). However, multiple articles (including those used in this review) continue to use the widely-accepted definition of seizure duration >30 minutes or >50% of time monitored spent in seizure, either clinical or electrographic seizure burden (4,6-22). Pediatric patients may be at higher risk of NCS when compared to adults due to lower seizure threshold, more limited communication, and more varied behavioral disturbances, making the diagnosis of NCS more challenging and suggesting the need for closer monitoring in this population (3,23-25). Electromechanical uncoupling, when electrical seizure activity persists despite resolution of clinical manifestations, is frequently reported in neonates and may persist into older children, which may further contribute to high rates of NCS in the PICU (26).
The reported frequency of NCS in critically ill children with acute encephalopathy ranges from 7-47% (1,3,7,25,27-40). Despite a reasonably high risk of NCS, the Pediatric Critical Care EEG Consortium revealed wide variability in how CEEGs are used in PICUs across North America. Results of this consortium, tasked with assessing current implementation of CEEG in major pediatric care centers in the US and Canada, showed that a clinical pathway guideline for CEEG placement and duration of use is only available in 31% of the 58 large academic centers surveyed. The most commonly reported indications for placement of CEEGs in the PICU are altered mental status (AMS) after convulsive seizure activity, AMS of unknown etiology, and AMS in the setting of a known acute primary neurologic condition, respectively. Even with growing literature on the indications for use, discrepancies remain within North America regarding access to CEEGs, cost, request of Neurology or Neuro ICU consultation, duration of data collection, and frequency of monitoring (1,2,4-6,18,20,27,28,32,39-42). Several limitations remain when implementing broader use of CEEG, including inadequate staffing of trained EEG technologists, neurologists, and clinical neurophysiologists (1,5). Regardless of institutional differences, detecting NCS via CEEG is essential to the acute management of critically ill children and continues to be the focus of ongoing research into the impact of NCS on neurologic outcome (1,2,8-19). This review summarizes the current literature regarding use and utility of CEEG in the PICU, as well as its influence on clinical management and, most importantly, its impact on patient outcomes.
Indications for CEEG
Identifying PICU patients at highest risk for NCS helps guide CEEG usage by prioritizing when this resource-intense monitoring tool should be used (1,5,41,42). Seizures among critically ill children with AMS are common, with the rate of NCS reported up to 47% and the rate of NCSE up to 35% (1,3,7,25,27-40). Multiple studies have suggested the potential significance of the co-diagnosis of epilepsy or congenital heart disease, the presence of clinical seizures prior to AMS, acute abnormalities on neuroimaging, and interictal EEG abnormalities (thought to represent evidence of an underlying seizure tendency) as having an association with higher rates of NCS and NCSE in PICU patients (4,23-25,27,29-31,34,35). A consensus statement on CEEG in critically ill adults and children was published in June 2015 by the Critical CEEG Task Force of the American Clinical Neurophysiology Society, making more formal monitoring recommendations; many of these recommendations are included in Table 1 (4).
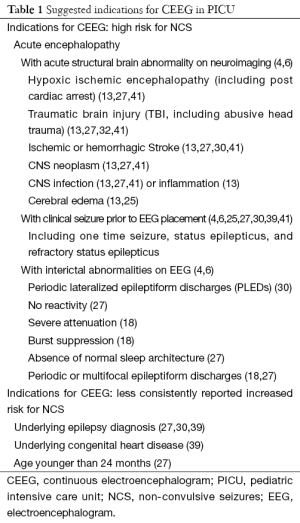
Full table
Two of the most significant risk factors consistently shown to be associated with NCS activity are clinical seizures prior to CEEG placement and acute neuroimaging abnormalities (4,10,25,30,39,41). In a study of 75 children referred to the PICU for possible NCSE, if both clinical seizures and acute neuroimaging changes were present, Greiner et al. identified an 82% probability of NCSE (25). This is in contrast to 34% NCSE in patients with clinical seizures and normal imaging, or 29% NCSE with abnormal imaging and no clinical seizure; if neither of these risk factors were identified, probability of NCSE was only 4% (25).
