Intraoperative MRI in pediatric neurosurgery—an update
Introduction
Social and technical trends in medicine are continuously driving the development of safer and less invasive neurosurgical procedures. Our knowledge of neuroanatomy, in conjunction with the high quality and non-invasive diagnostic modalities of CT and MRI, has better defined surgical targets and approaches. Effective neuro-imaging contributes to the arrival of patients with subtle—or even non-existent on—symptoms to our OR, increasing the stakes of neurosurgical intervention dramatically. Patient and/or parental expectations, education and access to understandable medical information on the internet have increased dramatically, effectively raising the bar for what should be accomplished by attending physicians (1-3). In turn, the need for procedural refinement drives surgical innovation, several aspects of which were reviewed in the pediatric neurosurgical context in a recent article (4). In this article we will focus on the use of intraoperative magnetic resonance imaging (ioMRI) in the pediatric neurosurgical context, beginning with a history of its development followed by a review of current ioMRI configurations and the available outcomes data in the pediatric neurosurgical realm. Outcomes from studies in adults have been included where relevant. Finally, we share some key points about our experience using this real-time, high-resolution modality in the care of young patients.
The double doughnut and the advent of ioMRI in the pediatric neurosurgical context
The use of ioMRI was initiated at the Brigham and Women’s Hospital in Boston, MA in collaboration with General Electric Medical Systems (SIGNA SP, General Electric Medical Systems, Milwaukee, WI, USA) (5-8). This collaboration produced a 0.5 T MRI capable of generating proton density, T1-, T2-, and T2*-weighted images of time-dependent quality in order to allow flexibility in minimizing imaging times while meeting navigation goals. The production model of the “double doughnut” allowed 58 cm of horizontal space for surgeons on either side of the operating table (between each circular magnet), and produced images with a 30-cm-diameter spherical volume. Surgeons viewed these MRI images using monitors placed within the operating gap above their heads (see Figure 1). Two significant issues required resolution in implementing the first ioMRI suite. First, all surgical equipment had to be compatible with the unique electromagnetic environment. Ferromagnetic tools were replaced with nonferrous items and were carefully tested for both potential kinetic properties as well as generation of image artifact before use in the ioMRI suite. Two classes of tools were defined: MR compatible and MR image compatible. MR-compatible tools are all devices that can be used within the 5-gauss line without fear of dangerous physical movement of the tool. Non-ferrous tools, such as titanium, plastic, and carbon fiber are all MR compatible. Some ferrous tools are MR compatible—typically stainless steel that is 300 grade or less, which includes many standard OR tools. MR image compatible tools must be composed of substances that generate minimal magnetic susceptibility artifacts (9,10). Plastic, silicone and carbon fiber create minimal artifacts but, depending on the need, may be reasonably MR image compatible. The second significant issue was how to handle the electricity-requiring instruments, which generate their own electric field; these needed to be switched on and off easily to avoid image degradation.
The double doughnut ioMRI configuration allowed images to be quickly generated at any time during the procedure without moving the patient or the MRI: a set of T2 images over the anatomy of intent adequate to answer questions about localization, lesion residual or hardware placement were generated in about 1 minute without manipulation of the patient’s operative site or position. Rapid, single slice acquisition was possible at a rate of up to one image every 3 seconds. The first pediatric brain tumor craniotomy using ioMRI was performed at the Brigham and Women’s double doughnut ioMRI suite in 1997 by Drs RM Scott and TM Moriarty. The patient was 3 years old with a deep-seated cerebellar tumor. The lesion had been previously unsuccessfully approached using frameless stereotactic navigation. No tumor was identified at the initial surgery and post-op MRI showed a surgical defect near, but not within, the lesion. The patient was then taken to the ioMRI where real time MR navigation provided a precise path to the lesion and a gross total resection was performed and confirmed by ioMRI.
From 2000 until 2010 a similar GE double doughnut ioMRI system (GE Medical Systems, Milwaukee, Wisconsin, USA) was in use at the Kosair Children’s Hospital, a part of Norton Healthcare (Louisville, KY, USA). Between 2000 and 2009 this system was used in 282 pediatric cases: 124 (44%) craniotomies, 71 intracranial cysts (25%) and 45 CSF diversion cases (16%) (11). As time passed, experience yielded lessons on ioMRI usage: not all tumors required ioMRI and the ergonomics and physical structure of the double doughnut limits some approaches. This resulted in a shift in types of cases: during the first 2 years 76% of ioMRI cases were craniotomies and 24% were cysts or CSF diversion cases, between 2007 and 2009 the percentages were 35% and 65%, respectively (12,13).
