Investigation and management of the hepatic glycogen storage diseases
Editor’s note:
“Rare Diseases Column” is chaired by Dr. Zhanhe Wu from The Children’s Hospital at Westmead, Australia, featuring articles related to rare diseases mostly genetic based, presented in early life disease, with chronic phase but frequently progressive, disabling and life threatening diseases. Article types of original articles, review articles, case reports, perspectives, etc. are welcomed to be submitted to the column.
Introduction
The glycogen storage disorders (GSD) comprise a range of disorders that affect the processing of glycogen. Glycogen itself is a highly branched glucose polymer that is osmotically inert (1). Glycogen therefore provides a reservoir of glucose for sudden energy demands and is particularly important for supplying glucose to the body from hepatocytes, during short-term fasting and to muscles in the early phase of exercise. As such, the GSDs conditions usually lead to a disruption of glucose supply to relevant organs. However, in some situations, because of the nature of the structure of glycogen, there are permanent pathological changes that ensue from dysfunctional glycogen storage. The GSD can no longer be considered one entity but are a group of different disorders related only by the dysfunctional processing of glycogen. That said, there are broad phenotypic classes that can be used to help target investigation and treatment. These include hepatomegaly with hypoglycaemia, intermittent myalgia and rhabdomyolysis and haemolytic anaemia. This paper will focus on hepatomegaly with or without hypoglycaemia.
The hypoglycaemic glycogen storage diseases (GSD)
GSD 0, I, III, VI, IX and Fanconi Bickel syndrome are all associated with hypoglycaemia. Figure 1 indicates the biochemical pathways involved and Table 1 the related genes. Of these GSD 0 has a small liver and the other conditions have acquired hepatomegaly (1).
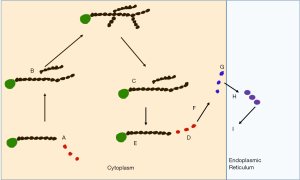
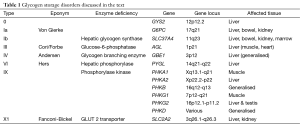
Full table
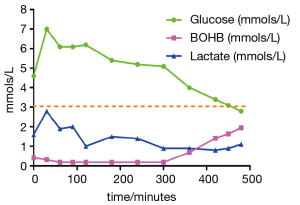
Typically, patients are referred to physicians either with recurrent hypoglycaemia, or with hepatomegaly. The severe forms of GSD in childhood are associated with very short fasting intervals of less than 4 hours (2). This is quite typical of GSD 1 but can also be seen in GSD III, VI and IX. Sometimes patients present in the newborn period with profound hypoglycaemia often with seizures. At this time, secondary metabolic disturbances and hepatomegaly may not be present and it can consequently be difficult to make a diagnosis. A suggested algorithm for observing biochemical flux is indicated in Table 2. It is always important to note the timing of the biochemical assessments of glucose, lactate and ketones in relation to the feeds in order to narrow down the potential diagnoses. Often the specialist is given a single glucose and lactate value in isolation which is meaningless. Table 2 and Figure 2 indicate how dynamic data may provide information for diagnosis and treatment.

Full table
It is important to identify an exact diagnosis as there are different treatments, complications and natural histories for the different types of disorder (3-5). Nowadays, a liver biopsy is seldom necessary unless to assess fibrosis. A genetic diagnosis can usually be made as indicated in Table 1. A history is important—if there is neonatal presentation, the timing of hypoglycaemia in relation to feeds needs to be established. Usually a hypoglycaemia screen is performed. This is important to identify other relevant diagnoses such as persistent hypoglycaemic hyperinsulinism of infancy (when there is often more glucose delivered than basal requirements) or fat oxidation defects where there should be hypoketotic hypoglycaemia with abnormal carnitine species. If these diagnoses appear excluded, often a glucagon challenge is performed. In GSD I, glucose cannot be produced by either gluconeogenesis or glycogenolysis and there is consequently no rise in glucose and lactate elevation. The other forms of GSD may have a glucose response to glucagon. GSDs more often present after the neonatal period when patients extend their fasting duration. In the more severe forms, the fasting duration maybe 4 hours, but in others up to 12 hours. Often there is a history of patients not being able to sleep through the night for several years. Patients can awaken overnight cold and clammy desperate for milk or later food. Some patients with GSD VI and IX in particular may not have any hypoglycaemia demonstrated (6,7). However, if symptomatic they too may awaken in the morning with similar symptoms and nausea. These symptoms may be related to either hypoglycaemia or ketosis. In all of these situations, a fasting duration for the individual can be identified from history. Clinical examination is imperative and hepatomegaly is seen in all of these disorders apart from GSD 0 after the first 6 months of life. Patients with Fanconi Bickel syndrome may have persistent vomiting, failure to thrive and rickets due to renal tubular wasting. In this situation, causes of rickets need to be established including assessing renal tubular function. Patients with GSD Ib may have recurrent infections due to neutropenia and neutrophil dysfunction (8).
