Non-accidental trauma in pediatric patients: a review of epidemiology, pathophysiology, diagnosis and treatment
Introduction
The history of non-accidental trauma (NAT) begins with forensic physicians and radiologists. In 1860, a French forensic physician, Ambroise Tardieu, published an article describing in detail 32 cases of physical abuse and neglect in which he was involved (1). In his article, he warns about the importance of all physicians being aware of suspicious clinical findings: “But it remains that these cases are multiplying, that they provoke indignation, that they must not catch off guard the physician, often the only one capable of denouncing the crime to the legal authorities, and that they must not remain unknown to the forensic expert enjoined by the courts to explain its true character and to unveil all the circumstances of the deed (1)”.
In 1946, John Caffey, a pediatric radiologist, reported the association of multiple fractures and subdural hemorrhages in six infants (2). He remained suspicious of a traumatic origin despite adamant denials from the parents. Frederic N. Silverman, also a radiologist, made the claim that “spontaneous” fractures in a child with a normal bony structure are caused by unrecognized trauma (3). Caffey subsequently urged his radiological colleagues to recognize that multiple fractures are caused by trauma and to beware of false histories given by parents. He later went on to coin the term “whiplash shaken infant syndrome” in 1974 (4). In 1962, C. Henry Kempe published “The Battered Child Syndrome,” describing the clinical features of physical abuse in children (5). This publication ultimately led to the creation of protection laws for children including the mandate for reporting maltreatment in the United States (6).
According to the Centers for Disease Control (CDC) and Prevention of the United States, abusive head trauma (AHT) can be defined as an injury to the skull or intracranial contents of a baby or child younger than 5 years due to intentional abrupt impact and/or violent shaking. Unintentional injuries resulting from negligent supervision, gunshot, and stabbing or penetrating trauma are excluded from this definition. According to the CDC, 25% to 30% of children with AHT die, and only 15% survive without any sequelae (7).
Traditionally, NAT injury in infants and toddlers has been described as shaken baby syndrome, although the terms inflicted Traumatic Brain Injury and non-accidental head injury been used in the literature. Only shaken baby syndrome, which has been defined as the triad of subdural hematoma, retinal hemorrhage and encephalopathy suggests a mechanism of injury in which there is tearing of the bridging veins secondary to shaking. This mechanism remains to be experimentally proven and is currently undergoing model experimentation (8). The term NAT will therefore be used throughout as it does not presume a mechanism.
The role physicians play in the management of NAT is twofold. First, a physician must maintain a healthy suspicion of NAT in the pediatric head injury population, as victims of abuse often initially present with head injuries. The error to not proceed with an abuse evaluation creates an obvious risk to the child of returning to an abusive environment. Conversely, the error of launching an unwarranted abuse evaluation can have unintended consequences, including prolongation of hospitalization, exacerbation of parental stress, compromise of the doctor-patient relationship, increased healthcare costs and potential exposure of children to additional sedation or radiation. Second, the incidence of malignant cerebral edema is higher in NAT patients when compared to those suffering from accidental trauma, leading to a higher frequency of surgical interventions in that group (8). This article will review the epidemiology of NAT as well as warning signs which should raise concern for NAT. We will also review the outcomes and future repercussions to children with a history of non-accidental trauma.
Epidemiology
NAT is a leading cause of childhood traumatic injury and death in the United States. In 2010, an estimated 2.07 per 100,000 children died as a result of abuse or neglect (9). Although NAT occurs in children of all ages, children between the ages 0 and 3 years are at the greatest risk for death (10). In 2010, the US Department of Health and Human Resources identified 695,000 children (9.2 patients per 1,000 children) as first-time patients of child abuse. In addition to these first-time patients, an unfortunate 754,000 children (10 patients per 1,000 children) were identified as recurrent patients (9).
The risk of unrecognized child abuse is obvious. In a study of 173 abused children with head injuries, 54 were not recognized as having been abused on initial presentation. Fifteen of these children were subsequently re-injured after the missed diagnosis and four of these children died (11). Deans et al. examined 1,572 patients who presented with NAT from 2000 to 2010 and 53 patients (3.4%) subsequently presented with recurrent NAT. These patients were more likely to be male (66% vs. 52%) and white (83% vs. 65%). Mortality was significantly increased in patients with recurrent NAT compared with a single episode (24.5% vs. 9.9%) (12).
In 2002, it is estimated that 1,400 children died from maltreatment in the United States, and AHT accounted for 80% of these deaths, making it the leading cause of death in this population (10).
Risk factors
Risk factors for NAT have been widely investigated and can be categorized as (I) risk factors intrinsic to child; (II) risk factors intrinsic to the perpetrator of abuse and (III) risk factors intrinsic to family structure and society (13). Race, gender, age and child’s health status have all been studied. There is no consensus regarding gender as a risk factor for NAT, although several studies have suggested that male children may be more likely to sustain fractures (14-17). The risk of NAT is inversely related to age, with the majority of victims being younger than 2 years (14,18-20). There is also no consensus regarding whether a particular race is at greatest risk of experiencing NAT, although black children have a greater risk of mortality from NAT (15,19-24). Children born prematurely or with multiple medical conditions are at a higher risk of experiencing NAT (15).
Factors that are intrinsic to the perpetrator of abuse include relationship to the child, and whether the perpetrator had been abused as a child. Perpetrators are likely to be young parents and female, however males are more likely to be responsible for episodes of NAT resulting in death (13).
The role of family structure has been investigated with mixed results. In one study, the eldest child had the highest risk of abuse but in another study the second child was identified as having the highest risk of abuse (14,25). Societal factors including socioeconomic status and lack of community support have been investigated. Several studies have shown no difference in abuse and non-abuse groups based on socioecomic status (15,23,25,26). Abuse is more common when the parents perceive that there is little community support and when families feel a lack of connection to the community. Other societal factors including increased decreased self-esteem, depression, history of suicide attempts, life stressors, parent in foster care or abandoned as a child, unplanned or unwanted pregnancy, engagement in criminal activity, less prenatal care, a history of relationship problems with other adults or a history of corporal punishment as a child, shorter birth intervals and increased number of separations from the child in the first year have been found to lead to an increase likelihood of NAT (14,17,25-32).
