Laser induced thermal therapy (LITT) for pediatric brain tumors: case-based review
Introduction
Over the past decades, neurosurgical advances have focused on developing minimally invasive procedures that would be as effective and as cost-efficient as open surgery but with less discomfort and morbidity for the patient. Even though the use of lasers is not new in neurosurgery, recent advances integrating Laser induced thermal therapy (LITT) with magnetic resonance imaging (MRI) to produce magnetic resonance thermal imaging (MRTI) have created new options for the treatment of tumors located in surgically challenging locations (either anatomically or functionally) that will represent an intrinsic comorbidity by the approach itself.
As new applications for this technology are developing and variations on its use are discussed, we want to present a case-based review of the current uses of minimally invasive MRI-guided laser interstitial thermal therapy (MRgLITT) ablation in pediatric brain tumors.
Laser induced thermal therapy (LITT)
LITT uses light absorption to create a precise and minimally invasive injury to targeted tissue inducing acute coagulation necrosis (1). It is hypothesized that the thermal ablation also may cause a concentric ring of injury with cellular apoptosis driven by DNA fragmentation (2).
LITT was investigated for treatment of cerebral neoplasms as early as 23 years ago (3). Different non-invasive methods using thermal energy to treat brain malignancies such as ultrasound, microwaves (3,4) and stereotactic radiofrequency appeared promising on filling the void between medical therapy and surgical resection (5-9). However, these technologies did not gain popularity due to a lack of precision in thermal source, limited ablative volume and an inability to monitor tissue damage on real time. Subsequently, LITT was performed with simultaneous T1-weighted MRI, allowing qualitative but not quantitative visualization of laser heating (10-14) with precise real time monitoring of the thermal ablation zone (15). Pre-clinical studies (1,16,17) had shown that lasers can deliver controlled, customizable, and precise thermal ablation that can be accurately monitored with MRI; this is called MRgLITT.
Technology
Two technologies have been reported for MRI guided LITT: the Visualase system (Visualase, Inc., Houston, TX, USA) and the Neuroblate® system (Monteris Medical Corporation, Plymouth, Minnesota) (18). Both systems can be used with intraoperative MRI, navigation or stereotactic systems and provide predictive thermal dosage lines to estimate ablation volume.
We and others (19-21) have previously reported the experience with the Visualase system; this is a FDA-cleared, MR-compatible LITT technology consists of a 15 W, 980 nm diode laser, cooling apparatus, and an image-processing workstation (21). The laser applicator is 1.6 mm in diameter, and contains a silicon fiberoptic core with a light-diffusing tip, surrounded by an outer cooling sheath. The cooling sheath helps prevent tissue carbonization by circulating room-temperature saline in the closed system, hence altering the location of deposition of light energy. The thermal energy induces damage to the tumor cells causing necrosis of the targeted tissue, as well as possibly cell apoptosis (1,22).
The MR-compatible applicator can be used during MR image acquisition. The workstation is positioned outside the MR scanning area, and is used to plan and monitor ablation. Proton resonance frequency shift relationship (4,21) is used to observe changes in temperature every three seconds (using single-plane viewing) or six seconds (using bi-planar viewing), making thermal imaging almost “real-time”. Color-coded temperature maps are overlaid on MR images. An “irreversible damage zone”, as defined by the Arrhenius rate model of thermal tissue destruction (1), is calculated based upon the time and temperature history data from individual voxels in the treatment zone. The model takes into account both the time and temperature dependency of protein denaturation rate processes to estimate cellular death (23). Additionally, the software can be used to associate limit temperatures with voxels in the image, which, if exceeded, will cause automatic deactivation of the laser. Maximal temperature limits with automatic shutoff can be set as an additional safety step for critical structures, to avoid damage (21).
Surgical technique
We have used general anesthesia for the pediatric patients, even though sedation and local anesthesia can be used for older and cooperative children. We prefer the stereotactic Leksell coordinate frame system (Elekta AB, Stockholm, Sweden) for small and/or deep targets (i.e., hypothalamic hamartomas). Alternatively, navigation systems can be used for lesions that are more superficial and where a margin of error of more than 2 mm is acceptable. Once the Leksell cranial frame is applied and the MR-lucent fiducial box is securely fastened to the head frame, we have typically transferred the patient directly from the operating room table to the MRI table.