At the end of the patient’s usual fast, blood tests should be performed. These should include electrolytes, liver function tests, full blood count, creatine kinase, uric acid, cholesterol, triglycerides and DNA should be stored. If there is hepatomegaly the size and texture should be noted. Often, the liver can be very large in older children, extending into the left loin. In some forms of GSD (GSD III, and IXc), hepatic fibrosis is not uncommon so an ultrasound should be performed and portal doppler flow assessed. Nephromegaly may be seen in GSD I and Fanconi Bickel syndrome.
There may be preliminary evidence of GSD on the basis of such blood tests—hyperlipidaemia with elevated uric acid typical of GSD I. The other forms often have hyperlipidaemia alone. The creatinine kinase may be elevated in GSD IIIa. If there is no evidence of hypoglycaemia, a prolonged fast can be performed. The diagnostic and therapeutic utility of this can be enhanced by immediately following the fasting test with an oral glucose tolerance test, as indicated by Figure 3. The role of liver biopsy has diminished in the diagnosis of GSD but there are still several instances when these are performed. This is usually in the scenario where there is isolated hepatomegaly with no apparent cause or where there is significant liver dysfunction leading to concern about cirrhosis. On microscopy, hepatocytes are often bulging with glycogen. Periodic acid Schiff stains non-membrance bound glycogen, which is digested by diastase leaving a vacuolated appearance. Lipid vacuoles are also frequently seen in most types, being most prominent in GSD I (5,9). Bridging fibrosis and micronodular cirrhosis can be seen in GSD III, IXc and rarely in IXa (6,7,10,11).

Enzyme assay can be performed for definitive diagnosis but is fraught with difficulties. Measurement of phosphorylase and phosphorylase kinase activity as well as glucose 6-phosphatase and transferase are particularly difficult and need to be performed by an experienced laboratory. For this reason many are turning to massively parallel sequencing to assess several of the GSD genes together (12,13).
Glycogen synthase deficiency (GSD 0)
GYS2 deficiency leads to patients not being able to lay down glycogen in the liver. Hypoglycaemia after short fasts may occur in childhood with seizures particularly with inter-current illness. Patients adapt physiologically by premature ketosis and this may appear exaggerated but this can prevent serious symptoms such as developmental delay even in the context of profound hypoglycaemia. Therefore, there is often ketosis after a routine overnight fast. When glucose is administered in this context, there is often prolonged hyperglycaemia as glycogen cannot be synthesized. Surplus glucose is converted anaerobically to lactate and increased flux through acetyl coA leads to hyperlipidaemia (1). Patients often present in early childhood when feed intervals are extended. There may be associated short stature. Patients often grow out of their hypoglycaemic tendency, but exaggerated ketosis remains (14).
Glycogen storage disease (GSD) type I
The physiology of GSD I means that patients are unable to make glucose either by gluconeogenesis or by glycogen breakdown. In response to hypoglycaemia, patients can form intra-hepatic glucose 6-phosphate but this cannot be exported in the form of glucose. There is consequently obligatory utilisation of this product within the cell. Primarily increased flux down the glycolytic pathway generates lactate by anaerobic respiration. Increased flux via acetyl coA generates lipids. In addition, there is increased flux via the pentose phosphate shunt to generate 5-carbon glucose molecules. These generate nucleic acids, which when catabolised, generate uric acid.
Patients can present in the newborn period but the cardinal secondary features including elevations of lipids, uric acid and hepatomegaly may be absent at this time. Patients are more likely to present with hypoglycaemic seizures at this age compared to later on. Pre-prandial lactate elevation can usually be demonstrated in severe cases. This does disappear after a feed so the timing of the sample is critical. Most patients have impairment of ketone body production through complex physiological mechanisms (15). If patients do not present in the neonatal period, they present later in infancy typically when parents try to extend the fasting interval beyond 4 hours. Infants can become demanding, waking and crying for feeds in response to hypoglycaemia. Once fed, they can become settled. Often parents succumb to the child’s wishes because the behaviour is intractable. By 6 months of age the protuberant abdomen is often quite obvious as is the cherubic face. Hence if the patients have not presented with clinical decompensation by then, they normally would present in the second 6 months of infancy.