Vinchon et al. found the association of perinatal illness with child abuse to be very significant. Antecedents of familial dysfunction, such as alcohol or drug abuse, psychiatric disorder, a history of violence, jail sentence, or child withdrawal, were identified in the child’s family in the majority (97%) of child abuse cases (33).
Many socioeconomic factors have also been studied. In a recent report on NAHT, 66% of families lived in the inner city and 76% received public assistance; however, the authors caution that all population segments are at risk (34). Huang et al. examined the relationship between the economy and NAT. They found a doubling in the rate of non-accidental head trauma during the economic recession as well as an increase in severity as measured by admission GCS (35).
General presentation of children with non-accidental trauma
Bruising
When evaluating children with bruises, the location, shape, and pattern of the bruising should be noted (36). Accidental bruises are typically found over bony prominences, such as the knees, elbow and forehead (37). Bruises on the cheeks, neck, genitals, buttocks and back are unlikely to be accidental and should be regarded as suspicious for inflicted injury (37,38). Bruises in this area may also represent underlying injuries, such as fractures, abdominal injury or intracranial hemorrhage, particularly in children younger than 2 years of age (39). The shape and pattern of bruises may also provide clues when distinguishing accidental versus abusive bruising. Bruises that are inflicted typically maintain the shape of the causative object (40). The hand commonly is used to injure children and can leave a negative imprint. Additional objects commonly used to inflict injury on children include belts, cords, shoes, and kitchen utensils, hangers and teeth. Patterned bruises generally do not occur during normal play and should raise suspicion of abuse (41).
There are several benign entities which may be confused with abusive bruises, such as Mongolian spots and hemangiomas (42,43). In addition, children with conditions such as idiopathic thrombocytopenic purpura and leukemia may present with unexplained bruises in different stages of healing that can be mistaken for signs of physical abuse (42).
Burns
A high percentage of childhood burns are due to abuse (2% to 35% overall; up to 45% for genital and perineal burns). The two kinds of burns most often seen in abused children are scald burns (from contact with hot liquids) and thermal burns (contact with hot objects).
In accidental burns, the head, neck, anterior trunk and arms are the most often affected. In cases of abuse, hands, legs, feet and buttocks were more likely to be involved. The anterior aspect of the hand was more likely to be involved in accidental burns and the dorsum of the hand was more likely involved in abuse cases (44).
Suspicious burns include patterned contact burns in clear shape of hot object (fork, clothing iron, curling iron, cigarette lighter) and classic forced immersion burn patterns with sharp stocking-and-glove demarcation and sparing of flexed protected areas. In addition, splash/spill burn patterns in children which is not consistent with the history of the child’s developmental level should be considered suspicious. Cigarette burns should always raise concern for abuse, as should any evidence of delay in seeking medical treatment (45,46).
Fractures
Fractures of the ribs are common in children who had been abused and result from violent squeezing, and their presence, in the absence of major chest trauma, strongly suggests physical abuse. Injuries to the long bones tend to be spiral or oblique fractures. Spiral fractures, indicative of a twist injury, of the humeral shaft are significantly more common in abused children. Classic metaphyseal chip fractures were uncommon. Femur fractures prior to the age of walking are particularly concerning and multiple fractures are various stages of healing is a red flag. One child in 8 aged under 18 months who sustains a fracture may be a victim of child abuse (45,47).
Spinal fractures are typically not considered to be a common site of trauma due to accidental trauma, however Knox et al. reviewed 342 cases of pediatric spine injury and found that NAT was the mechanism in 11 (3.2%). Spine injury was present in a total of 1.5% of NAT cases. The average age of these patients was 7 months old and all of these patients were under the age of 2. Of the patients under 2 years of age with spinal trauma, NAT was the mechanism in 38% of cases, which 64% of patients presenting with multilevel trauma. 73% of these patients also had associated head injury. The majority of these spinal fractures were managed conservatively (48).
Abusive head trauma (AHT) (shaken baby syndrome)
AHT remains the most common cause of death in children who are victims of NAT, and this usually occurs during the first year of life. The diagnosis of AHT is often missed since no history of head trauma is provided, and the signs and symptoms the child displays may be non-specific, such as vomiting, poor feeding, irritability or lethargy (49).
A distinction exists between primary and secondary injuries. The primary injury is a consequence of the initial trauma or impact of force and the secondary injury is a complication of the primary injury.
Primary injuries include epidural hemorrhages, subdural hemorrhages, subarachnoid hemorrhages, skull fractures, intraventricular hemorrhages, cortical contusions, diffuse axonal injury (DAI) and intraparenchymal hematomas. Epidural hemorrhages in children require a direct impact of forces and are generally venous bleeds which result from tears in the dural sinus or diploic veins, in contrast to epidural hemorrhages in adults which tend to be from injury to the middle meningeal artery (Figure 1A). Subdural hemorrhages do not require direct impact and may result from inertial shearing or rotational forces but most commonly result from abrupt deceleration. Subdural hemorrhages occur in a space created by the traumatic separation of the arachnoid from the dura mater and are caused by bleeding from bridging veins (Figure 1B) (50). Infants may be found to have hemispheric hypodensity (HH), also known as “big black brain” which is associated with subdural hemorrhage and consists of a hypodense cortex and underlying white matter in multiple cerebrovascular territories. The brain in these infants undergoes progressive atrophy rapidly (51-54). HH has been shown to be associated with elevated intracranial pressure as well as higher mortality. HH is likely associated with secondary insults and ischemia and is more common in infants with cardiac arrest, hypoxia and hypotension (55). Subarachnoid hemorrhage from trauma results from the rupture of subarachnoid or pial vessels (Figure 1C). Intraventricular hemorrhage may be visualized when a large intracerebral hematoma dissolves into the ventricle or when subependymal veins are torn. Cortical contusions involve the superficial cortical gray matter and tend to spare the subcortical white matter. Cortical contusions tend to involve the frontal and temporal regions due to the irregular contour of the skull base and are less common in children than adults due to the smoother skull base in children (Figure 1D). DAI results from sudden acceleration and deceleration forces which may be combined with rotational forces, which disrupts fiber tracts. Infants are more susceptible to DAI due to their large head to body ratio, weak neck musculature and thinner skull. DAI typically affects subcortical white matter, the corpus callosum, the brainstem and internal capsule (Figure 1E). Intraparenchymal hematomas can result from shearing-straining injuries due to rupture of small intraparenchymal blood vessels and typically occurs in the fronto-temporal white matter (50).