The patients have been transported to the MR suite and a volume image series is acquired (1.5-T GE Signa EXCITE scanner, GE Healthcare, Waukesha, WI); T1W localizers are acquired, followed by a T1W 3D SPGR volumetric study covering the full extent of the both patient anatomy and the MR-lucent “rods” of the Leksell fiducial box (TR =500, TE =12, FA =90 deg, FOV =240 mm × 240 mm, 1.2 mm slice thickness, 256 pix × 256 pix). All images are transferred to a Stryker Navigation image-guided surgery (IGS) workstation for co-registration of the image volumes acquired in the reference system of the MRI scanner to that of Leksell frame coordinate system. Target and entry loci are identified on multi-planar and 3D volume reconstructions of the MRI study, trajectories are planned, and the X, Y, Z, Arc, and Ring settings of the Leksell frame are calculated; all of these calculations are determined using the navigation software.
The patient is then transported back to the neurosurgery operating room, the arc is sterilely attached to the frame and appropriate coordinates are set. Local anesthesia is applied and a 5 mm skin cut is made. A Leksell’s drill guide set of 4.0-mm-diameter is used to guide a 3.2-mm-diameter Salczman drill through the cranium. A steel rod is inserted to test dural opening. If biopsy is indicated, the 2.5 mm drill guide set and a Sedan needle biopsy kit are used to stereotactically take tumor samples. Then, a specially constructed MR-compatible skull bolt (Figure 1) is fitted over a steel rod that is placed into the 2.1 mm Leksell drill guides to keep the cranial anchor at the calculated angle. The threaded end of the cranial anchor is screwed into the drilled hole, forming a rigid anchor point for insertion of the laser applicator, which is inserted through the cranial anchor and into the target using the Leksell’s frame guides. Additional laser probes can be inserted for the same session, considering that each laser will accomplish about 2 cm of ablative area. The arc is then carefully detached from the frame without altering the anchor’s angle.
With the laser applicator in place, steri-strips are used to mark the depth of the applicator in the MRI compatible skull bolt as well as the depth of the laser fiber in the cooling cannula. The patient is then transported back to the MR suite in the MRI-bed to begin the ablation. Extreme care on not bending or breaking the laser(s) on this step should be taken.
Once the patient is positioned in the MRI machine, the applicator cooling lines and the laser fiber are passed through a wall waveguide into the MRI control room, where they are connected to the cooling pump and laser, respectively. Pre-treatment images and T1-weighted volumetric images are acquired, and a T1 spin echo in a plane containing the applicator is chosen for target planning on the thermal therapy system. MRTI is accomplished using a fast RF-spoiled gradient recalled echo (GRE) sequence (field of view, 24 cm × 24 cm; matrix, 256 cm × 128 cm; echo time, 20 ms; repetition time, 45 ms; flip angle, 30 degrees; band width, 12.6 kHz), which required approximately 6 seconds for a single acquisition when two planes are selected for visualization of the treatment and 3 seconds if only one plane is used and which is run repeatedly during the treatment.
Laser therapy is performed using the Visualase Thermal Therapy System described previously. An initial treatment plan is designed by the neurosurgeon with one to two high-temperature limit points set near the applicator to limit temperatures below 90 °C (to avoid carbonization and thermal damage to the cooling cannula) and 1 to 3 low- temperature limits typically set either near the margin of the desired thermal ablation zone or at the border of critical structures to hold temperatures below 45 °C, protecting these structures from injury.
Initially, a low-power test pulse (3-4 W, 30-60 s) is applied that is sufficient to allow thermal visualization and further verification of the inner applicator position without causing permanent thermal damage. Subsequently, a treatment dose is applied per the criteria of the neurosurgeon. Laser ablation is either stopped manually by the neurosurgeon or automatically by the system if any of the temperature limits are exceeded. Depending on the size of the estimated thermal ablation zone as calculated by the system software, a second or third thermal ablation zone can be added by withdrawing the laser fiber inside the cooling cannula or the cooling cannula itself by several millimeters, usually no more than 5 mm, if an overlapped ablation is to be achieved. The initial estimated thermal ablation zone is saved by the software and superimposed onto the image of subsequent ablations to compile an image of the cumulative area of ablation.
