Advances in paediatric cancer treatment
Introduction
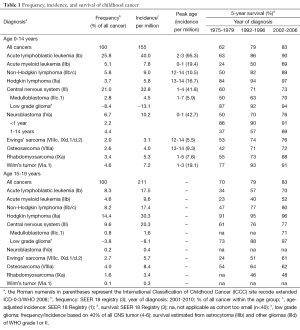
Cancer in children is rare with an incidence of 140-155 per million per year (age <15 years) (1,2) (Table 1). This translates to ~1 in 7,000 children is diagnosed with cancer each year. For the 15 to 19 years age group, the incidence is 210 per million with a different distribution of cancer diagnosis. Despite the rarity of cancer, malignant neoplasm is the most common cause of death after accidents in children aged 5 to 14 years, accounting for 23% of mortality (7).
Full table
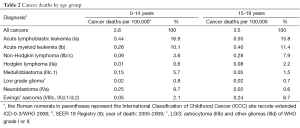
The introduction of chemotherapy in the 1960s has allowed the use of multi-modality approach, i.e., in conjunction with surgery and radiation, in the treatment of cancer. As a result, survival from childhood cancers, many of which was fatal in the pre-chemotherapy era, has increased dramatically from 20-30% in the 1960s (8) to 62% in the 1970s (mortality of 4.5/100,000) (3,9) (Tables 1 and 2). The current mortality is 2.6/100,000 and survival is 83%, meaning modern medicine can cure four out of five children with cancer. This is the end result of the tremendous effort dedicated to pediatric cancer research in the last 50 years.
Full table
One of the challenges of pediatric cancer research is the small disease population, comparing with adult cancer that is 40 times more frequent. To overcome this obstacle, multi-center clinical trials are essential to generate statistically meaningful results. For example, the Children Oncology Group (COG) represents the world’s largest organization devoted exclusively to childhood cancer research and comprises >200 member institutions, majority of which are based in the US. However, even with multi-center set up, it often takes five years to complete a phase III randomized controlled trial (RCT). Furthermore, it takes another six years to formally publish mature 5-year survival data (10-12). The CCG-1991 study enrolled 3,054 acute lymphoblastic leukemia (ALL) patients from 109 institutions between 2000 and 2005 and detected a <5% difference in survival. Results were published in 2011 (10). The HIT-SIOP PNET 4 Trial enrolled 340 children with medulloblastoma (MB) from 122 European centers between 2001 and 2006 and published the result in 2012 (12). It often takes over a decade for any progress established from clinical trials to become standard of care, and this has not taken into consideration the preceding ten years expended in preclinical and phase I/II studies.
We reviewed eight pediatric malignancies, in which significant advances in treatment were made in the last ten years, and most importantly are leading to improved standard of care. This included four hematologic malignancies and four solid tumors, comprising 60% of childhood cancer. The progresses were disseminated in published literature in the last decade and reflect clinical trials conducted between mid-1990 and mid-2000. We also explored ongoing studies with attention to trials conducted by COG, which have been developed based on knowledge gained in preclinical and early clinical studies in the last decade and discussed some of the more promising molecular targets for each of the eight cancers. Finally, we reviewed seven novel agents that have been most frequently pursued in childhood cancers.
Acute lymphoblastic leukemia (ALL)
Childhood leukemia has an incidence of 490 per million, of which 80% are ALL making it the commonest childhood cancer, peaking at 2 to 3 years old (1). With contemporary chemotherapy protocols, 5-year survival approaches 90% (Table 1). However, infant ALL with MLL/11q23 rearrangement have significantly worse outcome (EFS <30%) (13). The following is an overview of the recent advances in ALL treatment.