Enduring lessons from the double doughnut ioMRI
The following cases highlight some optimal uses of ioMRI technology. Case one is a 6-month-old child recently relocated to Kentucky who carried a fetal diagnosis by ultrasound of an arachnoid cyst. Immediate post-natal MRI and repeat MRI 6 months later showed a large but stable arachnoid cyst of the sellar and retro-clival region. On follow up in our center, an MRI was obtained which revealed a large arachnoid cyst centered in the interpeduncular and pontine cisterns and extended anteriorly to the sub-frontal space and caudally approximately to the mid-clivus. Ophthalmological and endocrine evaluations were within normal limits and the patient had no focal deficits and was meeting developmental milestones. Because of its large size, the parents elected to have the cyst shunted. The child was taken to the ioMRI suite for image-guided placement of a cyst-peritoneal shunt. Figure 2A is a pre-operative diagnostic MRI showing the large cyst. In the ioMRI but before incision, Figure 2B shows a good catheter trajectory and Figure 2C-E shows the image-guided placement of a drainage catheter. It is easy to appreciate the precision and real-time-interactive nature of the double doughnut. The catheter was passed through the brain and precisely into the foramen magnum to the apex of the cyst. Figure 2F is a 3-month post-operative MRI showing resolution of the arachnoid cyst. There were no complications and the patient demonstrated dramatically accelerated acquisition of developmental milestones post-operatively.

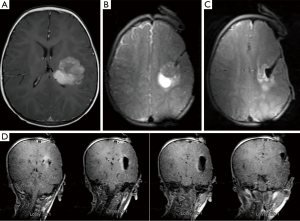
Case two is an example of how ioMRI can aid in tumor navigation and resection adequacy. This patient was a 13-year-old female complaining of a headache for which she received an MRI which demonstrated a right-sided antero-medial temporal lesion. The patient was without neurologic deficit but, because of family anxiety, the non-eloquent location and the indeterminate histopathology, resectional biopsy was elected. Once in the ioMRI, the craniotomy location was determined by pre-incision MR (Figure 3A). A small craniotomy was turned and a catheter was passed through brain parenchyma under image-guidance (Figure 3B,C). After localization with the catheter, a small corticectomy was made and successful resection is shown in Figure 3D. Pathology returned low-grade glioma with neuroepithelial features. The precision possible with interactive, real-time imaging of ioMRI with the patient not having to be transitioned for imaging purposes allows ioMRI to be as minimally invasive as is possible for the job at hand.
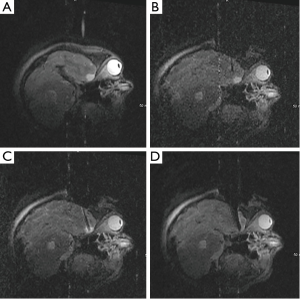
Case three illustrates the use of ioMRI in minimizing the impact of an operation on eloquent tissue. This patient was a 14-month-old male with a headache and a rapidly progressing right-sided hemiparesis. MRI demonstrated a large heterogeneous lesion involving the white matter of the left fronto-temporal region (Figure 4A). Starting anterior to the pre-central gyrus, the ioMRI was used to precisely navigate to the tumor (Figure 4B,C). Figure 4D shows complete resection over four sequential coronal slices. Pathology returned atypical teratoid/rhabdoid tumor. This patient is now 5 years out from surgery without evidence of disease.
Experience revealed several aspects of the double doughnut configuration which limited its viability. The double doughnut design presents real ergonomic challenges and completely precludes some angles of approach. Instruments compatible with operating within a 5-gauss line were often of inferior quality, and the environment imposed sub-optimal lighting and magnification solutions. Perhaps most importantly, experience in the double doughnut revealed that continuous imaging during most neurosurgical procedures is not necessary, raising the possibility of operating near a 5-gauss line rather than inside one. Because of these limitations, the double doughnut configuration is no longer commercially available. The market currently offers two types of solutions for the 5-gauss line constraint.