Once the surgeon is satisfied with the thermal ablation area, post-procedure FLAIR, T1-weighted gadolinium enhanced, or diffusion-weighted images are acquired to visualize the damage. If the margins of the thermal injury appeared sufficient, the patient is removed from the MRI machine and transported back to the recovery room for removal of the laser device, the MR-compatible skull anchor and the Leksell frame. The entry site is then re-approximated with a 3-0 Monocryl suture.
After the procedure, the patient is observed in the intensive care unit (ICU) or step-down care unit overnight, being discharged home on post-operative day 1 or 2. A 2-week dexamethasone taper is initiated in the operating room to prevent significant post-treatment cerebral edema. A proton pump inhibitor is also prescribed in conjunction with the steroids for gastric protection.
Follow-up visits have typically taken place at 15, 90, and 180 and 365 days after treatment. MRI of brain is performed 3, 6 and 12 months post-ablation to assess tumor volume. Additionally, the patients or their family members are encouraged to fill out quality of life (QOL) and performance questionnaires.
LITT in pediatric brain tumors
Twenty years ago, Kahn et al. (10) reported use of LITT for the treatment of glioma in eloquent regions that were not amenable to surgical resection. Since then, the use of LITT in neurosurgery has been described for the treatment of metastatic tumors, high- grade glioma (11,14,19,24-26) and radiation-induced tissue necrosis (27,28). Additional applications of LITT in the adult population were described by Jethwa et al. (29) in their initial series that included primarily supratentorial tumors, tumors in the deep gray matter, brainstem, posterior fossa, ventricular system and skull base. The first case report of LITT in the pediatric population was also reported by Jethwa in which they treated a thalamic PNET in 2011.
The use of LITT in children was initially limited to deep-seated brain lesions such as thalamic tumors or hypothalamic hamartoma. The ideal lesions for this treatment are deep-seated circumscribed tumors no bigger that 2.5- or 3-cm. where open surgical approach will involve collateral morbidity and where the resulting mass effect of the initial edema could be tolerated. However, the patient selection for this treatment has expanded as we have learned how to use the device more efficiently, and we have treated different types of tumors even in more superficial locations (unpublished series including thalamic tumors, bilateral choroid plexus xanthogranuloma, brainstem glioma, recurrent medulloblastoma, vermian tumors, hypothalamic pylocytic astrocytoma, hamartoma, frontal ganglioglioma, subependymal giant cell astrocytoma (SEGA) and mesial temporal cavernoma). Follow-up MRIs have shown volume reduction at 3, 6, 9, and 12 months post-ablation. Of note, patients with recurrent malignancies resistant to chemotherapy, i.e., medulloblastoma or hypothalamic pylocitic astrocytoma, have been successfully treated; therefore, if longer follow-up on these patients shows efficacy, LITT could eventually be considered as a first-line option of treatment for residual or recurrent tumors. The results are encouraging thus far, but additional long-term follow up is needed.
The application of LITT requires neurosurgical expertise in functional procedures. Planning on feasible, safe and effective laser-fiber trajectories, calculated damage and overlapping ablations is mandatory on surgical preparation. Advances in MRI imaging provide useful adjunct information when designing a treatment plan for pediatric brain tumors. The incorporation of MRI-tractography and functional images will add to the planning of the ablation zone in eloquent areas.