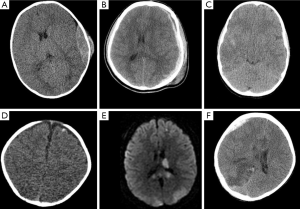
Secondary injuries include diffuse cerebral swelling, herniation, infarction, infection as well as chronic entities such as hydrocephalus or cerebrospinal fluid leak. Diffuse cerebral swelling is usually seen 24-48 hours following trauma and may occur more frequently in children than adults due to impaired autoregulation of perfusion and possibly an increased inflammatory response. Pediatric patients also generally have lower mean arterial pressures which results in greater likelihood for hypoperfusion and subsequent infarction (Figure 1F). Subfalcine, uncal or transtentorial herniation may all occur if significant mass effect develops either from the primary injury or from secondary diffuse cerebral edema. Hydrocephalus can also be a secondary injury which occurs when CSF absorption is impaired either due to obstruction or impaired resorption. Finally, skull fractures can lead to CSF leaks, particularly when the skull base is involved and predisposes the patient to pneumococcal meningitis (50).
After a literature review, Sieswerda-Hoogendoorn et al. observed that the main neurological manifestations resulting from abuse head trauma are as follows: altered state of consciousness (77%), seizures (43-50%), vomiting (15%), and developmental delay (12%) (56). The signs of AHT are often not recognized in less severe cases, so that it cannot be properly diagnosed. Hennes et al. highlight that some of the signs of AHT can mimic other diseases common in children, such as viral infections, colic, or food intolerance (57).
Children with mild encephalopathy may present with poor feeding, irritability, excessive crying or sleepiness. Those with moderate encephalopathy may present with lethargy, hypotonia, periods of apnea and diminished reflexes, such as grasping or sucking, while those with severe encephalopathy may present with seizures, stupor, coma or poorly reactive pupils (58).
When evaluating the child with intracranial hemorrhage, it is important to maintain a wide differential diagnosis, including accidental trauma or NAT, birth trauma, coagulopathy, congenital vascular malformations, spontaneous SDH, and metabolic deficiencies such as glutaric aciduria type I.
The possibility of spontaneous subdural hemorrhage in infants can complicate the determination of abusive trauma. The etiology of spontaneous subdural hemorrhage is thought to be related to benign enlargement of the subdural space (BESS), which typically begins with a rapid increase in head circumference at 2-3 months of age and results in head circumference above the 95th percentile at 3 years of age. A study of 177 children with BESS diagnosed on imaging were evaluated and four (2.26) were found to have evidence of subdural hemorrhage on computer tomography (CT) or magnetic resonance imaging (MRI). In 3 of the 4 children, a thorough evaluation did not reveal any additional signs of trauma and the SDH was determined to have occurred spontaneously (59).
Glutaric aciduria type I is a rare autosomal recessive neurometabolic disorder caused by a deficiency in glutaryl-CoA dehydrogenase, which affects the degradation of lysine, hydroxylysine, and tryptophan. The subsequent accumulation of glutaric acids results in hypotonia, acute striatal necrosis, frontotemporal atrophy and neurological deterioration. Infants may present with macrocephaly and bilateral SDH, which may be mistakenly diagnosed as non-accidental trauma (60).
Ocular manifestations
Ocular manifestations of NAT include periorbital hematoma, eyelid laceration, subconjunctival hemorrhage, subluxed or dislocated lens, cataracts, glaucoma, anterior chamber angle regression, iridiodialysis, retinal dialysis or detachment, intraocular hemorrhage, optic atrophy or papilledema. Retinal hemorrhages are often multilayered and occur in 60-85% of non-accidental head injuries. Multiple mechanisms of retinal hemorrhage have been postulated, including direct tracking of blood from intracranial hemorrhage, hemorrhage secondary to raised intracranial pressure or retinoschisis (61). Eisenbrey & Guilland regard retinal hemorrhage as diagnostic of child abuse when accompanied by intracranial injuries in the absence of a verifiable history (62).
A prospective study of children under 2 years of age with head injuries reviewed 150 cases and attempted to determine the sensitivity and specificity of retinal hemorrhage (RH) in the setting of NAT. Child abuse was found to be the cause of trauma in 57% of cases. The sensitivity of RH was estimated to be 75% and the specificity at 93.2% for the diagnosis of child abuse; the predictive positive and negative values were 89.4% and 82.9%, respectively. The retinal hemorrhages associated with accidental trauma were always mild and the authors found that the specificity of severe RH for the diagnosis of child abuse was 100% (34).
Estimating the probability of abuse
Several groups have attempted to develop algorithms to predict the likelihood of NAT. Maguire et al. sought to estimate the probability of AHT based on the combination of various clinical features. They performed an analysis of six studies, evaluating 1,053 total children under age 3 years, including 348 children who sustained AHT and were admitted with intracranial injury, including subdural hemorrhage, subarachnoid hemorrhage, contusions, DAI, and/or cerebral edema. The authors analyzed the probability of abuse if a child under age 3 also had one of the following clinical features: apnea; retinal hemorrhage; rib, skull and long-bone fractures; seizures and head and/or neck bruising. If a child who was admitted with intracranial injury did not have any of the clinical features above, the probability of abuse was 4%. The presence of rib fractures yielded the highest probability of abuse with an odds ratio of 45, and the presence of long-bone fractures yielded an odds ratio of 13.75. The presence of RH only increased the probability of abuse to 58% (odds ratio of 35). Head and/or neck bruising alone in the setting of intracranial injury increased the probability of abuse to 15% (63).
The authors then sought to determine the probability when several features were combined. If a child with intracranial injury had head and/or neck bruising as well as apnea, the probability of abuse was 54%. If apnea and RH were both present, the probability rose to 90%. The combination of seizure and rib fractures produced a probability of 90%. If three or more of the clinical features were present, the odds ratio was 100 and the positive predictive value for abuse was above 85% (63).
