
Initial use of voriconazole positively affects outcome of Candida parapsilosis bloodstream infection: a retrospective analysis
Introduction
Over the past 2 decades, Candida parapsilosis (C. parapsilosis) has become the second or the third most common Candida species from blood cultures worldwide and C. parapsilosis infection has been a persistent public health problem with great impact on the health care-associated costs and attributable mortality (1,2). In the UK and North America, Candida species accounts for more than 1/4 of all invasive fungal infections in low birth weight infants and Candida species infection has been a leading cause of neonatal mortality (3). Available studies have revealed the important differences among non-albicans species and Candida albicans (C. albicans), challenging the notion that lessons learned from study of C. albicans will be broadly applicable to other pathogenic Candida.
Fluconazole is generally effective for the treatment of candidaemia/invasive candidiasis (C/IC), but its wide use in clinical practice may be hampered by a potential increase in the infection of fluconazole-resistant Candida spp. (4-6).
It is difficult to investigate the optimal therapy for C. parapsilosis complex infection in randomized controlled clinical trials, and well-designed observational studies become important sources of findings on the therapy of C. parapsilosis complex infection. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tp-20-37).
Methods
Population
A total of 58 newborn infants with C. parapsilosis bloodstream infection (BSI) were admitted to the Department of Neonatal Pediatrics of Shanghai Children’s Hospital between 2014 and 2018, and these patients were included for retrospective analysis. There were 33 males and 25 females; 37 were born by cesarean section and 21 by natural delivery; the average gestational age was 32.6±3.5 weeks (range, 27–42 weeks) and the average birth weight was 1,869±836 g (range, 860–3,850 g). In addition, there were 6 full-term infants and 52 premature infants; 22 patients had very low birth weight and 6 had extreme low birth weight. The maternal diseases were as follows: premature rupture of membranes (n=16), gestational diabetes (n=7), gestational hypertension with or without eclampsia (n=8), Hashimoto thyroiditis (n=1), hypothyroidism (n=1) and gestational diabetes with hyperthyroidism (n=1).
Exclusion criteria were as follows: refusal of anti-fungal therapy (n=7): severe ischemic hypoxic encephalopathy complicated with epilepsy (n=2), recurrent bacterial pneumonia (n=1), severe suffocation (n=1), central hypopnea syndrome (n=1), neonatal necrotizing enterocolitis (NEC) with intestinal stenosis (n=1), bacterial septic shock (n=1).
The diagnostic criteria for fungal septicemia in neonates were as follows (7): (I) the culture of venous blood samples collected from at least 2 different sites showed C. albicans; (II) patients had clinical characteristics of sepsis, including changes in body temperature, feeding intolerance, weight loss, apnea and other manifestations.
According to the initial antifungal drugs used, the patients were divided into fluconazole group (n=30) and voriconazole group (n=21); after 7–10-day treatment, the anti-fungal drugs were replaced if blood fungal culture still showed positive. Fluconazole was intravenously administered at 12 mg/kg/dose, once daily, and voriconazole was intravenously administered at 7 mg/kg/dose, twice daily. Treatment continued for 2 weeks after blood culture showed negative. In the fluconazole group, 20 received voriconazole treatment after blood culture showed still positive following fluconazole treatment for 7–10-day, and the protocol of voriconazole treatment was above mentioned. In the case of fungal infection of central nervous system or other sites, the course of treatment was 4–12 weeks longer. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional board of Shanghai Children’s Hospital (NO.: 2020R046-E01) and informed consent was taken from all the patients.
Methodology
The clinical characteristics (such as gestational age, birth weight, obstetric conditions, clinical symptoms at onset, high-risk factors, clinical manifestations and complications) were collected based on medical records and compared between two groups. The time to blood culture showing negative, time of treatment for fungal sepsis, and the ratio of blood culture showing negative within 8 days after treatment were determined and compared between two groups.
Drug sensitive test
CLS (M27-A) protocol was used to determine the sensitivity to amphotericin B (AmB), fluconazole, 5-fluorocytosine (5-Fc), voriconazole, micafungin (MCF) and itraconazole (ICZ) by determining the minimum inhibitory concentration (MIC). The drug sensitivity was assessed according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and American Clinical and Laboratory Standards Institute (CLSI) criteria (8).
Statistical analysis
Statistical analysis was done using SPSS version 23. Continuous data with a normal distribution are expressed as mean ± standard deviation (SD) and compared with t-test. The data with non-normal distribution are expressed as median and interquartile range (IQR), and compared with non-parametric analysis. Categorical data are expressed as frequencies and percentages, and compared with Chi-square (χ2) test. A value of P<0.05 was considered statistically significant.
