
Chemical pleurodesis and somatostatin in treating spontaneous chylothorax in pediatric patients: a retrospective analysis and review of the literature
Introduction
Chylothorax is a rare disease resulting from the abnormal accumulation of lymphatic fluid in the thoracic cavity, and it is one of the most common causes of pleural effusion in young children. Although congenital chylothorax demonstrates prevalence in pediatric patients, occurring with an incidence of 1:6,000 to 1:24,000 live births, the mortality associated with this disease could reach 60%, uniquely combining with premature birth, low birth weight, fetal edema, lung hypoplasia, hypoproteinemia, or continuous chylous exudation (1-4). In this study, the author classified congenital chylothorax and idiopathic chylothorax (in 5–10% of pediatric patients) as spontaneous chylothorax for the cause of both types of chylothorax is not found.
Chylothorax has been clinically reported for nearly a century, but due to its low incidence and few case reports, there are still insufficient guidelines and clinical pathways on its medical management. From the earlier experience, conservative treatment like multiple organ function support, thoracic drainage, somatostatin (SST), and pleurodesis are the first line treatment. Few children will finally be suggested to accept surgery, including ligation of the thoracic duct when conservative treatment fails (5).
SST has been used in treating chylothorax for twenty years, but due to the few reports on chylothorax, its curative effect and medication regimen are still unclear. Povidone-iodine chemical pleurodesis (PICP) is a minimally invasive treatment used in treating pneumothorax or refractory pleural effusion with a high success rate and few complications, so it is expected to be the alternative method in treating pediatric chylothorax in recent years. With this background, we had performed a retrospective analysis to compare the efficacy and safety of PICP and SST in treating spontaneous chylothorax in pediatric patients and review our experience simultaneously. We presented the following article following the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tp-20-199).
Methods
Patients
Pediatric patients who were younger than 8-year-old and diagnosed with spontaneous chylothorax by chest X-ray, ultrasound, and cytological and biochemical analysis in the pleural effusion between January 2009 and May 2020, with no history of trauma, thoracic surgery other than thoracentesis and thoracic drainage, and other congenital and genetic diseases, were enrolled in this study.
After screening, 27 patients were enrolled and divided into the SST group and PICP group according to the therapeutic regimen. The data of their general condition, pleural effusion examination results, diagnosis, treatment, and adverse reactions were retrospectively analyzed and compared.
Written informed consent was obtained before the treatment. The ethical committee approved this study of Guangdong Second Provincial General Hospital (No. 20200615-YXKXYJ-LW-01-01). The research complied with the Declaration of Helsinki (as revised in 2013).
Diagnosis of chylothorax
The diagnosis of chylothorax in all the 27 children was confirmed by thoracic drainage after admission, which showed a milky or yellow turbid fluid on gross examination and positive chyle test when the patients accepted normal feeding, with increased levels of triglycerides ≥1.24 mmol/L, a total leukocyte count ≥1,000 cells/µL (1.0×106/L) or at least 80% lymphocytes, and sterile (6,7).
Basic support treatment
After admission, chest X-ray and ultrasound were performed as soon as possible to confirm the affected side of the thoracic cavity in all the patients, and a chest tube was put into the thoracic cavity to drain out the pleural effusion. Chyle testing, cytological and biochemical examination were checked on the effusion, and blood routine, serum electrolytes, lipid test, C-reactive protein, liver, kidney, and thyroid function test, and electrocardiography were also performed.
After the tests were finished, patients were then given electrocardiographic monitoring and oxygen treatment. Nutrition support therapy was carried out combining the way of internal and parental nutrition according to the daily need for fluid and energy from the patients’ age and weight. Both enteral and parenteral nutrition were adopted in the treatment. Patients were only allowed to eat carbohydrates, electrolytes, vitamins, vegetables, and fruits; we only allowed a limited number of milk-fat products (1/4 to 1/3 of the daily demand) and liquids (1/3 of the daily demand). The rest of the calories were supplied with the parental nutrition solution comprised of glucose, amino acids, fat emulsions, and vitamins. Human immunoglobulin and albumin were given according to the results of blood tests. Cephalosporin antibiotics were used to treat pneumonia. Blood routine and serum biochemical examination, liver and kidney function tests, chest X-ray, or ultrasound are rechecked routinely.