Other features of GSD I include increased bruising due to platelet dysfunction caused by decreased von Willebrand factor antigen (16). There are many complications that can ensue particularly when there is sub-optimal metabolic control. These include short-stature, myopathy, developmental delay, osteopenia, renal tubular dysfunction, polycystic ovarian disease, hepatic adenoma, hepatic carcinoma, diarrhoea, gout and renal stones (17-20). Most can be avoided with rigorous metabolic control apart from the renal tubular defects. Further discussion is provided in the Dietary management section.
GSD Ib is associated with other clinical features due to neutropenia and neutrophil dysfunction (8). This can present in infancy with recurrent infections. Poor wound healing is also seen and unfortunately sometimes the first time this is noted, is when a gastrostomy is placed for overnight feeds. The neutrophil defect is related to impaired glucose transport leading to an impaired respiratory burst, chemotaxis and calcium mobilisation (21). Persistence of neutropenia can lead to inflammatory bowel disease later on in childhood.
Glycogen storage disease (GSD) type III
Presentation is not typically in the newborn period but this can occur. The typical age of presentation is between 6 months to 3 years of age again with a history of awakening for feeds and not settling easily. In this disorder, there is no impairment of gluconeogenesis. This means that there is no acetyl coA accumulation and hence ketones can be formed. Ketosis after relatively short fasts is a feature. After glucose is given, there may be elevations of the plasma lactate. Glycogen debrancher is expressed in liver and muscle and hence myopathy and motor developmental delay may be a feature (22). If the muscle is involved, the creatine kinase is usually elevated although this may be only borderline in young patients. Patients are classified as having GSD IIIa if skeletal muscle is affected and IIIb if it isn’t. Motor developmental delay can occur in GSD IIIa and both forms can be associated with osteopenia. In adolescence, patients often become more stable from the hypoglycaemic perspective but it is at this age that the myopathy and cardiomyopathy often become worse. The myopathy of GSD IIIa is slowly progressive, manifesting often as exercise intolerance or fatigue. Exercise ability has been shown to be quite compromised in some individuals. In the author’s experience low impact exercise training may help endurance in such patients. The cardiomyopathy appears often as a progressive in susceptible individuals (23,24). In some situations, it has been recorded as the cause of death. Even though many patients are not prone to hypoglycaemia, some may develop liver dysfunction, liver cirrhosis and hepatocellular carcinoma. The bases of all these morbidities has not been defined (25,26).
Glycogen storage disease (GSD) type VI
Hepatic glycogen phosphorylase deficiency is the rarest form of GSD but it is probably the most under-diagnosed of the GSD. Most often it is not associated with hypoglycaemia but is more typically identified by patients that have isolated hepatomegaly, short stature and hyperlipidaemia. Typically a liver biopsy is performed for this non-specific hepatomegaly and the characteristic pathological finding of glycogen laden hepatocytes leads to the diagnosis. Mild liver dysfunction may be seen. Cardiomyopathy and hepatic fibrosis has been reported (27).
Glycogen storage disease (GSD) type IX (type IXa, IXb, IXc)
Phosphorylase kinase plays an important role in the regulation of glucose homeostasis. Ultimately, counter-regulatory hormones such as catecholamines activate glycogen phosphorylase kinase whilst simultaneously inhibiting glycogen synthase. The anabolic hormone insulin has the opposite effect. The enzyme is a tetramer with α, β, γ, and δ units. The α-subunit is encoded by 2 different genes with only PHKA2 being expressed in the liver. Similarly the γ-subunit is encoded by 2 genes with PHKG2 being expressed in liver (11,28). The remainder of the subunits are encoded by single genes with PHKB coding the β-unit (6). The catalytic site is housed in the γ unit with the remainder of the complexes being involved in regulation. No disorders of the δ unit are currently recognised. PHKA2 deficiency is an X-linked condition with a similar presentation to GSD VI. However some patients have more severe manifestations with hypoglycaemia. Liver cirrhosis has been reported (7). Most, however, have isolated hepatomegaly with fasting ketosis. This responds well to treatment which is often discontinued in adult life. PHKB deficiency is autosomal recessive condition although there have been cases with single mutations identified. It too is usually mild although mild hypertrophic cardiomyopathy has been reported. PHKG2 deficiency is a more severe disorder with a high preponderance to liver fibrosis. There have been several reports of this morbidity but this seems to stabilise over time with treatment. Long-term outcome data are not available.