In a review of 122 critically ill children, McCoy et al. reported an independent increase in risk of NCS after clinical seizure as well as in those with acute structural brain abnormalities on neuroimaging, however data on the combination of these two findings was not reported (41). Additional risk factors for NCS identified in that review were acute presentation of epilepsy, prior diagnosis of epilepsy and interictal epileptiform discharges on CEEG (41). In a similar review of 20 children of varying age and diagnoses with NCSE, Abend et al. reported that 75% of children had clinical seizure activity (isolated or status epilepticus) and 82% had abnormal neuroimaging (39). Additional risk factors for NCSE identified in this smaller review were prior diagnosis of epilepsy, congenital heart disease, and ischemic stroke (39). A larger review by Abend et al. in 2013 showed that of the critically ill children with NCS or NCSE on CEEG, 47% had clinical seizures prior to CEEG placement and 73% had an acute structural neurological disorder on imaging (10).
Therefore, while multiple potential risk factors are proposed for NCS and NCSE in critically ill children with AMS, the highest and most consistently reported risk is in children with clinical seizure prior to CEEG placement accompanied by acute structural neuroimaging abnormalities (10,25,39,41). This was further emphasized in a consensus statement on CEEG use in critically ill adults and children, published in June 2015 by the Critical CEEG Task Force of the American Clinical Neurophysiology Society, in which formal recommendations were made for initiating CEEG in critically ill children with AMS after clinical seizures and/or acute supratentorial brain injury (4). Table 1 summarizes common risk factors reported for NCS in critically ill children, including specific abnormalities identified on neuroimaging and interictal CEEGs.
Duration of CEEG monitoring
Deciding which patients to monitor with CEEG is the first step in detecting NCS in critically ill children, but variable access to CEEGs, EEG technicians, neurologists, and remote viewing opportunities continues to limit the utility of this neurodiagnostic tool (1,5,15). Therefore, it is important to discern ideal monitoring times, balancing practicality with maximal efficacy in identifying NCS.
In the recent survey on EEG utilization by Sanchez et al. of 58 major pediatric academic centers in North America, the optimal and practical duration of EEG monitoring and review frequency remains undecided (1,5,41). While the majority of large pediatric academic centers report ability to obtain EEGs twenty-four hours per day, EEG technicians may not be in house, and are only available by call outside of regular business hours 51% of the time (1). Once a CEEG is placed, the majority of survey respondents report that a technologist or physician typically reviews the data within one hour of placement (1,5). Beyond the first hour, CEEG data is continuously collected, but most commonly only reviewed twice per day (37% of institutions), unless clinical changes warrant earlier re-evaluation (1,5). Prompt review of the first hour is crucial to diagnosing NCSE rapidly and may identify up to 40-70% of NCS (37,41). Notably, only 10-15% of NCS may be detected in the first 20 minutes (27,41) but this increases to 80-100% of NCS detected within the first 24 hours of CEEG data (16,27,29,37,41). Monitoring for >24 hours has been shown to only identify 4-20% more cases of NCS in critically ill children when compared to 24 hour monitoring (27,42). Although the impact of continuous real-time EEG monitoring by appropriately trained personnel on early NCS and NCSE detection has not been specifically studied, it seems intuitive that this would facilitate earlier detection, and therefore treatment, in many instances.