PICP group
After admission, the patient’s food intake was controlled to reduce the amount of chyle exudation. PICP was possible when the daily amount of pleural effusion decreased and supported in a low volume. A chest X-ray or ultrasound was performed in each patient before the treatment to confirm that the affected side had sufficient lung recruitment. Then, a small amount of normal saline was used to clean the chest tube to ensure smooth drainage. A modified PICP treatment method was adopted for treatment (8), that is, to increase the amount of povidone-iodine (PI) sclerosant, reduce the concentration of PI, and reduce the stay time of the sclerosant in the thoracic cavity. The vital signs of the patients were watched closely, and the sclerosant was then injected slowly through the chest tube when the signs became stable, and the children were calm. Depending on the reaction of the child’s response, the sclerosant was wholly or partially injected. If the child had apparent cyanosis, difficulty breathing, and a noticeable change in the vital signs like a rapid drop in the blood oxygen saturation, the injection was stopped immediately, sclerosant should be drained out, and rescue treatment should be performed if it was necessary. After the treatment, their parents changed their position appropriately, and the chest tube was connected to the drainage bag for continuous drainage. A chest X-ray or ultrasound was rechecked within 12–24 hours to confirm the condition of the thoracic cavity. If the pleural effusion was significantly reduced or disappeared, the chest tube was closed for 1–2 weeks, and chest X-rays or ultrasounds was regularly rechecked to ensure no recurrence of the effusion. If the amount of pleural effusion was increased after reexamination, the chest tube was opened for drainage again, and PICP treatment could be considered when the patients had a steady general condition.
SST group
Eight patients were treated with SST simultaneously during the primary support treatment. Intravenous injection used SST with an intravenous infusion pump, with a starting dosage of 0.5 µg/kg/hour. If the child’s vital signs were stable, and no visible adverse reactions occurred, the dosage of SST could be gradually increased to 10 µg/kg/hour maximally. When the daily amount of pleural effusion decreased significantly and did not increase for about three days, the dosage could be gradually decreased until SST was stopped. During the treatment, the electrolytes, blood glucose, liver, kidney, and thyroid function should be re-examined regularly.
Clinical cure standards
After the treatment of PICP or SST, pleural effusion was absorbed, and no recurrence of effusion happened during the drainage tube was clamped for 1 to 2 weeks. No severe adverse reactions occurred during hospitalization (e.g., organ dysfunction). The results of the blood test and thyroid function were typical.
Treatment and follow-up after discharge
All patients were asked to keep on a low-fat diet after clinical cure, and their general condition was observed by their legal guardians and reported to us if it was abnormal in weight, mental state, daily activities, and any uncomfortable symptoms. Chest X-rays or ultrasound was rechecked for each patient on one month and three months after discharge. And they could have a regular diet if the pleural effusion had not recurred. The follow-up period was 1–10 years.
Statistical analysis
Data are presented as the minimum value to maximum value (in Tables 1,2) and mean ± standard deviation for categorical variables. Group comparisons performed on the mean length of stay, the mean total time of thoracic drainage, and parenteral nutrition were acquired using independent-samples t-test. All analyses were performed using IBM SPSS Statistics software (Version 22.0. IBM Corporation, NY, USA), with statistical significance set at P values less than 0.05.

Full table

Full table
Results
Patient clinical characteristics
Twenty-seven patients (18 males and 9 females) were enrolled in this study, aged between 19 hours and seven years old. All of them were admitted to the hospital due to the symptoms of shortness of breath, cyanosis, or anhelation. Five cases had accepted oxygen and hormone therapy for acute respiratory distress before, and 6 cases had suffered pneumonia repeatedly since birth.