Fanconi Bickel syndrome
Aetiology
Fanconi Bickel syndrome occurs due to deficiency of the transporter SLC2A2 which is expressed in enterocytes, hepatocytes, renal tubular cells and pancreatic beta-cells. Impairment of glucose export from cells leads to glycogen synthesis and storage within liver and kidney. Impairment of pancreatic beta-cells sensing glucose can lead to impaired insulin release, causing hyperglycaemia after feeds. There are some reports of neonatal diabetes occurring due to SLC2A2 mutations. However, the glucose transport defect is very complex with generalised tubulopathy leading to glycosuria. Renal tubular dysfunction leads to mineral wasting and consequent rickets (29). As well as post-prandial hyperglycaemia, fasting hypoglycaemia occurs due to impaired release of glucose from hepatocytes. The condition is therefore associated with severe somatic manifestation incorporating nephromegaly, hepatomegaly, hypoglycaemia, hyperglycaemia, rickets and short stature.
Dietary management
Fanconi Bickel syndrome is a different entity in terms of therapy and the reader should see other texts (29). The broad emphasis of dietary treatment in the remainder of glycogen storage disorders is to maintain normal blood glucose levels by carbohydrates administered from the diet. This can be achieved theoretically by knowing endogenous glucose requirements that were derived by several studies in the 1970’s (30). It is broadly considered that these are the targets of carbohydrate delivery namely 9 mg/kg/minute in neonates, 5 mg/kg/minute in childhood and 2 mg/kg/min in adults. The issue that often causes difficulties is the quality and rate of the carbohydrate given. For instance a bolus of 20 g glucose given is quite different to the same amount administered over 2 hours. The former would lead to resolution of hypoglycaemia promptly but cause insulin release. In GSD I this leads to rebound hypoglycaemia for which there is no physiological counter-regulation. In the other forms, this could lead to post-prandial lactate elevation which if recurrent in management may lead to complications.
Therefore the management should be centred around the administration of complex carbohydrates in the diet. In severe cases, this may have to be uncooked cornstarch (UCCS) but this may not always be tolerated under one year of age (31,32). It is the author’s preference to try and manage the diet using low glycemic products. Even these cannot be introduced before the baby has solids introduced effectively at 6 months of age. Up until this kind of age, glucose administered at 0.5 g/kg/hr is usually effective during the day and 0.4 g/kg/hr overnight. Usually, the diet can be fine-tuned by attempting to eliminate peaks and troughs in glucose levels using continuous monitoring systems (CGMS) (9). Caution needs to be taken with patients who have GSD Ib as the intradermal needle may lead to poor wound healing in this disorder.
The monitoring of the diet has been suggested by the European Study of GSD I (ESGSD I) as well a more recent American group (3,9). Broadly, clinical parameters of growth and indices of biochemical control should be monitored frequently in the under 5-year group—usually 3 monthly. In severe GSD I this may need to be much more frequent under 1-year of age. The uric acids, triglycerides, pre-prandial glucose, lactate or ketones (depending on what disorder is being managed) assist with management. For GSD III, VI and IX liver function can be quite deranged which also improves with tight metabolic control. It must be remembered that excessive glucose is not desirable and leads to complications too in these disorders. Other useful monitoring are ultrasounds to measure the size of the liver and monitor cirrhosis (where relevant), and for adenoma. Portal flow needs also to be assessed. Bone mineral densitometry should be performed when available. The intense nature of the dietary treatment in terms of carbohydrate delivery can mean that other macronutrients, micronutrients and vitamin deficiencies can occur (15). Deficiencies of vitamin B12, thiamine, riboflavin, selenium and essential fatty acids have been noted. A thorough nutritional need to take place periodical-at least yearly in childhood particularly in severe cases with diets skewed from the normal population. If UCCS forms a major part of the dietary therapy, multi-vitamin, mineral and essential fatty acid supplementation needs to be considered.