The cost of CEEG monitoring in the PICU must be considered as it increases with longer durations, according to US data recently published by Abend et al. (42). Their research attempted to provide cost-to-benefit analysis of CEEG in the ICU setting, ultimately suggesting a 24-hour period as the optimal duration (42). Unfortunately, cost-effectiveness of prolonged CEEG has yet to be prospectively assessed and such monitoring may be too resource intensive for many institutions. Nonetheless a minimum of immediate EEG review (at least within the first hour of hook up) and subsequent twice daily review is recommended by the 2015 Consensus Statement by the Critical CEEG Task Force of the American Clinical Neurophysiology Society (5,42). Additionally, it was recommended that 24-hour CEEG monitoring be used as the optimal study length, however the task force recognized that shorter or longer periods of monitoring may be indicated on a case by case basis (4). Similar to neonatal and adult neurocritical care practices, CEEG monitoring for 24+ hours after the last identified seizure or withdrawal of continuous anti-epileptic mediation helps demonstrate adequate seizure control (2,4,6).
NCS and acute management
Prolonged CEEG monitoring helps identify NCS in high risk pediatric patients, which subsequently impacts acute management, including medication options, continued EEG monitoring, imaging, or work up for other diagnoses (8-19). In a study of 100 PICU patients with AMS, acute medical management was modified based on results of CEEG in over half the cases (10). Of the 43% of patients in which seizures were identified on CEEG, anti-epileptic drugs (AEDs) were initiated. Furthermore, characterization of movements as non-epileptic was established in 21% of these patients, thereby avoiding unnecessary AED administration (10). Finally, identifying a need for urgent neuro-imaging occurred in 3% of cases (10). In the remainder of patients, no specific management modification was reported, but investigators felt ruling out NCS in a patient with AMS may allow the clinician to more confidently pursue other etiologies (10). The presence of NCSE specifically changed management in 92% of patients in a related study, either due to initiation or dose escalation of AEDs (25).
There have not been definitive studies regarding optimal AED management in the treatment of seizures (convulsive or nonconvulsive) in the PICU. The majority of clinicians continue to use benzodiazepines as first line agent as recommended by the 2012 guidelines for evaluation and management of status epilepticus (class 1, level A evidence) (6). The use of phenobarbital, fosphenytoin, levetiracetam, and valproic acid to treat seizures in the PICU has been studied extensively, although management approaches remain variable (6,8-11). One study reported that nearly half of all seizures were controlled with only one AED, but another 21% went on to require >4 AEDs for adequate seizure control (11). Though it is clear that using CEEG to identify NCS in PICU patients influences management, the details on which anti-epileptic to use varies based on the patient, the clinician, drug availability, and institutional guidelines (1,6,8-11).
NCS and patient outcomes
Increasing investigation into the impact of NCS and NCSE on neurological outcomes suggests that, similar to adult data, prolonged seizures negatively impact prognosis and outcomes in critically ill children (7-19,21,22). This is an important area of ongoing research, given earlier studies which suggested that children were more resilient to brain damage from seizures or status epilepticus and that morbidity was influenced more by severity of underlying illness than by seizure burden (43,44). More recent studies have instead shown that seizures, as an independent factor, do appear to negatively affect outcomes in critically ill children (4,7-22). Since the presence of NCS may be impossible to identify on clinical exam alone, prompt detection via CEEG is critical to initiating appropriate management (3,23-26). As yet, the full impact of CEEG use and the mechanism of brain injury, both in children and adults, from seizure activity remains uncertain (4,6,21,22). However, multiple studies into both short and long term outcomes have continued to show the negative impact of NCS and NCSE on outcomes in critically ill children independent of other factors (4,7-20).
Short term outcome measures for critically ill children with seizures include measures such as mortality, length of PICU stay, Pediatric Cerebral Performance Category (PCPC) scores, and King’s Outcome Scale for Childhood Head Injury (KOSCHI) scores at time of discharge (7,12,13,18,20). PCPC scores were designed as an assessment of neurologic function for critically ill children, while the KOSCHI score was developed as an outcome measure for pediatric patients after head injury (45,46). Duration of stay in PICU was significantly prolonged for patients with NCSE (median, 11 days), and statistically trended towards longer length of stay in patients with NCS (median, 8 days) when compared to critically ill children without NCS or NCSE (median, 5.5 days) (13). Multiple studies have shown that status epilepticus in critically ill children, whether convulsive or not, is associated with increased risk of mortality, and worse outcomes measures including worse PCPC and KOSCHI scores at discharge compared to deduced pre-admission scores (7,12,13,18,20). These studies used multivariate analyses of many possible factors including age, etiology, EEG background category, and acute and pre-existing neurologic disorders to focus on the independent risk of seizure or status epileptics on these outcomes measures. Topjian et al. found that NCS (in contrast to NCSE), did not result in increased mortality or worse short term neurologic outcomes (as measured by KOSCHI scores) (11).