Hymel et al. also sought to develop an algorithm for predicting abuse. They reviewed 209 children with head injuries, 95 of whom were found to be due to abuse. Five variables were found which could be used to identify 97% of children in their population who had previously been classified as those suffering from abusive trauma. The five variables used in the study include: acute respiratory compromise prior to admission; seizure or encephalopathy prior to admission; bruising of the ear, neck or torso; interhemispheric or bilateral subdural hemorrhage; any skull fracture other than isolated, unilateral, nondiastatic, linear, parietal skull fracture. The specificity, positive predictive value, negative predictive value, and negative likelihood ratio for an AHT clinical prediction rule incorporating these five variables were 0.27, 0.53, 0.91, and 0.12, respectively (64).
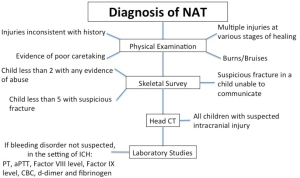
Physical exam
A general and focused physical exam is the starting point for any workup for NAT (Figure 2) Findings that raise suspicion are injuries inconsistent with the history, multiple injuries in various stages of healing, or any injuries pathognomonic for abuse, such as cigarette burns (46,47). Evidence of poor caretaking, sudden onset of mental status changes, bruises on an infant that is not yet cruising, bruises to the pinna, neck or abdomen and any injury to the genitalia should also raise suspicion. It is important to keep in mind that some findings may be concerning initially but are actually not signs of intentional injury. Intense crying, coughing or retching may cause petechiae on the face and shoulders. Mongolian spots may appear as bruising in the lumbosacral area, and coagulopathies may result in usual bruising (43). Pathologic bone disease (e.g., chronic renal disease, osteogenesis imperfecta, and rickets) may present with suspicious fractures (46).
Investigations
Laboratory data
The Committee on Child Abuse and Neglect addressed the appropriate evaluation for bleeding disorders in the setting of abusive trauma. The authors stress the importance of obtaining a family history of bleeding disorders as well as a past medical history in assessing for symptoms suggestive of a bleeding disorder, such as epistaxis, excessive bleeding following dental procedures or significant bleeding following circumcision or other surgery. Bleeding disorders which may mimic abuse include coagulation factor deficiencies, fibrinolytic defects, defects of fibrinogen, and platelet disorders. Most factor deficiencies will be detected by the prothrombin time (PT) and activated partial thromboplastin time (aPTT), with the exception of von Willebrand disease (vWD) and factor XIII deficiency. Mild hemophilia may not cause abnormal aPTT but may still result in significant bleeding after mild trauma. The initial testing panel recommended by the authors in the setting of intracranial hemorrhage where a bleeding disorder is not suspected includes PT, aPTT, Factor VIII level, Factor IX level, CBC, d-dimer and fibrinogen. The initial testing panel for intracranial hemorrhage (ICH) evaluates for conditions for which the probability for the condition resulting in ICH is greater than 1 per 5 million, but does not test for factor XIII deficieincy, vWD, fibrinolytic defects, hypofibrinogenemia and dysfibrinogenemia, as these conditions have either not been associated with intracranial hemorrhage or they are very rarely the cause (46).
Skeletal survey
A skeletal survey should be obtained in the following groups: any child less than 2 years of age with any evidence of abuse, any child less than 5 years of age with a suspicious fracture, or any older child who is unable to communicate areas of pain or trauma (e.g., intellectually disabled). In the event that a skeletal survey is negative but the suspicion remains high, a radionucleotide bone scan can be performed. A skeletal survey may also be repeated 7-10 days following the injury to reveal healing fractures that may have been missed (65).
Intracranial imaging
The American Academy of Pediatrics recommends that all children with suspected intracranial injury undergo cranial CT, MRI, or both. CT without intravenous contrast remains the imaging modality of choice for evaluating a child with acute neurologic findings or RH on physical examination. It is more sensitive with regard to acute intracerebral and extra-axial hemorrhages than is magnetic resonance imaging (MRI), and is also able to diagnose skull and facial fractures. CT is also more readily available and cost-effective when compared to MRI (65). However, brain MRI may be helpful as an adjunct for the evaluation of axonal shear injuries and for a more precise dating of intracranial hemorrhage. Diffusion-weighted imaging (DWI) is very helpful in the detection of early ischemic injury and is frequently used in trauma to help determine prognosis (66). In addition, magnetic resonance spectroscopy may be helpful in predicting outcomes in NAT. The N-acetylaspartate/creatinine ratios, N-acetylaspartate/choline ratios and lactate levels have been found to be significantly decreased in patients with poor outcome (67). The need for every patient to have a repeat CT scan following trauma has been challenged as concerns grow regarding the potential malignancy effects of radiation and has been found to be unnecessary in many patients (68). Current practice typically employs an initial head CT which may be followed by repeat CT in the case of concern for expanding hematoma or followed by MRI in cases where a patient’s neurologic exam remains abnormal and a diagnosis of DAI is being pursued.
Ophthalmologic examination
Unfortunately, obtaining a dilated fundoscopic exam in pediatric patients following non-accidental head trauma is complicated by the need for frequent neurologic assessments, including monitoring pupillary reflexes. The dilated fundoscopic examination is typically delayed at least 24 hours in the child with a GCS of 14-15 and even longer in children with poor neurologic exams. Interestingly, a recent studied evaluated the capability of MRI and the use of an orbits SWI protocol to diagnose retinal hemorrhages. Fifteen children with a history of AHT and the presence of retinal hemorrhage on dilated fundoscopic examination underwent an orbit SWI protocol and 80% (12) of these studies were positive for retinal hemorrhage (69). MRI may serve as a surrogate for diagnosing retinal hemorrhage in the patient who is unable to undergo a fundoscopic examination and requires an MRI for followup cranial or spine imaging.
Abdominal CT
If there are any concerns about abdominal trauma, liver and pancreatic enzymes should be ordered along with an abdominal CT and both urine and stool needs to be screened for blood (65).