The use of medium chain triglyceride (MCT), a modified Atkins diet and high protein diet has been postulated as being efficacious in the treatment in various forms of GSD particularly GSD III (33-35). Ultimately, alternative forms of energy delivery via ketones in particular are possible but needs to be carefully evaluated (36). Some patients with GSD I have impaired ketone production and hence MCT administration may not be effective. If the purpose of the dietary treatment is to generate ketones, clearly monitoring and suppressing this parameter is not relevant. It may be that these methods of energy delivery alter the glycemic load of any given meal leading to less variation in the plasma glucose level. Use of a heat modified waxy maize starch (Glycosade) seems to have a better profile of glucose and insulin release compared to UCCS (37,38). At this stage, these interventions are still experimental but may well be efficacious. There are no long-term data for guidance at the moment. There has been much debate about sucrose and lactose restriction as these have been shown to increase lactate in some individuals (9,39,40). However, the presence of lactate and ketones in the blood in patients with GSD provides organs with a source of energy when glucose has been exhausted. Whilst excessive production is associated with complications, some lactate and ketones are protective (41).
Myopathy is a particular problem with GSD III. A high protein diet has been proposed for this and certainly has merit in terms of leucine oxidation studies. It is probable that if this is used in conjunction with structured moderate impact consistent exercise that this would be effective. Whether use of MCT, a modified Atkins diet or a complex carbohydrate based diet is going to more effective long-term remains to be seen.
Cardiomyopathy and cirrhosis have been reported in GSD III, VI and IX The basis of these morbidities have not been identified but nonetheless treatments such as the use of ketones and MCT have been proposed to help cardiomyopathy and appear to be effective in a small number of cases.
Medical treatment
GSD I
Patients are prone to hypoglycaemia after relatively short fasts or may develop secondary physiology. In circumstances where fasting occurs, such as inter-current illness such as vomiting or pre-operatively, intravenous glucose based fluids need to be administered. These should be administered at rates based upon endogenous utilisation rates as discussed earlier.
Despite the best dietary treatment, patients with GSD are prone to complications as the secondary features are hard to control. There are also some complications that occur independently of therapy. For GSD I renal tubular dysfunction seems to be an inherent part of the disease. In early childhood, children have evidence of hyper-filtration with raised glomerular filtration rates (GFR), but this decreases with time with some developing end-stage renal failure (17). Monitoring for this needs to take place with formal dynamic filtration rate studies using substrates such as Chromium EDTA. Estimated GFR are unreliable in these patients with short-stature and body composition that differs from the normal population. Proteinuria should be assessed and if present treated with appropriate angiotensin blockade. In severe disease, dialysis or renal transplant may be required.
Increased nucleoside production (via 5 carbon sugars as shown in figure) results in uric acid production. If persistent, this can lead to gout and renal stone formation. Flux through this pathway is greater when metabolic control is poor and uric acid clearance is impaired as renal function deteriorates. For this reason, allopurinol may need to be used to help decrease uric acid levels in the plasma.
GSD I is associated with a bleeding tendency as discussed earlier and there have been several instances of persistent bleeding post-operatively requiring resuscitation. This combined with neutrophil dysfunction in GSD Ib means that surgery should be avoided where possible. Cautious evaluation and the use of antibiotics and haematological products need to be considered.
Whilst patients with GSD Ib may have neutropenia and neutrophil dysfunction, intervention is not always required unless there are poor wound healing or recurrent infections. Often, infections are worse with poor metabolic control. Low dose human granulocyte-colony-stimulating-factor (GCSF) may be required if there are significant problems. Long-term use of GCSF is associated with complications such as hypersplenism and malignancy.
Failure of medical therapy may require organ transplantation. There have been several instances of liver transplantation in both GSD Ia and Ib, as well as renal transplants (42). These are infrequently combined. Bone marrow transplantation has been performed in GSD Ib but is not a typical therapeutic option. For cardiomyopathy or cirrhosis, heart and liver transplantation may be indicated in GSD III. Gene therapy has been developed in several animal models but has not yet provided sufficient data in order to move to clinical trials.
Summary
The glucose forming hepatic GSDs are a complex group of disorders that can be identified by careful biochemical and clinical surveillance. Definitive testing by next generation sequencing is likely to be the best current method for accurate diagnosis. Therapy requires very careful dietetic management with prospective surveillance for complications. Outcome can be good but the dietary surveillance is constant and onerous with some complications occurring despite meticulous control.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has acted as a consultant for Vitaflo Ltd in 2008.