Interestingly, all of these studies defined SE or NCSE as >30 min of continuous clinical and/or electrographic seizure activity or >50% seizure burden in one hour (7,12,13,18,20). However, in the study by Payne et al. final data on seizure burden was further categorized as <20% (≤12 minutes) of time spent in seizure per hour, 20-50% per hour, or >50% per hour. This approach allowed a more detailed assessment as to the amount of time spent in seizure and the effect on short term neurological decline (measured by pre-admission versus discharge PCPC scores) in 259 critically ill children aged 0.3 to 9.8 years (20). Using a multivariate analysis to account for factors such as age, sex, diagnosis, and illness severity, it was shown that for every 1% increase in seizure burden per hour, the odds of neurological decline increased by 1.13. More specifically, there was a significant delineation for neurologic decline in patients before and after 20% seizure burden per hour (=12 minutes) (20). Patients who spent less than 12 minutes per hour in seizure had the same risk of neurologic decline as those with no seizures (around 60% of these patients had worsening PCPC score) versus children who spent >12 minutes per hour in seizure, who had a 98% risk of neurologic decline, regardless of whether they spent 20-50% per hour in seizure, or >50% per hour (20). This is an important distinction that argues for more rapid treatment of clinical and electrographic seizures, and supports the definition of status epileptics as >5 minutes duration as in Brophy et al.’s guidelines on the management of status epilepticus (4-6,20).
Long term outcome measures for PICU patients with seizures include Glasgow Outcome Scale with extended pediatric version scores (GOS-E Peds), Pediatric Quality of Life Inventory (PedsQL) scores, risk of acquiring an epilepsy diagnosis, and sustained neurologic deficits at follow up (12,14,18,31). The GOS-E Peds is an 8-point scale ranging from 1= good recovery and 5= death, with categories 1-3 more specifically divided into upper and lower categories for each (47). PedsQL is a 23 item measure to look at patient and parent perceptions of health related quality of life (48). Patients with status epilepticus, convulsive or not, had lower scores on both GOS-E Peds and PedsQL (47,48).
In a study of 204 comatose pediatric patients in Kenya and the UK, seizure activity was measured via 1-3 channel EEGs and seizure burden categorized as number of seizures, total duration of time in seizure throughout monitoring (not continuous), and duration of a single seizure event (continuous). Again, using multivariate analyses to minimize effect of other factors on outcomes, it was identified that patients with >139 seizures, a combined duration of 759 minutes, or a single seizure event lasting >6 hours had worse outcomes on follow up GOS-E Peds scores at one month follow up (31). Along this continuum, refractory status epilepticus has been associated with a higher risk of subsequent epilepsy diagnosis and sustained neurologic deficits, such as diffuse hypotonia, hemiparesis, and loss of developmental milestones (14,18).
A recent prospective study by Abend et al. includes some of the longest outcome data published in critically ill children with NCS and NCSE to date. This group evaluated neurobehavioral outcomes in children 1.2-3.8 years after an episode of critical illness and NCS activity. Neurobehavioral outcome measures such as adaptive behavior concerns, emotional or other behavioral issues, and executive functioning deficits were collected via the Adaptive Behavior Assessment System-II, Child Behavior Checklist, and Behavior Rating Inventory of Executive Function, respectively. Their results show that patients with NCS and NCSE had worse adaptive behavior in long term follow up, and, though not statistically significant, trended towards worse emotional issues, other behavioral problems, and executive functioning (22). This data suggests that increased seizure burden is associated with worse long term outcomes including quality of life, neurobehavioral issues, and long term disability in PICU patients with AMS. Given the high risk of NCSE in this population, the utility of CEEG is paramount to detect seizures, aid management, and may ideally help improve outcomes in critically ill children.