While the use of LITT for pediatric brain tumors has shown promising results thus far, additional research is necessary to further understand the long-term outcomes and to compare this treatment to other management strategies. In our experience, the power of the laser is applied differently according to the tumor type and location. While most of the lesions can be treated with 70% laser-power (of 15 Watts), more conservative ablations (60% laser-power or less) are recommended for brainstem lesions or lesions close to important white matter tracts. Tubers or solid tumors could tolerate a 75% laser-power ablation without producing post-ablation significant edema. In this regard, one important factor to consider is the use of the 980-nm laser in comparison to other forms of ablative treatment. For example, a 2.5-cm metastatic lesion would require a 16-minute ablation by radiofrequency, 73 minutes with a 1,064-nm Nd:YAG laser, but only 6 minutes with a 980-nm laser (29). Our experience suggests that the use of lower laser powers but longer ablations in tumors located in eloquent areas will further reduce the usually mild post ablation edema (21,29) that usually peaks at 3 to 4 days. This edema can be managed with dexamethasone taper and gastric protection over two weeks but additional cases are still needed to compare different forms, duration and laser powers in their effect upon edema.
In theory, any tumor type can be treated with thermal energy; however, the response to LITT is variable among different tumor types and among tumors of the same type. Jethwa et al, hypothesized that the response to thermal ablation is dependent on perfusion to the tumor, but this theory has not been rigorously tested (29).
Case illustration
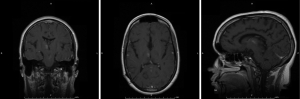
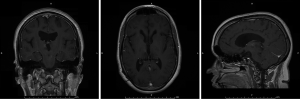
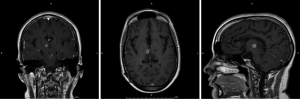
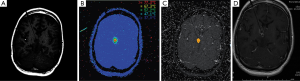
The patient was a sixteen year-old female who presented at the age of twelve with headaches and left arm intentional tremor. An MRI at that time showed tectal and bilateral thalamic lesions consistent with low grade glioma, in addition to hydrocephalus. She had a ventriculoperitoneal shunt placed to treat the hydrocephalus, and the patient was followed by Oncology with serial MRIs. Her tumor remained stable for four years, subsequent to which a new area of enhancement in the right thalamic region was found (Figure 2). At this time, due to the changes in tumor appearance on MRI, surgical options were discussed with the patient. Given deep location of the tumor, an MRI guided stereotactic approach was offered and for biopsy followed by laser ablation of the lesion. After biopsy, the Visualase laser fiber was placed at the center of the lesion; two thermal ablations (11 watts for thirty-one seconds and ten watts for thirty seconds) were performed. Post-ablation MRI showed a hyperemic ring surrounding the thalamic lesion (Figure 3), which is the expected appearance in these lesions post-ablation. There were no procedure-related complications, and the patient was discharged the next day. Pathology was consistent with a grade two ependymoma.
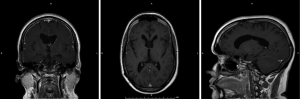
The tumor decreased in size by approximately 70% at three (Figure 4) and six months (Figure 5) and was barely visible at one year following ablation (Figure 6). The tumor has not recurred after two years and no other co-adjuvant treatment was done.
Conclusions
LITT offers a minimally invasive option of treatment of pediatric brain lesions that otherwise will require open procedures with significant comorbidity associated to the approach. As we are currently collecting the evidence of the different applications of this technology on different types of brain tumors in children, the results are promising and could eventually change some of the current protocols of treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- McNichols RJ, Gowda A, Kangasniemi M, et al. MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surg Med 2004;34:48-55. [PubMed]
- Heisterkamp J, van Hillegersberg R, Zondervan PE, et al. Metabolic activity and DNA integrity in human hepatic metastases after interstitial laser coagulation (ILC). Lasers Surg Med 2001;28:80-6. [PubMed]