After admission, pleural effusion was confirmed by chest X-ray or ultrasound, with 13, 6, and 8 cases on the left, right, and both sides, respectively. The examination of the pleural effusion showed that the mean leukocyte count was (9,826±9,482)×106/L, the mean lymphocyte ratio was (84.82±6.58)%, the mean albumin was 21.40±8.18 g/L, and the mean triglyceride was 7.11±6.63 mmol/L, with a result of positive chyle test and negative bacterial culture in all cases. The statistics of the general condition, diagnosis, and pleural effusion results of the patients in the PICP group and SST group were respectively described in Table 1. The pleural effusion collected from some patients for the first time was milky white or milky red. After diet control, the pleural effusion gradually turned yellowish and slightly turbid (see Figure 1).
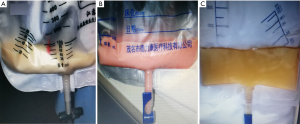
After treatment of necessary support, PICP and SST
All patients had stable vital signs during the necessary support treatment. The symptoms of dyspnea were alleviated after the effusion was drained out, and the amount of pleural effusion gradually decreased and maintained in a low volume after diet control (about 1/3 of the daily amount measured at the time of admission). Cephalosporin antibiotics cured pneumonia diagnosed in 6 children.
PICP treatment was successfully performed in all patients for 3.16±2.27 times. The sclerosant was used when the volume ranged from 20–50 mL during PICP, depending on the patient’s general condition. After the treatment, all the children had symptoms of chest pain, including crying and restlessness, which could be relieved by feeding, appeasement, and other ways to divert their attention. Moreover, low-grade fever was recorded in all cases, with the highest temperature of 37.8 °C, and cured by physical cooling and ibuprofen suspension. Twelve cases displayed short of breath, with the blood oxygen saturation decreasing minimally to 90% shortly after the injection of sclerosant, which returned to normal after the drainage of sclerosant. During the treatment, 19 children showed good general condition and physical activities. Their pleural effusion was finally absorbed without recurrence confirmed by the chest X-ray or ultrasound.
Eight patients in the SST group accepted SST for 14.75±9.08 d, with a dosage of 0.5–10 µg/kg/hour. During the treatment, all the patients had nausea and vomit, and 1 of them had diarrhea simultaneously. Symptomatic treatment, reducing the dosage and stopping using SST relieved the above symptoms. After the treatment, five patients reached the standards of clinical cure. Three patients were diagnosed with refractory chylothorax by continuously recurrent pleural effusion after treated by SST for 12–31 days, with a maximum dosage of 10 µg/kg/hour. Daily drainage volume in these child had decreased from 24–29 to 8–13 mL/kg/d after the treatment, but the pleural effusion still recurred and could not be absorbed completely. After the symptoms of gastrointestinal dysfunction found in the patients, SST was gradually stopped to avoid deterioration of the above adverse reactions. Considering a poor cardiopulmonary function and immunity and an elevated risk of anesthesia that these low-age patients had, PICP was adopted for treatment after SST instead of surgery. After 2–4 times of PICP treatment, the patients’ pleural effusion was gradually absorbed. Eight patients were clinically cured finally, and chylothorax did not recur in the follow-up period.
The statistical analysis results in the two groups were shown in Table 2. The chest X-ray results of each patient in the PICP group and SST group before and after treatment were shown in Figure 2, respectively. The pleural effusion was absorbed after a clinical cure.