Minimal residual disease (MRD)
The rapidity of clearance of leukemic cells from the bone marrow is a strong prognostic factor (14,15) and is best evaluated by MRD using quantitative flow cytometry or polymerase chain reaction (PCR) assay of immunoglobulin and T-cell receptor rearrangements. These techniques are sensitive to 1×10–5. After two decades of research, MRD is now widely used in risk stratification and provides a validated early measure of treatment response. MRD response to induction is best measured at day 15 and day 33 for pre-B ALL and at day 79 for T-cell ALL (16). Clinical trials currently evaluating treatment modifications based on MRD include the St Jude TOT XVI, AIEOP-BFM ALL 2009, DFCI-05001, COG-ALL0331, ALL-REZ BFM 2002.
Genome-wide analysis and targeted therapy
The collaborative TARGET research program has identified several key aberrations associated with high-risk ALL phenotypes, including BCR/ABL1, JAK, MLL, CRLF2 and IKZF1 (17). JAK kinases activation is present in 10% of BCR/ABL1-negative high-risk cases (18).
One of the best examples of targeted therapy is the use of tyrosine kinase inhibitors (TKI) to complement chemotherapy in BCR/ABL-positive ALL, which accounts for 3% of childhood ALL. Continuous exposure to imatinib in an intensive chemotherapy regimen yielded 3-year event-free survival (EFS) of 80%, more than twice that of historical controls (19). Second generation TKIs (dasatinib and nilotinib) with more potent BCR/ABL signaling suppression can overcome imatinib-resistance (20) and are being studied in a number of pediatric ALL studies. A COG phase III RCT of FLT inhibition with lestaurtinib (TKI) with chemotherapy is currently underway for MLL-rearranged infant ALL, which has been shown to express high levels of FLT3 mRNA (21) (NCT00557193). Lestaurtinib, which has also been shown to inhibit JAK2 (22,23), may potentially be active in JAK-mutated ALL.
Novel therapies
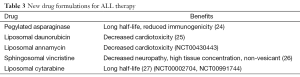
New formulations of the old armamentarium have been developed to improve delivery and reduce toxicities and can potentially be used in ALL therapy (Table 3). Continuous asparagine depletion has been associated with better EFS than intermittent depletion and a lower incidence of CNS relapse (28). Pegylated (PEG) asparaginase, approved for ALL treatment, has a long half-life and lowers the risk of allergic reactions and anti-asparginase antibody formation while maintaining efficacy similar to conventional E. coli asparaginase (24).
Full table
Second generation nucleoside analogues are part of a new repertoire of drugs against ALL. Nelarabine shows specific anti-leukemic effect in T-cell ALL (29) and is currently being evaluated in newly diagnosed (phase III: NCT00408005) and refractory T-cell ALL (phase IV: NCT00866671). Clofarabine is being studied in both de novo and recurrent disease (NCT00372619, NCT01228331). Furthermore, the Interfant-06 Study Group is conducting a RCT (NCT00550992) investigating the novel addition of AML-type therapy during intensification for MLL-rearranged infant ALL.
CNS-directed treatment
Intensification of systemic and intrathecal chemotherapy abrogates the need for prophylactic cranial irradiation without compromising survival (30,31). This removes the long-term side effects of radiotherapy-induced secondary malignancies and neurocognitive harm. The isolated CNS relapse rates were <3%. Risk factors for CNS relapse included t(1;19)/TCF3-PBX1, CNS involvement at diagnosis and T-cell immunophenotype.
Immunotherapy
Monoclonal antibodies are designed to potentiate chemotherapy, particularly in the setting of relapsed leukemia (32,33). This includes rituximab (anti-CD20), alemtuzumab (anti-CD52), epratuzumab (anti-CD22), inotuzumab ozogamicin (anti-CD22) for B-cell ALL. Blinatumomab (CD19/CD3-bispecific antibody) are particularly promising for T-cell ALL (34). Chimeric antigen receptor T cell (CAR-T cell) is an investigational novel approach to relapsed/refractory leukemia. Autologous T cells, which are genetically modified ex vivo to express a chimeric receptor that recognizes a surface antigen on the patient’s own tumor cells, home to the disease sites and persist with time (35). CD19-specific CAR-T cell therapy has been used with success in adult relapsed CLL (36) and has recently been used in two children with refractory ALL, albeit with toxicities (37). Killer-cell immunoglobulin-like receptor (KIR)—mismatch natural killer cell therapy is also another novel approach (38,39).