Literature review of current ioMRI options—low field
The first solution is to reduce the power of the magnet (low-field MRI) in order to keep the patient proximate to the magnet, sacrificing image quality for ease of imaging. There are currently two OR designs using the low-field solution. The first design grew out of work done at Tel Aviv University in Israel by Hadani et al. using a 0.12 T ioMRI consisting of a two discs with a gap in between accommodating the OR and area of the patient to be imaged (14). The discs are mounted directly onto an OR table linked to both an optical and MRI tracking system. When not in use, the apparatus is kept under the table and generates a minimal magnetic field, allowing the use of standard surgical instruments. When in use, the discs are raised to the level of the patient’s head, restricting the angle to the patient and requiring the use of MRI-compatible retractors and clips which minimize the hassle of transitioning to imaging. This system has the significant benefit of a much lower cost since the unit does not require major renovations and RF shielding of the OR. This system has been further developed and marketed by Medtronic (Medtronic, Minneapolis, MN, USA) as the 0.15 T PoleStar Surgical MRI System. The other low field alternative was developed in Erlangen, Germany by Fahlbusch et al. and made use of a two-room suite and an open 0.2 T MRI (Magnetom Open 0.2 T; Siemens AG, Erlangen, Germany) (15). In one room, standard operative equipment is used well away from the magnet. In the adjacent ioMRI room, there are two operative positions. The first is outside the 5-gauss line which allows most standard surgical instruments to be used, but requires MRI compatible retractors and clips as well as a modified microscope. From this position, the patient is easily and quickly transitioned into the scanner. The second position is with the patient within the magnet, allowing for immediate imaging. All surgical instrumentation in the second position must be fully compatible.
Data for the earliest series of patients in both of these configurations has been published in the last decade. For the Polestar, two series of patients, totaling 52 procedures and 49 pediatric patients have been published by Roth et al. in 2006 and Samdani et al. in 2005 (16,17). In Roth’s (16) series, using the PoleStar N-20, there were 20 craniotomies for 18 tumors and 2 cavernomas, 8 shunt placements, 1 biopsy, 1 ETV and 1 temporal lobectomy for epilepsy. Of the 20 craniotomies, 5 had complete resection on first scan, 10 patients had residual tumor with 8 having a discrepancy between amount on imaging and surgeon judgment, and in 5 patients the intraoperative images were of too low quality to be informative (assumed to be unidentified electromagnetic interference by the authors). In each case, lesion resections were confirmed by post-operative imaging. All eight intracranial catheters were successfully placed using the ioMRI in patients with either difficult or multi-cystic targets. The biopsy successfully produced diagnostic tissue, and radiographic adequacy of the biopsy site was easily confirmed in the OR. For the ETV, the ioMRI distorted the endoscopic images and MR guidance was abandoned. No infections or complications attributable to the ioMRI setting were reported, though the authors found that use of the ioMRI adds 30-45 minutes of positioning time and 10-15 minutes of imaging time. While the authors state that the posterior fossa is now able to be imaged by the N-20, none of the patients in this series received an operation for posterior fossa tumors.
In Samdani’s series (17), a PoleStar N-10 was used at 0.12 T. The authors performed 15 craniotomies, 2 shunts, and 1 each of transsphenoidal, craniotomy/transsphenoidal, cranioplasty, and endoscopic biopsy/fenestration. In 8 patients, the operative plan was modified secondary to ioMRI information. In 4 patients additional tumor was revealed and safely resected; in 1 patient, additional hippocampus was removed (for unclear reasons); in 1 patient, fenestration of compartmentalized hydrocephalus was confirmed; in 1 patient lack of communication between cyst and ventricle was visualized intraoperatively and in 1 patient, intraoperative imaging visualization delineated tumor adherence to the optic chiasm. Operative times were increased using ioMRI, but decreased later in the series. For the first 10 patients, ioMRI increased OR time by an average of 138 minutes; this average fell to 84 minutes for the last 10 patients. No complications or infections attributable to ioMRI were noted in this group, though 1 patient did have an ioMRI of too low quality to be used for decision-making. These authors did report successful ioMRI management of a posterior fossa pilocytic astrocytoma in this series in which total resection was obtained following an ioMRI-identified residual. Of note, both authors make mention that the PoleStar is uniquely suited to the pediatric population since they fit more easily between the discs of the under-table MRI system, which allows a greater ease of positioning and imaging of the skull base and posterior fossa.
In 2011, Senft et al. (18) published a prospective, randomized, parallel-group controlled trial of glioma resections done with a PoleStar system. This research group analyzed data from 49 patients with gliomas grade I though IV; 25 patients received resections without ioMRI (control group) and 24 received resections with the ioMRI (intervention group). Intraoperative MR led to extended tumor resection in 8 (33%) of the 24 intervention group patients and in 23 (96%) of these patients there were complete tumor resections on early post op, high-field MRI. For the 25 patients who were part of the control group, only 17 (68%) had a complete resection on post-op scanning (P=0.023). Extent of radiographic resection in the intervention group was greater even in a sub-analysis involving the 12 control patients who had operations using frameless stereotactic navigation (P=0.042). In the one patient without complete resection in the intervention group, the residual was estimated to be 1.2% of original tumor volume while 17 control group patients had post-op volumes between 0.7% and 13.5% of original tumor volume. Perhaps most significantly, at 6 months 16 (67%) of 24 patients in the ioMRI group were stable and eight (33%) had progressed, compared with nine (36%) of 25 patients who were stable in the conventional group and 16 (64%) who had progressed (including one death). Fewer patients had progressive disease in the ioMRI group than the conventional group with a significant P value of 0.046. There were 2 (8%) of 25 control patients and 3 (13%) of 24 intervention patients with new or aggravated post-operative deficits—this was not significantly different and none of the 3 intervention patients had their resection extended after ioMRI imaging.