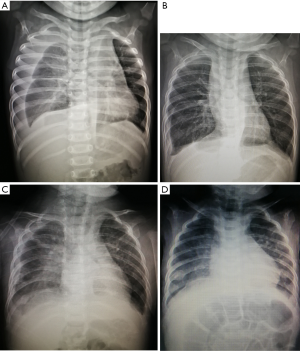
Discussion
Spontaneous chylothorax is a common cause of pleural effusion in young children, and its etiology is unclear, usually considered associated with the abnormal growth of lymphatic vessels in the thorax (9). Because the organ function of young children is not yet mature and the tolerance of their body is relatively weak, the condition of chylothorax in pediatric patients is often more complicated than adult patients, so an appropriate distinction needs to be made in the process of clinical diagnosis and treatment. Children with spontaneous chylothorax are usually faced with a higher possibility of premature delivery with low birth weight. They often need to be treated by mechanical ventilation, thoracentesis, and thoracic drainage immediately for the reason of anoxia causing by atelectasis and acute respiratory distress syndrome. Continuously thoracic drainage is more likely to result in malnutrition, electrolytes disorder, and decreasing in immunity due to the loss of lipids, albumin, globulin, vitamins, and electrolytes as well as lymphocytes and antibodies through the effusion (10). It has a high possibility to cause septicemia and nosocomial infection during the treatment. Therefore, life support must be adopted at first to support a stable general condition for pediatric patients with chylothorax.
As the incidence of chylothorax is low, and there have been few related studies, the treatment efficacy has not been sufficiently demonstrated. It is challenging and unprecise to perform surgeries like lymphangiography, embolization, or ligation of the thoracic duct on pediatric chylothorax patients because their chest wall lymphatics are small and have many collateral vessels. Moreover, pediatric patients with chylothorax often have a poor cardiopulmonary function, with an elevated risk of anesthesia. According to most of the previous studies, conservative treatment (e.g., necessary life support, thoracic drainage, SST, and chemical pleurodesis) is the first choice for spontaneous chylothorax in pediatric patients at present (5). Thus, it is highly relevant to explore a safe and effective treatment to promote the absorption of pleural effusion, the self-healing of pleural lymphatic fistula, and the reconstruction of thoracic lymphatic circulation.
SST (or octreotide) is a type of regulatory inhibitory polypeptides, which can act on receptors in splanchnic vessels to decrease intestinal blood flow, gastrointestinal motility, intestinal fat absorption and inhibit the release of gastrin, vasoactive intestinal peptide, secretin, motilin, and pancreatic polypeptide. Therefore, it can decrease the exudation of chylous lymphatic effusion in the thoracic cavity and promote the self-healing of pleural lymphatic fistula. In 1998, Rimensberger et al. (11) firstly used SST to cure an infant with chylothorax. Nevertheless, due to insufficient clinical experience and related researches, the dosage and cure rate of SST in treating spontaneous chylothorax vary widely in different case reports and series in early stage (11). In 2010 and 2018, Das et al. (12) and Bellini et al. (13) had performed a meta-analysis and systematic review of the efficacy of octreotide in treating spontaneous chylothorax, with a success rate of 70% and 53%, respectively. Adverse reactions reported in the study included nausea, bloating, diarrhea or constipation, hyperglycemia, skin flushing, liver dysfunction, necrotizing enterocolitis, and transient hypothyroidism (14,15). Therefore, avoiding adverse reactions is a critical point for SST treatment. A starting dosage of 0.5–1.0 µg/kg/hour with an increment of 1–2 µg/kg/hour to a maximum of 10 µg/kg/hour, continuing using for 2–5 weeks was recommended referring to the treatment experience in relevant studies in recent years (7,16,17). SST’s treatment in the dosage mentioned above had been observed with better curative efficacy and safety. Besides, SST had been used either intravenously in a high dosage (20 µg/kg/hour and even more) or subcutaneously in an extended course for over five months also had been reported clinically, and no apparent adverse reaction was reported (15,18). In conclusion, a larger sample of clinical trials still must demonstrate the appropriate dosage and course of treatment with SST in the treatment of spontaneous chylothorax in pediatric patients.