- Bettag M, Ulrich F, Schober R, et al. Stereotactic laser therapy in cerebral gliomas. Acta Neurochir Suppl (Wien) 1991;52:81-3. [PubMed]
- Jethwa PR, Lee JH, Assina R, et al. Treatment of a supratentorial primitive neuroectodermal tumor using magnetic resonance-guided laser-induced thermal therapy. J Neurosurg Pediatr 2011;8:468-75. [PubMed]
- Blume WT, Parrent AG, Kaibara M. Stereotactic amygdalohippocampotomy and mesial temporal spikes. Epilepsia 1997;38:930-6. [PubMed]
- Catenoix H, Mauguière F, Guénot M, et al. SEEG-guided thermocoagulations: a palliative treatment of nonoperable partial epilepsies. Neurology. 2008;71:1719-26. [PubMed]
- Guénot M, Isnard J, Ryvlin P, et al. SEEG-guided RF thermocoagulation of epileptic foci: feasibility, safety, and preliminary results. Epilepsia 2004;45:1368-74. [PubMed]
- Liscak R, Malikova H, Kalina M, et al. Stereotactic radiofrequency amygdalohippocampectomy in the treatment of mesial temporal lobe epilepsy. Acta Neurochir (Wien) 2010;152:1291-8. [PubMed]
- Schmitt FC, Voges J, Buentjen L, et al. Radiofrequency lesioning for epileptogenic periventricular nodular heterotopia: a rational approach. Epilepsia 2011;52:e101-5. [PubMed]
- Kahn T, Bettag M, Ulrich F, et al. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J Comput Assist Tomogr 1994;18:519-32. [PubMed]
- Schwabe B, Kahn T, Harth T, et al. Laser-induced thermal lesions in the human brain: short- and long-term appearance on MRI. J Comput Assist Tomogr 1997;21:818-25. [PubMed]
- Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser irradiation of recurrent glioblastomas. J Magn Reson Imaging 2005;22:799-803. [PubMed]
- Meister D, Hübner F, Mack M, et al. MR thermometry for laser-induced thermotherapy at 1.5 Tesla. Rofo 2007;179:497-505. [PubMed]
- Prudhomme M, Mattéi-Gazagnes M, Fabbro-Peray P, et al. MRI thermodosimetry in laser-induced interstitial thermotherapy. Lasers Surg Med 2003;32:54-60. [PubMed]
- Carpentier A, Chauvet D, Reina V, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med 2012;44:361-8. [PubMed]
- Kangasniemi M, McNichols RJ, Bankson JA, et al. Thermal therapy of canine cerebral tumors using a 980 nm diode laser with MR temperature-sensitive imaging feedback. Lasers Surg Med 2004;35:41-50. [PubMed]
- McNichols RJ, Kangasniemi M, Gowda A, et al. Technical developments for cerebral thermal treatment: water-cooled diffusing laser fibre tips and temperature-sensitive MRI using intersecting image planes. Int J Hyperthermia 2004;20:45-56. [PubMed]
- Hawasli AH, Ray WZ, Murphy RK, et al. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for subinsular metastatic adenocarcinoma: technical case report. Neurosurgery 2012 ;70:332-7; discussion 338. [PubMed]
- Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery 2008;63:ONS21-8; discussion ONS28-9.
- Curry DJ, Gowda A, McNichols RJ, et al. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav 2012;24:408-14. [PubMed]
- Tovar-Spinoza Z, Carter D, Ferrone D, et al. The use of MRI-guided laser-induced thermal ablation for epilepsy. Childs Nerv Syst 2013;29:2089-94. [PubMed]
- Heisterkamp J, van Hillegersberg R, Zondervan PE, et al. Metabolic activity and DNA integrity in human hepatic metastases after interstitial laser coagulation (ILC). Lasers Surg Med 2001;28:80-6. [PubMed]
- Welch AJ, van Gemert MJC, Star WM, et al. Definitions and overview of tissue optics. In: Welch AJ, van Gemert MJC. eds. Optical-thermal response of laser-irradiated tissue. New York: Plenum Press, 1995:15-46.
- Leonardi MA, Lumenta CB. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: clinical expierence. Minim Invasive Neurosurg 2002;45:201-7. [PubMed]
- Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol 2006;59:208-15. [PubMed]
- Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med 2011;43:943-50. [PubMed]
- Torres-Reveron J, Tomasiewicz HC, Shetty A, et al. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol 2013;113:495-503. [PubMed]
- Fabiano AJ, Alberico RA. Laser-interstitial thermal therapy for refractory cerebral edema from post-radiosurgery metastasis. World Neurosurg 2014;81:652.e1-4.
- Jethwa PR, Barrese JC, Gowda A, et al. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery 2012;71:133-44; 144-5.