More recently, in 2011 Paraskevopoulos et al. (19) reported on the use of a low field PoleStar integrated with navigated endoscopy to manage 5 infants: 4 with multi-cystic hydrocephalus presenting with malfunction and 1 patient with a quadrigeminal arachnoid cyst. In no patient were the ioMRI images degraded by the presence of the endoscope and there was a tight correlation between endoscopic anatomy and ioMRI anatomy intra-operatively. In addition, the ioMRI accurately documented the brain shifts accompanying manipulation of the fluid filled cysts. The patient with the arachnoid cyst was fenestrated and was doing well, shunt-free at a follow up of 6 months.
Similar to the PoleStar system, the open 0.2 T configuration has two series of patients totaling 74 patients and 78 procedures published by Nimsky et al. in 2003 and Kremer et al. in 2006 (20,21). In Nimsky’s series (21) of 33 pediatric cases, 9 patients had either cyst drainage or catheter placement, 6 had resection for pharmacoresistant seizures, 6 had sellar tumors (4 treated transsphenoidally, 2 by craniotomy), and 12 had tumors treated by craniotomy. ioMRI contributed passively to decision-making in all patients by confirming goal completion; in the following 5 patients, intraoperative imaging modified their surgical strategy. In 1 patient, intracystic application of contrast showed no communication requiring an additional catheter; 1 patient received intraoperative repositioning of an errant free-hand catheter; 1 patient demonstrated real-time collapse of a craniopharyngioma cyst with significant infolding of the cortex and aspiration was safely halted; in 2 tumor patients ioMRI prompted further resection. No complications were noted, however two patients developed delayed infections; the authors felt this was not attributable to the ioMRI environment since only 1 other patient in the entire 330 ioMRI patient series (including adults) had an infection, yielding an overall ioMRI infection rates of <1%. In no case was ioMRI imaging hampered by low image quality. The authors found that for procedures conducted in the adjoining room, transit times were 10-15 minutes each way for imaging while for procedures done just outside the 5-gauss line, transition times were 1-2 minutes. These authors did not comment on the feasibility of posterior sub-occipital approaches in this ioMRI environment.
Kremer’s group (20) used an identical ioMRI configuration on a series of 45 cases involving 41 pediatric patients. The largest group of patients—31 individuals and 35 cases—were tumors, including several posterior fossa tumors. In this group, the authors report surgical resection was extended after ioMRI in 21 cases (60%). The authors concluded that the use of ioMRI improved the rate of radical tumor resection to 29 patients (83%), as confirmed by early postoperative high-field MR examinations, with only six patients (17%) having small tumor remnants. Several children experienced post-operative neurologic deficits such as ataxia, mutism and nystagmus, 2 patients developed a CSF fistula and 1 patient experienced a wound infection; none of these complications were attributed to the ioMRI environment. In an additional 10 patients, ioMRI was used to guide 4 biopsies, 3 abscess aspirations, 2 catheter placements for cyst drainage, and 1 epilepsy surgery. In all cases, surgical goals were met safely and without post-operative complications. At no point was ioMRI unusable due to image quality in any of the 45 cases.
A much larger series of patients was reported on in 2011 by Muragaki et al. (22). They used a 0.3 T ioMRI which was able to generate informative images in 572 of 574 patients with gliomas of variable grade. This group used ioMRI that was integrated with intraoperative neurological monitoring, repeated histological evaluation of the tumor margins and neurochemical navigation using 5-aminolevulinic acid in a technique they call “information-guided management of gliomas”. This technique enabled maximal possible tumor resection in 569 of their 574 cases—defined as radiographically complete tumor removal when possible and intentionally leaving behind tumor residual in vital functionally important brain areas. Despite the low field strength, the authors report that the 0.3 T ioMRI demonstrated a high sensitivity for residual glioma and that in no case did high-field post-operative MRI images show unexpected residual tumor.