PICP is a minimally invasive treatment mainly used in conservative therapy for pneumothorax and refractory pleural effusion in adult patients (19), with a highly successful rate of up to 80–90% (20,21). It was firstly reported in the treatment of pediatric chylothorax in 2003 (22) and gradually adopted in recent years with its highly successful rate and excellent curative effect illustrated in some case reports (23-25). During the treatment, PI sclerosant was injected into the thoracic cavity to induce aseptic pleurisy and create adhesions between parietal and visceral pleura, and the thoracic cavity was closed, therefore; this was done to prevent chylous effusion exudates into the thoracic cavity. However, it was reported that the incidence of chest pain after PICP could reach 31–55% (20,21) and side effects caused by sclerosant like allergic reactions, thyrotoxicosis, and nephrotoxicity may occur. While other studies pointed out that the above side effects were usually rare, as long as the concentration and dosage of the PI sclerosant were well controlled to avoid being absorbed excessively by the pleura during the treatment process (26). Therefore, the proper use of PI sclerosant also needs further studies.
In this study, the efficacy of PICP and SST in treating spontaneous chylothorax in pediatric patients was compared. Statistics results showed no significant differences in the mean length of stay (P=0.664), the mean time of thoracic drainage (P=0.440), and the mean time of parenteral nutrition (P=0.793) between PICP group and SST group, which indicated two methods had a specific curative effect. However, when it comes to the adverse reactions and side effects, the symptoms displayed by the patients between PICP group and SST group were different in our study Short-term of low-grade fever, and mild chest pain were the main adverse reactions found in 19 patients who accepted PICP treatment and could be relieved by the ways to divert the children’s attention and physical and drug treatment. Also, anhelation and decreased blood oxygen saturation recorded in 12 cases after PICP treatment were alleviated and returned to normal after the sclerosant was drained out. This condition was identified as an artificial compression on the affected lung caused by the sclerosant during the PICP process, but not an adverse reaction in fact, which could be solved by eliminating the compression after drainage of the effusion. In the SST group, gastrointestinal dysfunction was the main adverse reactions in our study. All the eight children had been found nausea and vomiting, and one patient had mild diarrhea simultaneously after SST treatment. Compared with chest pain and fever, it is more challenging treating gastrointestinal dysfunction in pediatric patients, not to mention the need to use SST to treat chylothorax. Finally, symptomatic treatment cured these eight children, reducing the dosage or stopping using SST. To avoid PI and SST related adverse reactions, we had adopted a modified PICP treatment and controlled the dosage of SST within 10 µg/kg/hour. Twenty-seven patients were discharged after meeting the clinical cure standards, and no complications and recurrence of chylothorax were reported during the follow-up period.
During the treatment, 3 cases of refractory chylothorax were found in the SST group. The daily volume of pleural effusion, the duration of conservative treatment, and the patient’s condition should be carefully considered for the diagnosis of refractory chylothorax. In some earlier studies, refractory chylothorax was defined in the case of drainage volume more than 10 mL/kg/day after 2–4 weeks of conservative treatment (27,28). Moreover, no significant improvement in drainage volume over ten days, or drainage volume of pleural effusion over 30 mL/kg/d, was considered meeting the surgical indications by some researchers (29). Minimally invasive treatment like chemical pleurodesis with PI and other sclerosants (27,28) and surgeries like thoracic duct ligation and (29,30) diaphragmatic fenestration (31) had been reported before. In this study, three children were diagnosed with refractory chylothorax for failing to be cured with SST used intravenously for 12–31 days. Their daily drainage volume had decreased from 24–29 to 8–13 mL/kg/d after treating by SST, but their pleural effusion could not be absorbed completely. Surgery was not adopted considering the elevated risk of anesthesia, and PICP treatment finally cured them. However, up to now, there are still sporadic reports of refractory chylothorax in pediatric patients. More research is needed to prove the treatment protocols of refractory chylothorax.