Neurosurgical intervention in NAT
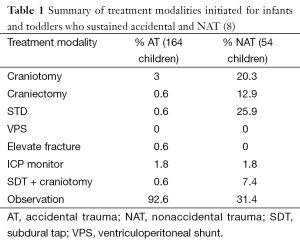
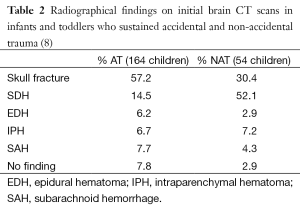
In a study by Adamo et al., 54 children who sustained NAT were reviewed and compared to 218 children who sustained accidental trauma. Interestingly, 69% of the children in the NAT group required neurosurgical operative intervention, compared to 7% in the accidental trauma group, reflecting the greater severity of injuries at admission (Table 1). More children in the accidental trauma group presented with skull fracture (57%) whereas patients in the NAT group were more likely to present with SDH (52%), likely reflecting the inertial force required to tear a bridging cortical vein as opposed to the direct impact required to create a skull fracture (Table 2). The authors also correlated hypodense SDH with the likelihood of NAT (8).
Full table
Full table
Outcomes in children with NAT
Traumatic brain injury continues to be a significant cause of death and disability in infants. It is estimated that 1,200-1,400 children are shaken every year, which results in death in 25-30% of cases. Unfortunately, most of those who survive are left with permanent disability, including blindness, seizures and cerebral palsy. In a series of 100 children under the age of 2 who sustained head injuries, the mortality rate was 15-38%. The rate of permanent cognitive deficit was 50% and only 30% of the children made a full recovery (70).
Compared with children with non-abusive head trauma, mortality and morbidity are consistently greater in children with AHT (71-73). Younger age at injury, more severe initial injuries and higher rates of secondary injuries from hypoxia and/or ischemia may contribute to the worse outcomes observed after AHT (8,70,71,74-77).
In a study of 37 children with traumatic brain injury who underwent decompressive craniectomy, 14 children were victims of NAT (38%). The mean age of the NAT group was 2.2 years and the mean age of the accident group was 8 years. The children with NAT had significantly increased mortality (35.7% vs. 4.3%) when compared to the accident group which resulted in a 12-fold increase in the odds of death. The children with NAT who survived also had significantly increased poor outcome (57.2% vs. 30.4%) based on a King’s Outcome Scale for Closed Head Injury score of 1,2, or 3 (78).
Risen et al. compared the functional changes during inpatient rehabilitation in 33 children with AHT with 33 age-matched children with non-abusive head trauma. The patients in the abusive trauma group were more likely to have associated injuries including cerebral infarction (6 times), cardiorespiratory arrest (4 times) and status epilepticus (6 times) when compared to the non-abusive trauma group. The authors evaluated attainment of independent ambulation and expressive language. They found that independent ambulation was acquired 14 months after discharge in the AHT group and 9 months after discharge in the non-abusive head trauma group. Nearly all of the children with non-abusive head trauma attained independent ambulation and expressive language after discharge from inpatient rehabilitation, compared to two thirds of children in the AHT group that gained independent ambulation, and three quarters that gained expressive language. Both groups reached independent ambulation and expressive language after the developmentally expected age range. The authors note that children in both cohorts were similar with respect to gains made during inpatient rehabilitation and stress the potential for significant benefit from inpatient rehabilitation (79).
Seizure and NAT
Barlow et al. conducted a retrospective study of 44 children with non-accidental head trauma in order to elucidate the relationship of NAT and seizures. Thirty-two of the 44 children (73%) had early posttraumatic seizures, with 6 of these children having seizures prior to presentation and 21 of the 44 having a seizure within 24 hours of presentation. All had onset of seizures within 3 days of admission and all had stopped by day 10. Thirteen children had episodes of status epilepticus. Sixteen children had intractable seizures, which did not respond to conventional treatment. Six of the 44 children died during admission. On follow up, 14 children were developing normally, while 6 had mild neurodevelopmental delays, 8 had moderate neurodevelopmental delays and 8 had severe neurodevelopmental delays. The authors found that the presence of seizures was significantly related to outcome. Only one child with intractable seizures had a normal outcome and the number of children without seizure was significantly overrepresented in the normal neurodevelopment group. At follow-up, 8 children had epilepsy, which was defined as intractable in 5 cases (80).
A retrospective study of 54 cases of confirmed AHT reported clinical seizures in 33% of these patients in the first 7 days after hospital admission, and electrographic only seizures in three of the eight patients (38%) who underwent continuous EEG monitoring (cEEG) (81). Hasbani et al. addressed the question of the prevalence of electrographic seizure (ES) in children with AHT. Of the 32 children with AHT diagnosed during the study period, 21 (66%) underwent cEEG monitoring. ES seizures were common, occurring in 12 of these patients (57%). Importantly, in eight of these 12 children (67%), the seizures were either electrographic only and would not have been detected without cEEG monitoring (82). The results of the present study suggest that cEEG monitoring is indicated early (within hours not days) after admission in children with suspected AHT, that this monitoring should continue a minimum of several hours in order to minimize secondary brain injury following trauma. cEEG monitoring may also be used in cases of refractory elevated ICP when barbiturate therapy is utilized. Serum levels of barbituates are not predictive of therapeutic response and cEEG remains the most reliable guide for dosing of barbituate therapy with the goal of titrating to burst-suppresion (83). Finally, cEEG may also be an important aid in determing prognosis after TBI. Several EEG background features, such as EEG reactivity, EEG variability and the presence of sleep architecture have been associated with outcome. A recent review suggests that cEEG monitoring in children with acute TBI may improve outcomes through early seizure detection and treatment and suggests that a standard protocol be developed for all severe TBI patients. Several factors which increase the likelihood of seizures following trauma, including GCS ≤8, a history of NAT, a history of previous convulsive seizures and intracranial hemorrhage may be an indication for cEEG monitoring (84). The current evidence suggests that cEEG should be made a priority for patients younger than age 2 with an intracranial injury and concern for NAT (85). Many questions remain regarding the best treatment regimen for these seizures and if treatment improves outcome (86).
Seizure prophylaxis
Current guidelines for management of severe TBI in children (GCS <8) recommend considering prophylactic antiepileptic drugs in patients at high risk for early post traumatic seizure (EPTS), although many studies fail to show a benefit in preventing early post traumatic seizure (87-89). Accurate identification of children at risk for EPTS can be difficult (88,90,91). Younger age and more severe injury remain the most consistently reported risk factors (90-93).