Results
General demographic characteristics
There were no significant differences in the general demographic characteristics (gender/age in days/gestational age/weight), maternal and intrapartum factors (delivery method/gestational diabetes/gestational hypertension/asphyxia/premature rupture of membranes) between voriconazole group and fluconazole group (P>0.05) (Table 1).
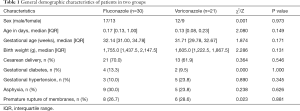
Full table
Clinical manifestations
There were no significant differences in the clinical manifestations at onset (fever/apnea/feeding intolerance), laboratory findings [granulocyte reduction/C-reactive protein (CRP) increase/blood platelet count (BPC) reduction/hepatic dysfunction/electrolyte abnormality/fungal meningitis/shock/coagulation abnormality/gastrointestinal diseases/neonatal respiratory distress syndrome (NRDS)/bronchopulmonary dysplasia (BPD)/patent ductus arteriosus (PDA)] and imaging findings (cranial imaging) between two groups (P>0.05).
Baseline diseases were as follows: perinatal asphyxia (n=13), NRDS (n=24), neonatal NEC (n=7), congenital megacolon (n=3), jejunal volvulus (n=1), ileum perforation (n=1), congenital intestinal malrotation (n=1), and congenital esophageal atresia (n=1). There were no marked differences in these diseases between two groups (P>0.05).
Potential risk factors for fungal septicaemia were as follows: intravenous nutrition (n=51), broad-spectrum antibiotics (35/51; 68.6%), endotracheal intubation (14/51; 27.5%), central venous catheterization (29/51; 56.9%), and prenatal dexamethasone (14/51; 27.5%). There were no marked differences between two groups in these factors (P>0.05). Fundus examination showed slightly retinal vein tortuosity in 2 cases and arterial narrowing in 2 cases (Table 2).
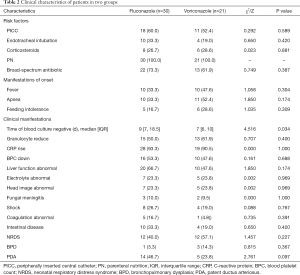
Full table
The drug sensitivity examination showed the MIC was <0.062 µg/mL for voriconazole, <0.5 µg/mL for AmB, <1 µg/mL for fluconazole, <4 µg/mL for 5-Fc, <0.125 µg/mL for ICZ, and <2 µg/mL for MCF. This suggested that C. parapsilosis was sensitive to these drugs.
Treatment results
The median time to blood culture showing negative was 7 (IQR, 6–10) days in the voriconazole group, which was significantly shorter than in the fluconazole group [9 (IQR, 7–18.5) days, P=0.034]. The overall median time negative blood culture was 8 days. After 8-day antifungal therapy, the negative blood culture was noted in 16 patients and positive blood culture in 5 patients in the voriconazole group, with effective rate of 76.1%. In the fluconazole group, the negative blood culture was found in 12 patients and positive blood culture in 16, with the effective rate of 40%. The proportion of patients with negative blood culture in the voriconazole group was significantly higher than in the fluconazole group (χ2=6.535, P=0.011). None died in the voriconazole group but 4 deaths were observed in the fluconazole group (Table 3).

Full table
The median time of treatment for fungal sepsis was 22 (IQR, 20–26) days in the voriconazole group, which was significantly shorter than in the fluconazole group [32 (IQR, 23.5–40) days; P=0.000] (Table 4).
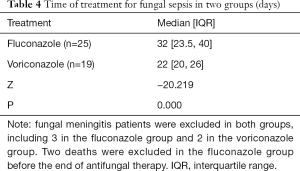
Full table
Discussion
Fungal BSI is an important morbidity in neonates with the mortality ranging from 21% to 76% (9,10). The candidaemia accounts for 30–50% of causes of fungal BSI related death, which is significantly higher than that reported after most bacterial infections (11). C. albicans is the most common fungus, followed by C. parapsilosis. In recent years, the incidence of infection caused by C. tropicalis and C. glabrata is increasing. Recent studies have reported that C. parapsilosis has become the first or second cause of candidaemia in the Latin America, Asia and Europe (12-15).
C. parapsilosis is a commensal fungus in the human skin and it has been widely understood because of its potent ability to form biofilms on the indwelling devices such as central venous catheters. Recent studies have also highlighted the importance of fatty acid synthesis and storage in the pathogenesis of C. parapsilosis infection. The incidence of C. parapsilosis infection in neonates is significantly higher than in other high-risk populations. This suggests a unique susceptibility of neonates to C. parapsilosis infection. In the neonatal intensive care unit (NICU), the risk factors for the development of invasive candidiasis infection include very low birth weight, parenteral nutrition, use of central catheters, abdominal surgery, NEC, mechanical ventilation, endotracheal intubation, and exposure to broad-spectrum antibiotics/steroids (11,16-19). Fungal colonization is an important predisposing factor for developing invasive disease (20). Of note, the C. parapsilosis colonization is several weeks later than the C. albicans colonization in neonates (21), which is consistent with fact that C. parapsilosis is a rare cause of early-onset sepsis in neonates.