Our study has limitations. With the limited sample size, the results and conclusion in our paper could not represent the efficacy of the two treatment methods, and publication bias is prone to appear in the SST group. Moreover, research approaches like random control and prospective observation did not be applied in this study. The theory of PICP and SST treatment is also different. PICP works in closing the pleural cavity and induces the reconstruction of the lymphatic circulation by causing pleurisy (32,33), and SST mainly promotes the self-healing of the lymphatic fistula by reducing the generation of lymph fluid and the pressure of lymphatic vessels. With the results and theory, we think that PICP is safer, more direct, and comprehensive, with slight adverse reactions, expected to become a new possibility for minimally invasive treatment of chylothorax in pediatric patients. As for refractory chylothorax cases, the two methods may be combined to explore a comprehensive treatment method. More randomized controlled trials with larger samples are needed for further study to prove the curative efficacy of PICP and SST treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-199
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-199
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-199). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research complied with the Declaration of Helsinki (as revised in 2013) and was approved by the ethical committee of Guangdong Second Provincial General Hospital (No. 20200615-YXKXYJ-LW-01-01). Written informed consent was obtained before the treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Christofe NM, Pessotti CFX, Paiva L, et al. Incidence and Treatment of Chylothorax in Children Undergoing Corrective Surgery for Congenital Heart Diseases. Braz J Cardiovasc Surg 2017;32:390-3. [PubMed]
- Papoulidis P, Vidanapathirana P, Dunning J. Chylothorax, new insights in treatment. J Thorac Dis 2018;10:S3976-S3977. [Crossref] [PubMed]
- Concheiro-Guisan A, Alonso-clemente S, Suarez-albo M. The Practicality of Feeding Defatted Human Milk in the Treatment of Congenital Chylothorax. Breastfeed Med 2019;14:648-53. [Crossref] [PubMed]
- Lai Y, Zheng X, Yuan Y, et al. A modified pleurodesis in treating postoperative chylothorax. Ann Transl Med 2019;7:549. [Crossref] [PubMed]
- Tutor JD. Chylothorax in infants and children. Pediatrics 2014;133:722-33. [Crossref] [PubMed]
- Le Tuan L, Nguyen NC, Tran VH, et al. An uncommon therapeutic option for a challenging cause of pleural effusion. Breathe (Sheff) 2019;15:e69-76. [Crossref] [PubMed]
- Zaki SA, Krishnamurthy MB, Malhotra A. Octreotide Use in Neonates: A Case Series. Drugs R D 2018;18:191-8. [Crossref] [PubMed]
- Long WG, Cai B, Liu Y, et al. Povidone-iodine chemical pleurodesis in treating spontaneous chylothorax in pediatric patients. Ann Palliat Med 2020;9:1004-12. [Crossref] [PubMed]
- Lee CJ, Tsao PN, Chen CY, et al. Prenatal Therapy Improves the Survival of Premature Infants with Congenital Chylothorax. Pediatr Neonatol 2016;57:127-32. [Crossref] [PubMed]
- Bender B, Murthy V, Chamberlain RS. The changing management of chylothorax in the modern era. Eur J Cardiothorac Surg 2016;49:18-24. [Crossref] [PubMed]
- Roehr CC, Jung A, Proquitté H, et al. Somatostatin or octreotide as treatment options for chylothorax in young children: a systematic review. Intensive Care Med 2006;32:650-7. [Crossref] [PubMed]
- Das A, Shah PS. Octreotide for the treatment of chylothorax in neonates. Cochrane Database Syst Rev 2010;08:CD006388. [PubMed]
- Bellini C, Cabano R, De Angelis LC, et al. Octreotide for congenital and acquired chylothorax in newborns: A systematic review. J Paediatr Child Health 2018;54:840-7. [Crossref] [PubMed]
- Dehghan K. Idiopathic Chylothorax in a Term Neonate and Successful Treatment with Octreotide and Medium Chain Triglyceride - Enriched Formula: A Case Report. Int J Pediatr 2019;7:9535-40.