Liesemer sought to determine other risk factors for early post traumatic seizure. They reviewed the cases of 275 children with moderate to severe traumatic head injury and found that 34 (12%) had a seizure within the first 7 days. Focal seizure activity was the most common, followed by generalized tonic-clonic seizures. NAT was identified in 38% of children with EPTS but only 8% of those without seizures, which resulted in a threefold increase in risk of early post traumatic seizure in the NAT group. The presence of subdural hematoma was found to be significantly associated with early post traumatic seizure when compared to children without seizure (65% vs. 39%). The increase of post traumatic seizure in children with NAT may be related to the fact that the presence of subdural hematoma confers a higher risk of early post traumatic seizures. Age <2 years old resulted in a threefold increase risk of early post traumatic seizure and severe TBI yielded an eightfold increase in the risk of early post traumatic seizure. The administration of an antiepileptic drug reduced the risk of EPTS by 80% (94).
Conclusions
All physicians play an important role in the management of NAT, from early recognition of potentially suspicious injuries to the management of malignant edema. It is essential for all physicians to maintain a high suspicion for NAT in the management of pediatric patients as the risks of malignant cerebral edema remains high. The future research on NAT will likely focus on further development of algorithms to predict NAT as well as the potential benefit of antiepileptic medications in reducing the incidence of clinically silent seizures.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roche AJ, Fortin G, Labbé J, et al. The work of Ambroise Tardieu: the first definitive description of child abuse. Child Abuse Negl 2005;29:325-34. [PubMed]
- Caffey J. Multiple fractures in the long bones of infants suffering from chronic subdural hematoma. Am J Roentgenol Radium Ther 1946;56:163-73. [PubMed]
- Silverman FN. The roentgen manifestations of unrecognized skeletal trauma in infants. Am J Roentgenol Radium Ther Nucl Med 1953;69:413-27. [PubMed]
- Caffey J. The whiplash shaken infant syndrome: manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics 1974;54:396-403. [PubMed]
- Kempe CH, Silverman FN, Steele BF, et al. The battered-child syndrome. JAMA 1962;181:17-24. [PubMed]
- Lopes NR, Eisenstein E, Williams LC. Abusive head trauma in children: a literature review. J Pediatr (Rio J) 2013;89:426-33. [PubMed]
- Center of Disease and Control. Child Maltreatment: Fact-sheet. Atlanta: National Center for Injury Prevention & Control; 2007. [cited 14 Feb 2014]. Available online: http://www.cdc.gov/ncipc/factsheets/cmfacts.htm
- Adamo MA, Drazin D, Smith C, et al. Comparison of accidental and nonaccidental traumatic brain injuries in infants and toddlers: demographics, neurosurgical interventions, and outcomes. J Neurosurg Pediatr 2009;4:414-9. [PubMed]
- US Department of Health and Human Services, A.f.C.a.F., Administration on Children, Youth, and Families, Children’s Bureau, Child Maltreatment 2010. [Accessed 7 Feb 2014]. Available online: http://www.acf.hhs.gov/
- Feldman KW, Bethel R, Shugerman RP, et al. The cause of infant and toddler subdural hemorrhage: a prospective study. Pediatrics 2001;108:636-46. [PubMed]
- Jenny C, Hymel KP, Ritzen A, et al. Analysis of missed cases of abusive head trauma. JAMA 1999;281:621-6. [PubMed]
- Deans KJ, Thackeray J, Askegard-Giesmann JR, et al. Mortality increases with recurrent episodes of nonaccidental trauma in children. J Trauma Acute Care Surg 2013;75:161-5. [PubMed]
- Mulpuri K, Slobogean BL, Tredwell SJ. The epidemiology of nonaccidental trauma in children. Clin Orthop Relat Res 2011;469:759-67. [PubMed]
- Baldwin JA, Oliver JE. Epidemiology and family characteristics of severely-abused children. Br J Prev Soc Med 1975;29:205-21. [PubMed]
- Leventhal JM, Thomas SA, Rosenfield NS, et al. Fractures in young children: distinguishing child abuse from unintentional injuries. Am J Dis Child 1993;147:87-92. [PubMed]
- Perez-Arjona E, Dujovny M, Vinas F, et al. CNS child abuse: epidemiology and prevention. Neurol Res 2002;24:29-40. [PubMed]
- Worlock P, Stower M, Barbor P. Patterns of fractures in accidental and non-accidental injury in children: a comparative study. BMJ 1986;293:100-2. [PubMed]
- Agran PF, Anderson C, Winn D, et al. Rates of pediatric injuries by 3-month intervals for children 0 to 3 years of age. Pediatrics 2003;111:e683-92. [PubMed]
- Lauer B, Ten Broeck E, Grossman M. Battered child syndrome: review of 130 patients with controls. Pediatrics 1974;54:67-70. [PubMed]
- Cappelleri JC, Eckenrode J, Powers JL. The epidemiology of child abuse: findings from the Second National Incidence and Prevalence Study of Child Abuse and Neglect. Am J Public Health 1993;83:1622-4. [PubMed]
- Lane WG, Rubin DM, Monteith R, et al. Racial differences in the evaluation of pediatric fractures for physical abuse. JAMA 2002;288:1603-9. [PubMed]
- Leventhal JM, Larson IA, Abdoo D, et al. Are abusive fractures in young children becoming less common? Changes over 24 years. Child Abuse Negl 2007;31:311-22. [PubMed]
- Falcone RA Jr, Brown RL, Garcia VF. The epidemiology of infant injuries and alarming health disparities. J Pediatr Surg 2007;42:172-6; discussion 176-7. [PubMed]