Conclusions
NCS are common among critically ill children with AMS and nearly impossible to diagnose without CEEG monitoring. Risk factors associated with NCS and NCSE remain varied; however the significance of acute encephalopathy with clinical seizure prior to CEEG placement, acute structural brain abnormalities on neuroimaging, and interictal abnormalities on CEEG is continually reinforced in the scientific literature. Monitoring high-risk PICU patients for at least 24 hours on CEEG appears to be ideal based on existing literature; however shorter duration CEEG or incremental routine EEG studies may ultimately prove to be more practical and cost-effective for some cases. The diagnosis of NCS should prompt caregivers to re-evaluate management and initiate or escalate AEDs to improve seizure control as indicated, although evidence-based treatment algorithms remain limited. The impact of NCS and NCSE on neurologic outcome continues to be area of significant investigation, as increased seizure burden has been associated with higher mortality, longer PICU stays, and greater short term and long term disability in critically ill children. Ultimately, a better understanding of the mechanisms involved in the development of NCS and NCSE is fundamental to improving clinical management in critically-ill children and optimizing CEEG usage across the highly diverse landscape in which it is utilized.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG Monitoring: Current Resources and Practice in the United States and Canada. J Clin Neurophysiol 2013;30:156-60. [PubMed]
- Abend NS, Dlugos DJ, Hahn CD, et al. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care 2010;12:382-9. [PubMed]
- Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Non-convulsive seizures are common in critically ill children. Neurology 2011;76:1071-7. [PubMed]
- Herman ST, Abend NS, Bleck TP, et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part I: Indications. J Clin Neurophysiol 2015;32:87-95. [PubMed]
- Herman ST, Abend NS, Bleck TP, et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part II: Personnel, Technical Specifications, and Clinical Practice. J Clin Neurophysiol 2015;32:96-108. [PubMed]
- Brophy GM, Bell R, Claasen J, et al. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit Care 2012;17:3-23. [PubMed]
- Arndt DH, Lerner JT, Matsumoto JH, et al. Subclinical Early Post-Traumatic Seizures Detected by Continuous EEG Monitoring in a Consecutive Pediatric Cohort. Epilepsia 2013;54:1780-88. [PubMed]
- Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol 2009;66:723-8. [PubMed]
- Abend NS, Dlugos DJ. Treatment of Refractory Status Epilepticus: Literature Review and a Proposed Protocol. Pediatr Neurol 2008;38:377-90. [PubMed]
- Abend NS, Topjian AA, Gutierrez-Colina AM, et al. Impact of continuous EEG monitoring on clinical management in critically ill children. Neurocrit Care 2011;15:70-5. [PubMed]
- Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short Term Outcome in Critically Ill Children. Crit Care Med 2013;41:215-23. [PubMed]
- Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology 2014;82:396-404. [PubMed]
- Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology 2013;81:383-91. [PubMed]
- Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia 2008;49:615-25. [PubMed]
- Gutierrez-Colina AM, Topjian AA, Dlugos DJ, et al. EEG Monitoring in Critically Ill Children: Indications and Strategies. Pediatric Neurol 2012;46:158-61.