Early diagnosis and aggressive treatment improve the outcome of fungal BSI, which is mainly based on isolation of predominant fungus and drug sensitivity test. The symptoms of suspected fungal BSI include fever, lethargy, thrombocytopenia, glucose instability, feeding intolerance, increased requirement for mechanical ventilation and apnoea. Our results showed the symptoms of suspected C. parapsilosis BSI varied among individuals and were characterized by fever (27.4%), apnea (25.5%) and abdominal distension (27.4%).
The treatments of candidemia and other infections of invasive candidiasis is still a challenge in clinical practice. The most common first-line treatment is fluconazole, followed by lipid formulation AmB, caspofungin, voriconazole and other antifungal drugs. The prophylactic use of fluconazole is still controversial (22-24). In the present study, the incidence of fluconazole resistance was high in the initial treatment of C. parapsilosis infection with fluconazole. A recent study found that more C. parapsilosis were resistant to fluconazole as compared to C. albicans (16/32 vs. 1/16, P<0.003 Fisher exact test) (25). AmB deoxycholate is the second most commonly used antifungal drug. In the 2016 America guideline for the management of candidiasis, AmB deoxycholate (1 mg/kg/d) is recommended for the treatment of disseminated candidiasis in neonates. Limited evidence shows the lipid formulation of AmB is preferred because of its high toxicity (26,27). This guideline also recommends the initial treatment of candidiasis with echinomycin antifungal drugs, but these antifungal drugs are not yet very accessible due to its high cost. The adverse events are largely unknown in neonates, but they may include hepatitis and electrolyte abnormalities.
In our study, the initial use of voriconazole achieved good clinical response: rapid presence of negative blood culture, fewer complications and almost no seriously adverse reactions. Despite the in vitro drug sensitivity test showed the C. parapsilosis was sensitive to fluconazole, some patients developed resistance to fluconazole during the clinical treatment. Because MIC interpretive breakpoints for fluconazole and Candida spp. from the clinical data came largely (80%) from mucosal infections and there were very few infections involving strains with elevated fluconazole MICs (28). Loss of allelic variation in the ERG11 promoter may result in a resistant strain that is homozygous for the mutated gene (29).
Voriconazole is a synthetic second-generation, broad-spectrum triazole derivative of fluconazole. It can inhibit the cytochrome P450 (CYP)-dependent 14α-sterol demethylation in the fungi, thereby disrupting the biological synthesis of ergosterol. In Europe, intravenous and/or oral voriconazole is recommended for the treatment of invasive aspergillosis, candidaemia in non-neutropenic patients and fluconazole-resistant serious invasive Candida spp. infection in paediatric patients older than 2 years. Voriconazole is metabolized by hepatic CYP2C19 (the major route). The most common adverse effects of voriconazole include visual disturbance, neurologic/psychiatric disorders, hepatotoxicity, gastrointestinal effects, and skin disorders (30). In the present study, some patients developed mild to moderate liver damage, nearly no kidney damage and mild electrolyte imbalance after 3-day intravenous voriconazole treatment; only 2 patients showed temporary fundal vascular tortuosity or thinning, and all the patients were generally well tolerant. The median time to negative blood culture in the voriconazole group was also significantly shorter than in the fluconazole group; the incidence of negative blood culture after 8-day treatment was higher in the voriconazole group than in the fluconazole group; the complications and adverse effects were comparable between two groups. These findings indicate that the therapeutic effects of voriconazole are superior to those of fluconazole in the treatment of C. parapsilosis BSI.
In conclusion, voriconazole are safety and efficacy in the treatment of C. parapsilosis BSI. Our findings provide a new option for the management of invasive fungal infections and offer evidence on the clinical studies of anti-fungal treatment.
Acknowledgments
Funding: The work was funded by the Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (2019) (No. CXYJY2019MS003).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-37
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-37
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-37). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional board of Shanghai Children’s Hospital (NO.: 2020R046-E01) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Das I, Nightingale P, Patel M, et al. Epidemiology, clinical characteristics, and outcome of candidemia: experience in a tertiary referral center in the UK. Int J Infect Dis 2011;15:e759-63. [Crossref] [PubMed]
- Bassetti M, Taramasso L, Nicco E, et al. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One 2011;6:e24198. [Crossref] [PubMed]
- Gacser A. Adhesins in Candida parapsilosis: Understudied players in virulence. Virulence 2016;7:65-7. [Crossref] [PubMed]
- Cleveland AA, Farley MM, Harrison LH, et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008-2011. Clin Infect Dis 2012;55:1352-61. [Crossref] [PubMed]
- Pfaller MA, Messer SA, Woosley LN, et al. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 2013;51:2571-81. [Crossref] [PubMed]
- Pfaller MA, Jones RN, Castanheira M. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006-2011. Mycoses 2014;57:602-11. [Crossref] [PubMed]
- Chinese Society of Critical Care Medicine. Guidelines for diagnosis and treatment of invasive fungal infections in critically ill patients (2007). Chin J Int Med 2007;46:960-6.