References
- Lee PJ, Bhattacharya K. Glycogen Storage Diseases. Oxford: Oxford University Press, 2013. Available online: http://oxfordmedicine.com/view/10.1093/med/9780199204854.001.1/med-9780199204854
- Lee PJ, Dixon MA, Leonard JV. Uncooked cornstarch--efficacy in type I glycogenosis. Arch Dis Child 1996;74:546-7. [PubMed]
- Rake JP, Visser G, Labrune P, et al. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr 2002;161 Suppl 1:S20-34. [PubMed]
- Schippers HM, Smit GP, Rake JP, et al. Characteristic growth pattern in male X-linked phosphorylase-b kinase deficiency (GSD IX). J Inherit Metab Dis 2003;26:43-7. [PubMed]
- Kishnani PS, Austin SL, Arn P, et al. Glycogen storage disease type III diagnosis and management guidelines. Genet Med 2010;12:446-63. [PubMed]
- Goldstein J, Austin S, Kishnani P, et al. Phosphorylase Kinase Deficiency. In: Pagon RA, Bird TD, Dolan CR, et al. eds. GeneReviews. Seattle, WA: University of Washington, 1993.
- Tsilianidis LA, Fiske LM, Siegel S, et al. Aggressive therapy improves cirrhosis in glycogen storage disease type IX. Mol Genet Metab 2013;109:179-82. [PubMed]
- Visser G, Rake JP, Labrune P, et al. Consensus guidelines for management of glycogen storage disease type 1b - European Study on Glycogen Storage Disease Type 1. Eur J Pediatr 2002;161 Suppl 1:S120-3. [PubMed]
- Kishnani PS, Austin SL, Abdenur JE, et al. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med 2014;16:e1. [PubMed]
- Burwinkel B, Rootwelt T, Kvittingen EA, et al. Severe phenotype of phosphorylase kinase-deficient liver glycogenosis with mutations in the PHKG2 gene. Pediatr Res 2003;54:834-9. [PubMed]
- Burwinkel B, Shiomi S, Al Zaben A, et al. Liver glycogenosis due to phosphorylase kinase deficiency: PHKG2 gene structure and mutations associated with cirrhosis. Hum Mol Genet 1998;7:149-54. [PubMed]
- Mundy H, Smith F, Cullup T, et al. Next generation sequencing (NGS) fpr glycogen storage diseases (GSDS); The first UKGTN approved NGS diagnsotic strategy. Journal of Inherited Metabolic Diseases 2011;34:S173.
- Wang J, Cui H, Lee NC, et al. Clinical application of massively parallel sequencing in the molecular diagnosis of glycogen storage diseases of genetically heterogeneous origin. Genet Med 2013;15:106-14. [PubMed]
- Weinstein DA, Correia CE, Saunders AC, et al. Hepatic glycogen synthase deficiency: an infrequently recognized cause of ketotic hypoglycemia. Mol Genet Metab 2006;87:284-8. [PubMed]
- Bhattacharya K. Dietary dilemmas in the management of glycogen storage disease type I. J Inherit Metab Dis 2011;34:621-9. [PubMed]
- Mühlhausen C, Schneppenheim R, Budde U, et al. Decreased plasma concentration of von Willebrand factor antigen (VWF:Ag) in patients with glycogen storage disease type Ia. J Inherit Metab Dis 2005;28:945-50. [PubMed]
- Lee PJ, Dalton RN, Shah V, et al. Glomerular and tubular function in glycogen storage disease. Pediatr Nephrol 1995;9:705-10. [PubMed]
- Lee PJ, Patel A, Hindmarsh PC, et al. The prevalence of polycystic ovaries in the hepatic glycogen storage diseases: its association with hyperinsulinism. Clin Endocrinol (Oxf) 1995;42:601-6. [PubMed]
- Lee PJ, Leonard JV. The hepatic glycogen storage diseases--problems beyond childhood. J Inherit Metab Dis 1995;18:462-72. [PubMed]
- Lee PJ, Patel JS, Fewtrell M, et al. Bone mineralisation in type 1 glycogen storage disease. Eur J Pediatr 1995;154:483-7. [PubMed]
- Jun HS, Weinstein DA, Lee YM, et al. Molecular mechanisms of neutrophil dysfunction in glycogen storage disease type Ib. Blood 2014;123:2843-53. [PubMed]