- Lyman JM, McGwin G, Malone DE, et al. Epidemiology of child homicide in Jefferson County, Alabama. Child Abuse Negl 2003;27:1063-73. [PubMed]
- Smith JA, Adler RG. Children hospitalized with child abuse and neglect: a case control study. Child Abuse Negl 1991;15:437-45. [PubMed]
- Altemeier WA, O’Connor S, Vietze PM, et al. Antecedents of child abuse. J Pediatr 1982;100:823-9. [PubMed]
- Egeland B, Jacobvitz D, Sroufe LA. Breaking the cycle of abuse. Child Dev 1988;59:1080-8. [PubMed]
- Garbarino J, Crouter A. Defining the community context for parent-child relations: the correlates of child maltreatment. Child Dev 1978;49:604-16. [PubMed]
- Smith SM, Hanson R. Interpersonal relationships and child-rearing practices in 214 parents of battered children. Br J Psychiatry 1975;127:513-25. [PubMed]
- Oliver JE. Successive generations of child maltreatment: social and medical disorders in the parents. Br J Psychiatry 1985;147:484-90. [PubMed]
- Benedict MI, White RB, Cornley DA. Maternal perinatal risk factors and child abuse. Child Abuse Negl 1985;9:217-24. [PubMed]
- Muller RT, Hunter JE, Stollak G. The intergenerational transmission of corporal punishment: a comparison of social learning and temperament models. Child Abuse Negl 1995;19:1323-35. [PubMed]
- Vinchon M, Defoort-Dhellemmes S, Desurmont M, et al. Accidental and nonaccidental head injuries in infants: a prospective study. J Neurosurg 2005;102:380-4. [PubMed]
- Dashti SR, Decker DD, Razzaq A, et al. Current patterns of inflicted head injury in children. Pediatr Neurosurg 1999;31:302-6. [PubMed]
- Huang MI, O’Riordan MA, Fitzenrider E, et al. Increased incidence of nonaccidental head trauma in infants associated with the economic recession. J Neurosurg Pediatr 2011;8:171-6. [PubMed]
- Hornor G. Physical abuse: Recognition and reporting J Pediatr Health Care 2005;19:4-11. [PubMed]
- Herendeen PM. Evaluation of physical abuse in children. Solid suspicion should be your guide. Adv Nurse Pract 2002;10:32-6. [PubMed]
- Sugar NF, Taylor JA, Feldman KW. Bruises in infants and toddlers: Those who don’t cruise rarely bruise. Arch Pediatr Adolesc Med 1999;153:399-403. [PubMed]
- Thompson S. Accidental or inflicted? Pediatr Ann 2005;34:372-81. [PubMed]
- Jenny C. Cutaneous management of child abuse. In: Reece RM, Ludwig S. eds. Child abuse medical diagnosis and management (2nd ed.). Philadelphia: Lippincott Williams & Wilkins, 2001:23-45.
- Harris TS. Bruises in children: normal or child abuse? J Pediatr Health Care 2010;24:216-21. [PubMed]
- Bays J. Conditions mistaken for child physical abuse. In: Reece RM, Ludwig S. eds. Child abuse and neglect: Medical diagnosis and management (2nd ed.). Philadelphia: Lippincott Williams & Wilkins, 2001:177-206.
- Tanner JL, Dechert MP, Frieden IJ. Growing up with a facial hemangioma: parent and child coping and adaptation. Pediatrics 1998;101:446-52. [PubMed]
- Hobbs CJ. When are burns not accidental? Archives of Disease in Childhood 1986;61:357-61. [PubMed]
- Carpenter SL, Abshire TC, Anderst JD, et al. Evaluating for suspected child abuse: conditions that predispose to bleeding. Pediatrics 2013;131:e1357-73. [PubMed]
- Montrey JS, Barcia PJ. Nonaccidental burns in child abuse. South Med J 1985;78:1324-6. [PubMed]
- Worlock P, Stower M, Barbor P. Patterns of fractures in accidental and non-accidental injury in children: a comparative study. Br Med J (Clin Res Ed) 1986;293:100-2. [PubMed]
- Knox J, Schneider J, Wimberly RL, et al. Characteristics of spinal injuries secondary to nonaccidental trauma. J Pediatr Orthop 2014;34:376-81. [PubMed]
- Duhaime AC, Christian CW, Rorke LB, et al. Nonaccidental head injury in infants--the “shaken-baby syndrome”. N Engl J Med 1998;338:1822-9. [PubMed]
- Pinto PS, Meoded A, Poretti A, et al. The unique features of traumatic brain injury in children. review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications, and their imaging findings--part 2. J Neuroimaging 2012;22:e18-41. [PubMed]
- Duhaime AC, Bilaniuk L, Zimmerman R. The “big black brain”: radiographic change after severe inflicted head injury in infancy. J Neurotrauma 1993;10:S59.
- Cohen RA, Kaufman RA, Myers PA, et al. Cranial computed tomography in the abused child with head injury. AJR Am J Roentgenol 1986;146:97-102. [PubMed]
- Duhaime AC, Durham S. Traumatic brain injury in infants: the phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”). Prog Brain Res 2007;161:293-302. [PubMed]
- Dias MS, Backstrom J, Falk M, et al. Serial radiography in the infant shaken impact syndrome. Pediatr Neurosurg 1998;29:77-85. [PubMed]
- Foster KA, Recker MJ, Lee PS, et al. Factors Associated with Hemispheric Hypodensity after Subdural Hematoma following Abusive Head Trauma in Children. J Neurotrauma 2014. [Epub ahead of print]. [PubMed]
- Sieswerda-Hoogendoorn T, Boos S, Spivack B, et al. Educational paper: Abusive Head Trauma part I. Clinical aspects. Eur J Pediatr 2012;171:415-23. [PubMed]
- Hennes H, Kini N, Palusci VJ. The epidemiology, clinical characteristics and public health implications of shaken baby syndrome. In: Lazoritz S, Palusci VJ, eds. The shaken baby syndrome: A multidisciplinary approach. Binghamton: The Haworth Maltreatment & Trauma Press, 2001:19-40.