Literature review of current ioMRI options—high field
The second strategy for managing the 5-gauss constraint is to use high-field ioMRI but keep the patient more distant from the MRI during the operation and tolerate longer transport times as well as more detailed patient preparation. This was adopted by two separate research groups who managed the logistics of this strategy in different ways. In 1999, Sutherland et al. reported on a high-field ioMRI (created by Magnex Scientific, Abingdon, Oxon, UK) in which the magnet comes to and from the patient (23). Currently, both 1.5 and 3 T machines are available with a modified bore for better patient positioning and the accommodation of larger patients and patients in different positions. The MRI is mounted on reinforced ceiling track beams and is housed in an alcove adjacent to as many as two operating rooms with the option of using the alcove as a diagnostic MRI room. The ioMRI can be brought into the room in 90 seconds, and preparation for imaging, including safety inspection, draping, and positioning of the magnet, requires approximately 15 to 20 minutes. Generation of T1, spin-echo, and T2 images takes anywhere from 2 to 8 minutes, and getting the surgical site ready for continued operation after imaging takes approximately 2 minutes. Only select instruments need to be ioMRI compatible because most of the operative procedure takes place outside the magnetic field. In addition to standard imaging, the high-field-strength magnet is readily able to incorporate images generated by MRA, functional MRI (fMRI), diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), and MR spectroscopy (MRS) as needed into preoperative, intraoperative, or postoperative planning. The limitations of this configuration derive from the need to manipulate the patient and move the MRI prior to imaging. The time delay, though not enormous, does limit the number of images one can reasonably generate during a procedure. In order to expand the use of high-field ioMRI to procedures requiring continuous or very frequent imaging (e.g., biopsies or tricky catheter placements), a few centers have started to operate from the back end of the scanner using MRI compatible surgical equipment that has undergone vast improvements since the first sets were created in the mid-1990’s. This configuration is currently marketed by IMRIS, Inc (Winnipeg, Manitoba, Canada).
The option of moving the patient to an available ioMRI was developed simultaneously in Minneapolis (Minnesota, USA), Erlangen and Hannover (Germany), and at UCLA (24). Both Phillips (Philips Medical Systems, Best, Netherlands) and Siemens (Siemens Medical Solutions, Erlangen, Germany) offer 1.5 T and 3 T ioMRIs, Siemens does so in conjunction with Brainlab (Brainlab AG, Feldkirchen, Germany). In these systems, the operative wound is covered sterilely and the head coil placed on top before the patient is placed into the MRI scanner, located through double doors. The process of transferring patients to the MRI takes about 20-25 minutes, with imaging times identical to those of a diagnostic magnet. Similar to the moving magnet, these systems allow standard surgical tools to be used in the adjacent OR, allow multiple imaging modalities (MRS, fMRI, DTI, etc.) to be integrated into the operative plan and also allow “back end” procedures to be done with specialized equipment with the patient in the scanner for near continuous imaging.
The first series of pediatric patients treated in a high-field ioMRI suite emerged from Hall’s group in Minneapolis (25). Ten patients were treated in a 1.5 T ioMRI receiving 2 brain biopsies, 5 craniotomies for tumor resection, 2 laminectomies for treatment of a thoracic syrinx and placement of 1 reservoir for a cystic brain stem tumor. In no cases was image quality an issue, all surgical goals were met and no complications or safety events occurred within the ioMRI environment. One child developed a scalp infection; the authors state that the overall infection rate for all patients (85 at that point) within the ioMRI environment was 2%. A second series from the Minneapolis group was published three years later, dealing with posterior fossa lesions in 9 pediatric patients (26). Seven patients received a craniotomy for tumor resection; 4 had complete radiologic resection at first scan, 3 had ioMRI images which demonstrated residual and had further resection at the same sitting, 2 of these three had maximal resection limited by vital structures while 1 patient went on to total resection based on the intraoperative images. The entirety of the resections took place outside the 5-gauss line. Of the remaining 2 patients, 1 had a biopsy and 1 had a craniopharyngioma cyst aspirated and injected with bleomycin; both procedures accomplished their surgical goals. The authors noted that transporting the patients from outside the 5-gauss line into the magnet was not trivial but even rostral aspects of lesions were reachable surgically from that operative location. No complications, infections or imaging issues hindered any procedure.