Acute myeloid leukemia (AML)
AML represents 5% of childhood malignancy and 20% of childhood leukemia. While cure of childhood AML has tripled since the 1970’s, current survival of 70% makes it one of the less curable cancers in children. Chemotherapy intensification, response-directed therapy and better supportive care including routine anti-fungal prophylaxis have improved survival by 15% in the last decade, a substantial progress compared to other childhood cancers (Table 1).
AML can be categorized into three genetically defined prognostic groups (40). Favorable risk comprises acute promyelocytic leukemia (APL), myeloid leukemias of Down Syndrome (MLDS), core binding factor (CBF) AML with t(8;21) and inv(16), and AML with NPM1 and CEBPA mutations. APL is characterized by t(15;17)/PML-RARA and its unique sensitivity to all-trans-retinoic acid (ATRA) and arsenic trioxide, both of which target the PML-RARA fusion protein (41). Children with Down syndrome (DS) (Trisomy 21) have a 20-times increased risk of developing leukemia and are particularly susceptible to chemotherapy and treatment-related complications (42). High risk aberrations include monosomy 5 and 7, some 11q23/MLL rearrangements and FLT3/ITD. Intermediate risk is neither favorable nor high risk. CBF, APL and MLL represent 50% of pediatric AML.
MLL-rearranged pediatric AML generally has an unfavourable outcome [overall 5-year EFS 44%; overall survival (OS) 56%] (43). However, it is a heterogeneous disease with the MLL gene having over 60 different translocation partners (44) and 5-year EFS ranging from 11% to 92% for different translocation partners (43). t(9;11) (p22;q23)/MLL-AF9 is the most common rearrangement (50%) and has a 5-year EFS of 50%. The 3% of patients with t(1;11) (q21;q23)/MLL-AF1q have the best outcome (5-year EFS 93%). In contrast, t(6;11) (q27;q23)/MLL-AF6 (5% of cases) has the lowest survival (5-year EFS 11%) (43,45).
Internal tandem duplication (ITD) of the receptor tyrosine kinase FLT3 results in activating mutations. FLT3-ITD-positive patients have significantly inferior progression-free survival (PFS) than FLT wild-type patients (4-year PFS 31% vs. 55%; P<0.001) (46). However, prognosis is influenced by ITD allelic ratio (AR) (mutant to wild-type ratio) and NPM1 status. Patients with high ITD-AR (>0.4) were reported to have significantly worse outcome than those with low AR (PFS 16% vs. 72%) (46). In contrast, NPM1 mutations were found to improve survival of patients with FLT-ITD. In the UK MRC AML 10 and 12 trials, young adults who were FLT-ITD-positive had significantly better outcome when concurrent NMP1 mutations were present (5-year DFS 31% vs. 15%) (47).
Advances in AML treatment in the last decade include:
- In APL, addition of ATRA to anthracycline monotherapy or multi-agent chemotherapy containing idarubicin and high-dose cytarabine increased survival to 85-90% (48,49). Arsenic trioxide has been shown to be very effective when incorporated into adult APL therapy (50,51). COG is currently conducting a phase III trial of introducing arsenic to a multi-agent regimen containing ATRA, idarubicin, and cytarabine in newly diagnosed APL (NCT00866918) (52);
- Reducing chemotherapy intensity did not compromise outcome in MLDS with EFS ranging from 79 to 83% and OS 84 to 91% in three different studies (53-55);
- Risk-adapted therapy utilizing cytogenetic risk stratification improved outcome, yielding EFS from 56 to 61% and OS from 66 to 75% in a number of large studies (56-59);
- MRD-positivity by flow cytometry after first course of chemotherapy predicts survival. Relapse-free survival (RFS) was found to be significantly worse in MRD-positive (RFS 14-43%) than in MRD-negative patients (RFS 65-85%) in three large studies (57,60,61). AML trials evaluating response-guided therapy based on MRD include NOPHO-DBH AML 2012 (NCT 1828489) and COG-AAML-1031 (NCT01371981).