- Ferriero DM. Neonatal brain injury. N Engl J Med 2004;351:1985-95. [PubMed]
- McKeag H, Christian CW, Rubin D, et al. Subdural hemorrhage in pediatric patients with enlargement of the subarachnoid spaces. J Neurosurg Pediatr 2013;11:438-44. [PubMed]
- Bishop FS, Liu JK, McCall TD, et al. Glutaric aciduria type 1 presenting as bilateral subdural hematomas mimicking nonaccidental trauma. Case report and review of the literature. J Neurosurg 2007;106:222-6. [PubMed]
- Green MA, Lieberman G, Milroy CM, et al. Ocular and cerebral trauma in non-accidental injury in infancy: underlying mechanisms and implications for paediatric practice. Br J Ophthalmol 1996;80:282-7. [PubMed]
- Eisenbrey AB. Retinal hemorrhage in the battered child. Childs Brain 1979;5:40-4. [PubMed]
- Maguire SA, Kemp AM, Lumb RC, et al. Estimating the probability of abusive head trauma: a pooled analysis. Pediatrics 2011;128:e550-64. [PubMed]
- Hymel KP, Willson DF, Boos SC, et al. Pediatric Brain Injury Research Network (PediBIRN) Investigators: Derivation of a clinical prediction rule for pediatric abusive head trauma. Pediatr Crit Care Med 2013;14:210-20. [PubMed]
- Section on Radiology, American Academy of Pediatrics. Diagnostic imaging of child abuse. Pediatrics 2009;123:1430-5. [PubMed]
- Ashwal S, Wycliffe ND, Holshouser BA. Advanced neuroimaging in children with nonaccidental trauma. Dev Neurosci 2010;32:343-60. [PubMed]
- Aaen GS, Holshouser BA, Sheridan C, et al. Magnetic resonance spectroscopy predicts outcomes for children with nonaccidental trauma. Pediatrics 2010;125:295-303. [PubMed]
- Schnellinger MG, Reid S, Louie J. Are serial brain imaging scans required for children who have suffered acute intracranial injury secondary to blunt head trauma? Clin Pediatr (Phila) 2010;49:569-73. [PubMed]
- Zuccoli G, Panigrahy A, Haldipur A, et al. Susceptibility weighted imaging depicts retinal hemorrhages in abusive head trauma. Neuroradiology 2013;55:889-93. [PubMed]
- Duhaime AC, Christian C, Moss E, et al. Long-term outcome in infants with the shaking-impact syndrome. Pediatr Neurosurg 1996;24:292-8. [PubMed]
- Hymel KP, Makoroff KL, Laskey AL, et al. Mechanisms, clinical presentations, injuries, and outcomes from inflicted versus noninflicted head trauma during infancy: results of a prospective, multicentered, comparative study. Pediatrics 2007;119:922-9. [PubMed]
- Minns RA, Jones PA, Barlow KM. Outcome and prognosis of nonaccidental head injury in infants. In: Minns RA. eds. Shaking and other nonaccidental head injuries in children. London: MacKeith Press, 2006:364-414.
- Keenan HT, Hooper SR, Wetherington CE, et al. Neurodevelopmental consequences of early traumatic brain injury in 3-year-old children. Pediatrics 2007;119:e616-23. [PubMed]
- Kieslich M, Marquardt G, Galow G, et al. Neurological and mental outcome after severe head injury in childhood: a longterm follow-up of 318 children. Disabil Rehabil 2001;23:665-9. [PubMed]
- Ewing-Cobbs L, Prasad M, Kramer L, et al. Inflicted traumatic brain injury: relationship of developmental outcome to severity of injury. Pediatr Neurosurg 1999;31:251-8. [PubMed]
- Goldstein B, Kelly MM, Bruton D, et al. Inflicted versus accidental head injury in critically injured children. Crit Care Med 1993;21:1328-32. [PubMed]
- Ichord RN, Naim M, Pollock AN, et al. Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion weighted imaging. J Neurotrauma 2007;24:106-18. [PubMed]
- Oluigbo CO, Wilkinson CC, Stence NV, et al. Comparison of outcomes following decompressive craniectomy in children with accidental and nonaccidental blunt cranial trauma. J Neurosurg Pediatr 2012;9:125-32. [PubMed]
- Risen SR, Suskauer SJ, Dematt EJ, et al. Functional outcomes in children with abusive head trauma receiving inpatient rehabilitation compared with children with nonabusive head trauma. J Pediatr 2014;164:613-9.e1-2.
- Barlow KM, Spowart JJ, Minns RA. Early posttraumatic seizures in non-accidental head injury: Relation to outcome. Dev Med Child Neurol 2000;42:591-4. [PubMed]
- Goldstein JL, Leonhardt D, Kmytyuk N, et al. Abnormal neuroimaging is associated with early in-hospital seizures in pediatric abusive head trauma. Neurocrit Care 2011;15:63-9. [PubMed]
- Hasbani DM, Topjian AA, Friess SH, et al. Nonconvulsive Electrographic Seizures are Common in Children With Abusive Head Trauma. Pediatr Crit Care Med 2013;14:709-15. [PubMed]
- Winer JW, Rosenwasser RH, Jimenez F. Electroencephalographic activity and serum and cerebrospinal fluid pentobarbital levels in determining the therapeutic end point during barbiturate coma. Neurosurgery 1991;29:739-41; discussion 741-2. [PubMed]
- Gallentine WB. Utility of continuous EEG in children with acute traumatic brain injury. J Clin Neurophysiol 2013;30:126-33. [PubMed]
- Arndt DH, Lerner JT, Matsumoto JH, et al. Subclinical early posttraumatic seizures detected by continuous EEG monitoring in a consecutive pediatric cohort. Epilepsia 2013;54:1780-8. [PubMed]
- Wainwright MS. Timing is everything: whether and when to use continuous electroencephalograms in abusive head trauma comes into focus. Pediatr Crit Care Med 2013;14:726-8. [PubMed]
- Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 19. The role of anti-seizure prophylaxis following severe pediatric traumatic brain injury. Pediatr Crit Care Med 2003;4:S72-5. [PubMed]
- Lewis RJ, Yee L, Inkelis SH, et al. Clinical predictors of post-traumatic seizures in children with head trauma. Ann Emerg Med 1993;22:1114-8. [PubMed]
- Young KD, Okada PJ, Sokolove PE, et al. A randomized, double-blinded, placebo controlled trial of phenytoin for the prevention of early posttraumatic seizures in children with moderate to severe blunt head injury. Ann Emerg Med 2004;43:435-46. [PubMed]
- Hahn YS, Fuchs S, Flannery AM, et al. Factors influencing posttraumatic seizures in children. Neurosurgery 1988;22:864-7. [PubMed]
- Ratan SK, Kulshreshtha R, Pandey RM. Predictors of posttraumatic convulsions in head-injured children. Pediatr Neurosurg 1999;30:127-31. [PubMed]
- Ong LC, Dhillon MK, Selladurai BM, et al. Early post-traumatic seizures in children: Clinical and radiological aspects of injury. J Paediatr Child Health 1996;32:173-6. [PubMed]
- Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma 1992;9 Suppl 1:S287-92. [PubMed]
- Liesemer K, Bratton SL, Zebrack CM, et al. Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: rates, risk factors, and clinical features. J Neurotrauma 2011;28:755-62. [PubMed]