A large series was published in 2009 by Levy et al. in Calgary with 105 ioMRI procedures in 98 patients (27). There were 100 cranial procedures: 73 craniotomies for supratentorial lesions, 17 craniotomies for infratentorial lesions, 3 transsphenoidal tumor resections, 3 ioMRI guided endoscopic procedures, 2 craniofacial tumor resections, 1 depth electrode placement and 1 Ommaya placement. Of the 5 spinal cases, 4 were tumor resections and 1 was a placement of a syringosubarachnoid shunt. Of the 55 neoplastic cases, 49 received intradissection (ID)-MR imaging; 49% of these (24/49) resulted in further resection. All of these went on to complete resection save 2 patients who had residual disease adjacent to sensitive structures. Imaging prompted further resection in 4 of 9 vascular lesion receiving ID-MR, in 2 of 4 pontine cavernous malformations and in 23% of epilepsy resections. The authors report no difficulty with patient positioning, no ioMRI related complications and no infections; one safety event occurred when a Greenberg retractor clamp was left on the table during an ID-MR but no injuries or damage occurred. An abstract covering a second large series was published by Honeycutt et al. in 2009 (28). These authors make use of a 1.5 T IMRIS system installed in 2007. Their series consisted of 55 tumor extirpations, 26 Chiari I decompressions, 17 epilepsy resections, and 2 spine procedures. Though details are not available for these patients, the authors state that ioMRI images prompted additional surgery in 34 procedures (34%) while decreasing their immediate tumor reoperation rate to 0%. The authors conclude that ioMRI has facilitated aggressive tumor and epilepsy resections.
Most recently, Shah et al. (29) published their comparison of 42 prospectively collected patients who received resection of either brain tumors or focal dysplasia using an IMRIS 1.5 T ioMRI to 103 retrospectively collected patients who received similar resections without the ioMRI. Mean duration of surgery was understandably longer in the ioMRI group at 350 minutes versus 243 minutes (P<0.0001) but more interestingly, the mean length of stay post-operatively was 8.2 days for the ioMRI group versus 6.6 days (P=0.05). In terms of outcomes, there were 8 (7.75) re-operations in the without-ioMRI group but none in the ioMRI group, which only trended towards statistical significance in a 1-tailed test with P=0.06. Complication rates were similar in both groups and the authors comment that reducing the rate of re-operation may help reduce the costs of patient care. In 2013, Avula et al. (30) published a similar study which included only pediatric patients for whom the decision to attempt complete resection of a tumor had been made at a prior tumor board. Retrospective data were collected on 36 ioMRI patients and these were compared to 36 matched non-ioMRI patients resected before the ioMRI was in use. In non-io-MRI patients, 7 had unequivocal evidence of residual tumor on post-operative scans and 5 (14%) received repeat resections within 6 months. In ioMRI patients, 11 had unequivocal residual tumor on ioMRI scans with 10 of these patients receiving further resection during that operative episode. None of the ioMRI patients required repeat surgery during the following 6 months which, compared to the repeat surgery rate within 6 months for the non-ioMRI patients, was statistically significant (P=0.003). These authors conclude that by reducing the number of repeat surgeries in children both monetary and emotional costs are reduced.
High-field MRI offers a wider variety of imaging protocols than low-field and these can be integrated into the navigation. In a paper with relevance to pediatric neurosurgical practice, Sommer et al. (31) describe their experience avoiding awake craniotomies in extra-temporal lesional epilepsy surgery by integrating fMRI information on eloquent anatomy into the ioMRI environment. Twenty percent of the 25 patients received further intraoperative resection based on ioMRI information. Post-operative MRI revealed that all patients had complete resection of their putative epileptogenic lesion, it is important to note that published rates of incomplete extra-temporal lesionectomies range between 71% and 85%. Transient complications occurred in 20% and permanent ones in 12% of patients, which compares favorably with complication rates in traditional awake craniotomy extra-temporal lesional seizure resections. The authors conclude that the fMRI/ioMRI method “may lead to avoidance or marked reduction of postoperative neurological deficits”.
Discussion
In 1994, only one institution had an ioMRI—the 0.5 T double doughnut at the Brigham and Women’s Hospital in Boston, MA. Since that time, the technology has spread quickly (especially when one takes cost into account) to over 100 centers, expanding beyond the academic center to large non-academic clinical centers (12,28). Other than cost, the attributes of an ioMRI system are clear: real time imaging without an increase in infectious or complicative risks. As such, it is no surprise that despite the costs of ioMRI in an increasingly cost-conscious healthcare system, the spread of this technology will continue. What is not clear is which type of ioMRI system is best for which uses, a question that should be readily answerable in the coming years as centers with ioMRI have time to collect data on significant numbers of patients, both adult and pediatric. The literature to date suggests that any type of ioMRI configuration is enormously helpful in attaining surgical goals while limiting exposures, with low-field ioMRIs running a higher risk of image distortion severe enough to disallow operative decision-making. It is reasonable to expect, however, that as experience increases with these low-field ioMRI systems that this difficulty will be overcome. Our ioMRI experience has led us to believe that these low-field systems have a particularly strong role to play in CSF diversion/cyst aspiration and in certain types of tumors.