- Saito M, Kamoda T, Kajikawa D, et al. High Dose Octreotide for the Treatment of Chylothorax in Three Neonates. J Neonatal Biol 2016;5:218. [Crossref]
- Azam MN, Majhi SK, Mishra BN, et al. Bilateral spontaneous chylothorax in a newborn and response to octreotide therapy. Int J Contemp Pediatr 2017;4:658-60. [Crossref]
- Church JT, Antunez AG, Dean A, et al. Evidence-based management of chylothorax in infants. J Pediatr Surg 2017;52:907-12. [Crossref] [PubMed]
- Çakır U, Kahvecioğlu D, Yıldız D, et al. Report of a case of neonatal chylothorax that responded to long-term octreotide treatment, and review of the literature. Turk J Pediatr 2015;57:195-7. [PubMed]
- Mierzejewski M, Korczynski P, Krenke R, et al. Chemical pleurodesis - a review of mechanisms involved in pleural space obliteration. Respir Res 2019;20:247-53. [Crossref] [PubMed]
- Agarwal R, Khan A, Aggarwal AN, et al. Efficacy & safety of iodopovi-done pleurodesis: a systematic review & meta-analysis. Indian J Med Res 2012;135:297-304. [PubMed]
- Matus I, Ho P. Ambulatory Iodopovidone Instillation Via Indwelling Pleural Catheters For Malignant Pleural Effusions. J Bronchology Interv Pulmonol 2019;26:290-2. [Crossref] [PubMed]
- Brissaud O, Desfrere L, Mohsen R, et al. Congenital idiopathic chylothorax in neonates: chemical pleurodesis with povidone-iodine (Betadine). Arch Dis Child Fetal Neonatal Ed 2003;88:F531-3. [Crossref] [PubMed]
- Borcyk K, Kamil A, Hagerty K, et al. Successful management of extremely high-output refractory congenital chylothorax with chemical pleurodesis using 4% povidone-iodine and propranolol: a case report. Clin Case Rep 2018;6:702-8. [Crossref] [PubMed]
- Hmami F, Oulmaati A, Bouchikhi C, et al. Congenital chylothorax: rapid and complete response to polyvidone iodine. Arch Pediatr 2014;21:1002-5. [Crossref] [PubMed]
- Murki S, Faheemuddin M, Gaddam P. Congenital chylothorax--successful management with chemical pleurodesis. Indian J Pediatr 2010;77:332-4. [Crossref] [PubMed]
- Bagheri R, Noori M, Rajayi M, et al. The effect of iodopovidone versus bleomycin in chemical pleurodesis. Asian Cardiovasc Thorac Ann 2018;26:382-6. [Crossref] [PubMed]
- Scottoni F, Fusaro F, Conforti A, et al. Pleurodesis with povidone iodine for refractory chylothorax in newborns Personal experience and literature review. J Pediatr Surg 2015;50:1722-5. [Crossref] [PubMed]
- Kasdallah N, Kbaier H, Ben Salem H, et al. Povidone-iodine Pleurodesis for refractory Congenital Chylothorax: a review of literature. Tunis Med 2016;94:834. [PubMed]
- Matsuo S, Takahashi G, Konishi A, et al. Management of Refractory Chylothorax After Pediatric Cardiovascular Surgery. Pediatr Cardiol 2013;34:1094-9. [Crossref] [PubMed]
- Khelif K, Maassarani F, Dassonville M, et al. Thoracoscopic Thoracic Duct Sealing with LigaSure in Two Children with Refractory Postoperative Chylothorax. J Laparoendosc Adv Surg Tech A 2007;17:137-9. [Crossref] [PubMed]
- Kumar TKS, Balduf K, Boston U, et al. Diaphragmatic fenestration for refractory chylothorax after congenital cardiac surgery in infants. J Thorac Cardiovasc Surg 2017;154:2062-8. [Crossref] [PubMed]
- Yamakawa M, Doh SJ, Santosa SM, et al. Potential lymphangiogenesis therapies: Learning from current antiangiogenesis therapies - A review. Med Res Rev 2018;38:1769-98. [Crossref] [PubMed]
- Sáinz-Jaspeado M, Claesson-welsh L. Cytokines regulating lymphangiogenesis. Curr Opin Immunol 2018;53:58-63. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