Molecular target
FLT3-ITD reported in 10% of pediatric AML (40) is a candidate for targeted therapy with TKI. In a phase I study, sorafenib (multikinase inhibitor) in combination with clofarabine and cytarabine induced complete remission in relapsed AML patients with FLT3-ITD (62). In a current COG phase III trial for newly diagnosed AML (NCT01371981), FLT3-ITD-positive patients receive sorafenib.
Targeting CD33 using gemtuzumab ozogamicin (GO) (Mylotarg®), a calicheamicin-conjugated CD33 antibody, has promising activity in AML. In pediatric AML, GO induced complete remission in 35% of refractory disease (63,64) and was safe in de novo AML when combined with chemotherapy (65). In adults, GO improved survival in newly diagnosed AML in some but not all large studies (65-70). Pediatric trials of GO in de novo AML are ongoing (NCT00372593, NCT00476541).
Non-Hodgkin lymphoma (NHL)
NHL constitutes 6% of childhood cancer and is most common in the second decade of life. NHL can be classified according to phenotype (B-cell vs. T-cell) and differentiation (71). Unlike adults NHL that is generally low/intermediate grade, pediatric NHL is frequently high grade. It falls into three categories: (I) mature B-cell NHL including Burkitt/Burkitt-like lymphoma and diffuse large B-cell lymphoma (DLBCL); (II) lymphoblastic lymphoma (LL) (mostly precursor T-cell); and (III) anaplastic large cell lymphoma (ALCL) (mature T-cell or null-cell). Burkitt lymphoma (BL) is most common, accounting for one-third of pediatric NHL.
Currently, cure from childhood NHL is 90%. The steady increase in survival over the last 40 years was due to the initial recognition that LL was best treated with two years of ALL therapy (72,73) and later on due to increasingly effective risk-stratified therapies evolving from large multi-centre clinical trials including BFM-NHL90 (74), NHL-BFM95 (75) and FAB/LMB96 (76-78). Survival is excellent for low-stage LL (>90%) (74), BL (>85%) and DLBCL (90%) (79). However, survival is inferior for BL with CNS involvement (75%) (76), primary mediastinal B-cell lymphoma in DLBCL (73%) (80) and ALCL (70-85%) (81,82).
Advances in the treatment of childhood NHL include:
- Reducing multi-agent chemotherapy to only two courses and omitting intrathecal chemotherapy while maintaining an excellent cure rate of 99% in completely resected localized BL (75,77);
- Reducing treatment in early responding patients with intermediate-risk B-NHL (78);
- Use of multi-agent chemotherapy tailored to disease burden and initial response in B-NHL (79);
- Substituting cranial irradiation with high-dose methotrexate for CNS prophylaxis that is essential for T-cell LL, thus avoiding the long-term side-effects of cranial irradiation (83-85).
Molecular therapy
BL and DLBCL, both mature B-cell phenotype, express high levels of CD20. Rituximab (CD20 antibody) in combination with chemotherapy improved survival in adult DLBCL (86). It is an approved drug for the treatment of DLBCL. In pediatric BL, rituximab has activity as a single agent (87) and can be safely combined with chemotherapy (88). Based on these studies, an international collaborative study INT-B-NHL ritux 2010 (NCT01516580) is currently evaluating rituximab with chemotherapy for high-risk BL and DLBCL in children.
Rituximab may also be useful as a second-line therapy in post-transplant lymphoproliferative disorder (PTLD) or BL secondary to Epstein-Barr virus reactivation with immunosuppression after solid organ or stem cell transplantation. The benefit of rituximab with low dose chemotherapy has been described in a small number of pediatric refractory PTLD (89,90).