- Pfaller MA, Diekema DJ, Andes D, et al. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 2011;14:164-76. [Crossref] [PubMed]
- Rodriguez D, Almirante B, Park BJ, et al. Candidemia in neonatal intensive care units: Barcelona, Spain. Pediatr Infect Dis J 2006;25:224-9. [Crossref] [PubMed]
- Xavier PC, Chang MR, Nunes MO, et al. Neonatal candidemia in a public hospital in Mato Grosso do Sul. Rev Soc Bras Med Trop 2008;41:459-63. [Crossref] [PubMed]
- Quindós G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev Iberoam Micol 2014;31:42-8. [Crossref] [PubMed]
- Ece G, Samlioglu P, Akkoclu G, et al. The evaluation of the distribution of yeast like fungi 'Candida Species' at a tertiary care center in western Turkey. Int J Med Sci 2012;9:617-20. [Crossref] [PubMed]
- Ericsson J, Chryssanthou E, Klingspor L, et al. Candidaemia in Sweden: a nationwide prospective observational survey. Clin Microbiol Infect 2013;19:E218-21. [Crossref] [PubMed]
- Al-Sweih N, Khan Z, Khan S, et al. Neonatal candidaemia in Kuwait: a 12-year study of risk factors, species spectrum and antifungal susceptibility. Mycoses 2009;52:518-23. [Crossref] [PubMed]
- Moretti ML, Trabasso P, Lyra L, et al. Is the incidence of candidemia caused by Candida glabrata increasing in Brazil? Five-year surveillance of Candida bloodstream infection in a university reference hospital in southeast Brazil. Med Mycol 2013;51:225-30. [Crossref] [PubMed]
- Manzoni P, Jacqz-Aigrain E, Rizzollo S, et al. Antifungal prophylaxis in neonates. Early Hum Dev 2011;87 Suppl 1:S59-60. [Crossref] [PubMed]
- Brecht M, Clerihew L, McGuire W. Prevention and treatment of invasive fungal infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2009;94:F65-9. [Crossref] [PubMed]
- Spiliopoulou A, Dimitriou G, Jelastopulu E, et al. Neonatal intensive care unit candidemia: epidemiology, risk factors, outcome, and critical review of published case series. Mycopathologia 2012;173:219-28. [Crossref] [PubMed]
- Sardana V, Pandey A, Madan M, et al. Neonatal candidemia: a changing trend. Indian J Pathol Microbiol 2012;55:132-3. [Crossref] [PubMed]
- Singhi S, Rao DS, Chakrabarti A. Candida colonization and candidemia in a pediatric intensive care unit. Pediatr Crit Care Med 2008;9:91-5. [Crossref] [PubMed]
- Parm U, Metsvaht T, Sepp E, et al. Risk factors associated with gut and nasopharyngeal colonization by common Gram-negative species and yeasts in neonatal intensive care units patients. Early Hum Dev 2011;87:391-9. [Crossref] [PubMed]
- Manzoni P, Leonessa M, Galletto P, et al. Routine use of fluconazole prophylaxis in a neonatal intensive care unit does not select natively fluconazole-resistant Candida subspecies. Pediatr Infect Dis J 2008;27:731-7. [Crossref] [PubMed]
- Poikonen E, Lyytikainen O, Anttila VJ, et al. Secular trend in candidemia and the use of fluconazole in Finland, 2004-2007. BMC Infect Dis 2010;10:312. [Crossref] [PubMed]
- Sarvikivi E, Lyytikainen O, Soll DR, et al. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. J Clin Microbiol 2005;43:2729-35. [Crossref] [PubMed]
- Ballot DE, Bosman N, Nana T, et al. Background changing patterns of neonatal fungal sepsis in a developing country. J Trop Pediatr 2013;59:460-4. [Crossref] [PubMed]
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;62:e1-50. [Crossref] [PubMed]
- Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 2013;73:919-34. [Crossref] [PubMed]
- Pfaller MA, Diekema DJ, Sheehan DJ. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin Microbiol Rev 2006;19:435-47. [Crossref] [PubMed]
- White TC. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14alpha demethylase in Candida albicans. Antimicrob Agents Chemother 1997;41:1488-94. [Crossref] [PubMed]
- Chen K, Zhang X, Ke X, et al. Individualized Medication of Voriconazole: A Practice Guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Ther Drug Monit 2018;40:663-74. [PubMed]