Cysts and difficult CSF diversion situations can be vexing neurosurgical problems. While static frameless stereotaxy can aid surgeons in these cases, they cannot update anatomical relationships as fluid is removed and/or redirected and they do not provide post-placement catheter position verification. In addition, for particularly deep cysts that approximate dense and fragile anatomy, frameless stereotactic navigation is of no use at all. In the case related above, we were able to safely advance a catheter into highly sensitive anatomy and confirm that as fluid was drained no potentially damaging tissue shifts were occurring. The ioMRI is also particularly useful in small (hard to locate) tumors which are poorly differentiated from their surrounding parenchyma by sight (often low-grade gliomas). By using the ioMRI in the above tumor case, we were able completely resect the lesion with minimal damage to the surrounding tissue and then confirm complete resection before the dura was closed. As many authors have pointed out, total resection for pediatric tumors is crucial for attaining progression free survival (12). Large tumors as well can benefit from ioMRI as margins can be obscured by the surrounding edema and large shifts in anatomy take place as the tumor is de-bulked. Often, the ioMRI can guide a quick de-bulking and can then help to identify tissue planes obscured by edema in order to dissect around the margins of large tumors.
The recent development of integrating high-field ioMRI with fMRI and other modalities for seizure resection is likely going to expand in the coming years. Allowing safe, reliably total, resection of lesion seizure foci without the risks of awake craniotomy promises a more objective and targeted approach to difficult cases of extra-temporal seizure resection. In children, whose age makes awake craniotomies harder to achieve, this modality may offer a real chance to expand the availability of near-eloquent resection.
Given the intuitive appeal of ioMRI and its quick adoption despite the costs, the neurosurgical community has leapfrogged past a formal assessment of the cost-effectiveness of this technology. As more centers install this technology, some important questions which might deserve some attention are as follows. First, it will be important to address important ioMRI techniques and surgical nuances in order to increase overall operative efficiency in the ioMRI environment. Second, it will be important to further clarify the strengths and weakness of both different field strengths as well as operative suite configurations to allow interested centers to pick the most appropriate system for their needs. Finally, it may be a benefit at the population level to determine how many ioMRI operative suites are most cost efficient. The market forces to which the healthcare system is subject might best be mitigated by a careful, profession-driven, analysis in order to avoid the redundancy of an extremely expensive technology.
Summary
Even in an era of cost-conscious medicine, ioMRI technology begun to spread globally. Limited literature indicates that this technology is safe and can bring multiple benefits to several types of neurosurgical procedures. In the pediatric context, the present authors have found the ioMRI is most helpful for:
- Small, discrete tumors with poor differentiation from surrounding brain tissue;
- Large tumors with surrounding edema that:
- Obscure differentiation from the surrounding brain parenchyma, and/or;
- Cause mass effect that will alter the anatomy significantly from preoperative images after craniotomy.
- Catheter/CSF diversion when:
- The target is small, and/or;
- The target is surrounded by eloquent anatomy, and/or;
- Verification of catheter placement and not merely CSF return is required (i.e., multiloculated or intraventricular cysts).
With time, experience should better clarify which field strengths, OR configurations and techniques are best suited to the many potential procedures which might benefit from an ioMRI environment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dawn AG, Lee PP. Patient expectations for medical and surgical care: a review of the literature and applications to ophthalmology. Surv Ophthalmol 2004;49:513-24. [PubMed]
- Neuberger J. The educated patient: new challenges for the medical profession. J Intern Med 2000;247:6-10. [PubMed]
- Sitzia J, Wood N. Patient satisfaction: a review of issues and concepts. Soc Sci Med 1997;45:1829-43. [PubMed]
- Ahn ES, Scott RM. Transcendent leadership: recent advances in pediatric neurosurgery. Clin Neurosurg 2007;54:19-22. [PubMed]