- DiMauro S, Spiegel R. Progress and problems in muscle glycogenoses. Acta Myol 2011;30:96-102. [PubMed]
- Sentner CP, Caliskan K, Vletter WB, et al. Heart Failure Due to Severe Hypertrophic Cardiomyopathy Reversed by Low Calorie, High Protein Dietary Adjustments in a Glycogen Storage Disease Type IIIa Patient. JIMD Rep 2012;5:13-6. [PubMed]
- Moon JC, Mundy HR, Lee PJ, et al. Images in cardiovascular medicine. Myocardial fibrosis in glycogen storage disease type III. Circulation 2003;107:e47. [PubMed]
- Bali DS, Goldstein JL, Fredrickson K, et al. Variability of disease spectrum in children with liver phosphorylase kinase deficiency caused by mutations in the PHKG2 gene. Mol Genet Metab 2014;111:309-13. [PubMed]
- Demo E, Frush D, Gottfried M, et al. Glycogen storage disease type III-hepatocellular carcinoma a long-term complication? J Hepatol 2007;46:492-8. [PubMed]
- Roscher A, Patel J, Hewson S, et al. The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada. Mol Genet Metab 2014;113:171-6. [PubMed]
- Burwinkel B, Tanner MS, Kilimann MW. Phosphorylase kinase deficient liver glycogenosis: progression to cirrhosis in infancy associated with PHKG2 mutations (H144Y and L225R). J Med Genet 2000;37:376-7. [PubMed]
- Santer R, Groth S, Kinner M, et al. The mutation spectrum of the facilitative glucose transporter gene SLC2A2 (GLUT2) in patients with Fanconi-Bickel syndrome. Hum Genet 2002;110:21-9. [PubMed]
- Bier DM, Leake RD, Haymond MW, et al. Measurement of "true" glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes 1977;26:1016-23. [PubMed]
- Chen YT, Cornblath M, Sidbury JB. Cornstarch therapy in type I glycogen-storage disease. N Engl J Med 1984;310:171-5. [PubMed]
- Chen YT, Bazzarre CH, Lee MM, et al. Type I glycogen storage disease: nine years of management with cornstarch. Eur J Pediatr 1993;152 Suppl 1:S56-9. [PubMed]
- Mayorandan S, Meyer U, Hartmann H, et al. Glycogen storage disease type III: modified Atkins diet improves myopathy. Orphanet J Rare Dis 2014;9:196. [PubMed]
- Valayannopoulos V, Bajolle F, Arnoux JB, et al. Successful treatment of severe cardiomyopathy in glycogen storage disease type III With D,L-3-hydroxybutyrate, ketogenic and high-protein diet. Pediatr Res 2011;70:638-41. [PubMed]
- Das AM, Lücke T, Meyer U, et al. Glycogen storage disease type 1: impact of medium-chain triglycerides on metabolic control and growth. Ann Nutr Metab 2010;56:225-32. [PubMed]
- Fernandes J, Pikaar NA. Hyperlipemia in children with liver glycogen disease. Am J Clin Nutr 1969;22:617-27. [PubMed]
- Bhattacharya K, Orton RC, Qi X, et al. A novel starch for the treatment of glycogen storage diseases. J Inherit Metab Dis 2007;30:350-7. [PubMed]
- Bhattacharya K, Mundy H, Lilburn MF, et al. A pilot longitudinal study of the use of waxy maize heat modified starch in the treatment of adults with glycogen storage disease type I: a randomized double-blind cross-over study. Orphanet J Rare Dis 2015;10:18. [PubMed]
- Fernandes J, van de Kamer JH. Hexose and protein tolerance tests in children with liver glycogenosis caused by a deficiency of the debranching enzyme system. Pediatrics 1968;41:935-44. [PubMed]
- Fernandes J. The effect of disaccharides on the hyperlactacidaemia of glucose-6-phosphatase-deficient children. Acta Paediatr Scand 1974;63:695-8. [PubMed]
- Fernandes J, Berger R, Smit GP. Lactate as a cerebral metabolic fuel for glucose-6-phosphatase deficient children. Pediatr Res 1984;18:335-9. [PubMed]
- Bhattacharya N, Heaton N, Rela M, et al. The benefits of liver transplantation in glycogenosis type Ib. J Inherit Metab Dis 2004;27:539-40. [PubMed]