- Hyllienmark L, Amark P. Continuous EEG monitoring in a paediatric intensive care unit. Eur J Paediatr Neurol 2007;11:70-5. [PubMed]
- Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 1996;47:83-9. [PubMed]
- Kravljanac R, Jovic N, Djuric M, et al. Outcome of status epilepticus in children treated in the intensive care unit: a study of 302 cases. Epilepsia 2011;52:358-63. [PubMed]
- Raspall-Chaure M, Chin RF, Neville BG, et al. Outcome of paediatric convsulsive status epilepticus: a systematic review. Lancet Neurol 2006;5:769-79. [PubMed]
- Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429-38. [PubMed]
- Abend NS. Electrographic status epilepticus in children with critical illness: Epidemiology and outcome. Epilepsy Behavior 2015;49:223-7. [PubMed]
- Abend NS, Wagenman KL, Blake TP, et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy Behavior 2015;49:238-44. [PubMed]
- Dan B, Boyd SG. Nonconvulsive (Dialeptic) Status Epilepticus in Children. Curr Pediatr Rev 2005;1:7-16.
- Raspall-Chaure M, Chin RF, Neville BG, et al. Epidemiology of Convulsive Status Epilepticus in Children: A Critical Review. Epilepsia 2007;48:1652-63. [PubMed]
- Greiner HM, Holland K, Leach JL, et al. Nonconvulsive status epilepticus: The encephalopathic pediatric patient. Pediatrics 2012;129:e748-55. [PubMed]
- Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743-8. [PubMed]
- Jette N, Claasen J, Emerson RG, et al. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol 2006;63:1750-5. [PubMed]
- Abend NS, Chapman KE, Gallentine WB, et al. Electroencephalographic Monitoring in the Pediatric Intensive Care Unit. Curr Neurol Neurosci Rep 2013;13:330. [PubMed]
- Schreiber JM, Zelleke T, Gaillard WD, et al. Continuous Video EEG for Patients with Acute Encephalopathy in a Pediatric Intensive Care Unit. Neurocrit Care 2012;17:31-8. [PubMed]
- Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive Seizures in the Pediatric Intensive Care Unit: Etiology, EEG, and Brain Imaging Findings. Epilepsia 2006;47:1510-8. [PubMed]
- Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med 2012;38:853-62. [PubMed]
- Hasbani DM, Topjian AA, Friess SH, et al. Nonconvulsive Electrographic Seizures are Common in Children With Abusive Head Trauma. Pediatr Crit Care Med 2013;14:709-15. [PubMed]
- Alehan FK, Morton LD, Pellock JM. Utility of electroencephalography in the pediatric emergency department. J Child Neurol 2001;16:484-7. [PubMed]
- Tay SK, Hirsch LJ, Leary L, et al. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia 2006;47:1504-9. [PubMed]
- Shahwan A, Bailey C, Shekerdemian L, et al. The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia 2010;51:1198-204. [PubMed]
- Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology 2009;72:1931-40. [PubMed]
- Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia 2011;52:1130-6. [PubMed]
- Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol 2005;32:162-5. [PubMed]
- Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol 2007;37:165-70. [PubMed]
- Abend NS, Beslow LA, Smith SE, et al. Seizures as a Presenting Symptom of Acute Arterial Ischemic Stroke in Childhood. J Pediatr 2011;159:479-83. [PubMed]
- McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia 2011;52:1973-8. [PubMed]
- Abend NS, Topjian AA, Williams S. How much does it cost to identify a critically ill child experiencing electrographic seizures? J Clin Neurophysiol 2015;32:257-64. [PubMed]
- Maytal J, Shinnar S, Moshe SL, et al. Low morbidity and mortality of status epilepticus in children. Pediatrics 1989;83:323-31. [PubMed]
- Gross-Tsur V, Shinnar S. Convulsive status epilepticus in children. Epilepsia 1993;34 Suppl 1:S12-20. [PubMed]
- Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121:68-74. [PubMed]
- Crouchman M, Rossiter L, Colaco T, et al. A practical outcome scale for paediatric head injury. Arch Dis Child 2001;84:120-4. [PubMed]
- Beers SR, Wisniewski SR, Garcia-Fillon P, et al. Validity of pediatric version of Glasgow Outcome Scale-Extended. J Neurotrauma 2012;29:1126-39. [PubMed]
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126-39. [PubMed]