- Black PM, Moriarty T, Alexander E 3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery 1997;41:831-42. [PubMed]
- Alexander E 3rd, Moriarty TM, Kikinis R, et al. Innovations in minimalism: intraoperative MRI. Clin Neurosurg 1996;43:338-52. [PubMed]
- Alexander E 3rd, Moriarty TM, Kikinis R, et al. The present and future role of intraoperative MRI in neurosurgical procedures. Stereotact Funct Neurosurg 1997;68:10-7. [PubMed]
- Moriarty TM, Kikinis R, Jolesz FA, et al. Magnetic resonance imaging therapy. Intraoperative MR imaging. Neurosurg Clin N Am 1996;7:323-31. [PubMed]
- Shellock FG, Shellock VJ. Additional information pertaining to the MR-compatibility of biopsy needles and devices. J Magn Reson Imaging 1996;6:411. [PubMed]
- Bendel LP, Shellock FG, Steckel M. The effect of mechanical deformation on magnetic properties and MRI artifacts of type 304 and type 316L stainless steel. J Magn Reson Imaging 1997;7:1170-3. [PubMed]
- Mutchnick IS, Moriarty TM. Neurosurgical uses for intraprocedural magnetic resonance imaging. Top Magn Reson Imaging 2005;16:383-95. [PubMed]
- Moriarty TM, Titsworth WL. The evolution of iMRI utilization for pediatric neurosurgery: a single center experience. Acta Neurochir Suppl 2011;109:89-94. [PubMed]
- Vitaz TW, Hushek S, Shields CB, et al. Changes in cyst volume following intraoperative MRI-guided Ommaya reservoir placement for cystic craniopharyngioma. Pediatr Neurosurg 2001;35:230-4. [PubMed]
- Hadani M, Spiegelman R, Feldman Z, et al. Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms. Neurosurgery 2001;48:799-807; discussion 807-9. [PubMed]
- Steinmeier R, Fahlbusch R, Ganslandt O, et al. Intraoperative magnetic resonance imaging with the magnetom open scanner: concepts, neurosurgical indications, and procedures: a preliminary report. Neurosurgery 1998;43:739-47. [PubMed]
- Roth J, Beni Adani L, Biyani N, et al. Intraoperative portable 0.12-tesla MRI in pediatric neurosurgery. Pediatr Neurosurg 2006;42:74-80. [PubMed]
- Samdani AF, Schulder M, Catrambone JE, et al. Use of a compact intraoperative low-field magnetic imager in pediatric neurosurgery. Childs Nerv Syst 2005;21:108-13. [PubMed]
- Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncology 2011;12:997-1003. [PubMed]
- Paraskevopoulos D, Biyani N, Constantini S, et al. Combined intraoperative magnetic resonance imaging and navigated neuroendoscopy in children with multicompartmental hydrocephalus and complex cysts: a feasibility study. J Neurosurg Pediatr 2011;8:279-88. [PubMed]
- Kremer P, Tronnier V, Steiner HH, et al. Intraoperative MRI for interventional neurosurgical procedures and tumor resection control in children. Childs Nerv Syst 2006;22:674-8. [PubMed]
- Nimsky C, Ganslandt O, von Keller B, et al. Preliminary experience in glioma surgery with intraoperative high-field MRI. Acta Neurochir Suppl 2003;88:21-9. [PubMed]
- Muragaki Y, Iseki H, Maruyama T, et al. Information-guided surgical management of gliomas using low-field-strength Intraoperative MRI. Acta Neurochir Suppl 2011;109:67-72. [PubMed]
- Sutherland GR, Kaibara T, Louw D, et al. A mobile high-field magnetic resonance system for neurosurgery. J Neurosurg 1999;91:804-13. [PubMed]
- Hall WA, Truwit CL. Intraoperative Magnetic Resonance Imaging. Acta Neurochir Suppl 2011;109:119-29. [PubMed]
- Hall WA, Martin AJ, Liu H, et al. High-field strength interventional magnetic resonance imaging for pediatric neurosurgery. Pediatr Neurosurg 1998;29:253-9. [PubMed]
- Lam CH, Hall WA, Truwit CL, et al. Intra-operative MRI-guided approaches to the pediatric posterior fossa tumors. Pediatr Neurosurg 2001;34:295-300. [PubMed]
- Levy R, Cox RG, Hader WJ, et al. Application of intraoperative high-field magnetic resonance imaging in pediatric neurosurgery. J Neurosurg Pediatr 2009;4:467-74. [PubMed]
- Honeycutt J, Roberts R, Macomber D, et al. Consecutive pediatric intraoperative high-field MR imaging cases. Cook Children’s Hospital Clinical Research Day Abstract Booklet [Internet]. 2009 [cited 2011 Sep 12]. p. 12. Available online: https://www.cookchildrens.org/SiteCollectionDocuments/Research/RD_Abstract-Booklet_2009.pdf
- Shah MN, Leonard JR, Inder G, et al. Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr 2012;9:259-64. [PubMed]
- Avula S, Pettorini B, Abernethy L, et al. High field strength magnetic resonance imaging in paediatric brain tumour surgery — its role in prevention of early repeat resections. Childs Nerv Syst 2013;29:1843-50. [PubMed]
- Sommer B, Grummich P, Coras R, et al. Integration of functional neuronavigation and intraoperative MRI in surgery for drug-resistant extratemporal epilepsy close to eloquent brain areas. Neurosurgical Focus 2013;34:E4. [PubMed]