Advanced-stage disease is common in ALCL and has less favourable outcome. ALCL expresses CD30 and frequently harbors the t(2;5)/NPM-ALK aberration. Brentuximab vedotin (Bv) (SGN-35, Adcetris®), an antibody-drug conjugate of chimeric CD30 antibody and monomethylauristatin E, resulted in an objective response of 86% and complete remission of 57% in an adult phase II trial of relapsed systemic ALCL (91). Crizotinib (ALK inhibitor) elicited response in eight of nine ALCL in a phase I pediatric study (92). COG is currently conducting a phase I study of crizotinib in combination with chemotherapy for relapsed ALCL (NCT01606878) and a phase II trial where newly diagnosed ALCL patients are randomized to either crizotinib or Bv with chemotherapy (NCT01979536).
Hodgkin lymphoma (HL)
HL is the most common cancer in the 15 to 19 years age group and is four to five times more frequent than in the <15 years age group. It is characterized by the Reed-Sternberg multinucleated giant cell or its variants and histologic subtypes are defined by the number of Reed-Sternberg cells, characteristic inflammatory milieu and degree of fibrosis. HL is categorized into classical and nodular lymphocyte predominant HL.
HL was fatal until the 1960s when the MOPP nitrogen mustard containing chemotherapy regimen was introduced (93). The cure rate of HL in children has been >90% in the last two decades and is one of the most curable childhood cancers. Unfortunately, survivors of childhood HL are at significant risk of long-term treatment-related morbidity and mortality. In a study of ~2,700 childhood HL survivor, 23% of deaths were from secondary malignant neoplasms and 14% from cerebrovascular and heart disease. The 30-year cumulative incidence of secondary malignant neoplasms was significantly higher in females than in males (26% vs. 11%) due to the high incidence of invasive breast cancer in female survivors treated with radiation (94). Hence, to reduce long-term side effects while maintaining excellent survival, pediatric oncologists have adopted a risk-adapted approach for HL and attempted to reduce or omit radiation. However, risk assignment and definition of response has not been uniform in clinical trials and has made comparison of outcomes across trials challenging. Study results should therefore be interpreted in the context of risk stratification strategy and chemotherapy regimen, as well as response criteria and timing.
Advances in the treatment of pediatric HL in the last decade include (Table 4):

Full table
- Reduction in chemotherapy exposure in patients who are in complete response (CR) after two cycles of chemotherapy, referred to as rapid early responder (RER) (95,96);
- Omission of involved-field radiation therapy (IFRT) in low-risk RER (97-99);
- modification of chemotherapy in RER according to gender, based on the principle that less gonadal toxic alkylating therapy in males would reduce the risk of infertility and avoiding IFRT in female would reduce the risk of breast cancer (99,100).
The rapidity of early response is prognostic in HL (96,97,99). Positron emission tomography (PET) has been evaluated as an interim imaging modality in adult HL and was more superior than other response assessments (101). COG has recently completed four HL trials treating ~2,400 patients of different risks while addressing the utility of PET. Mature results are yet to be published. There are also ongoing clinical trials in North America and Europe addressing the utility of PET for rapid early response and the omission of IFRT in RER [NCT01858922, NCT00846742, 2006-000995-33 (102)]. All these trials should provide valuable data on the value of PET to predict outcome and whether EFS can be maintained after adjusting therapy based on early PET response.
Molecular therapy targeting CD30 expressed by the Hodgkin/Reed-Sternberg cells may be effective in classical HL. In addition to ALCL, Bv is currently being studied in a number of phase I and II trials for pediatric relapsed HL (NCT01780662, NCT01393717, NCT01492088, NCT01508312) and in newly diagnosed unfavourable risk HL patients (NCT01920932).
Medulloblastoma (MB)
MB is a malignant embryonal neuroectodermal tumor in the cerebellum and comprises 15% of childhood brain tumors. Clinical staging stratified MB into standard (60%) and high-risk (40%) (metastases, residual volume ≥1.5 cm2, large cell/anaplastic histology) (103). Current survival is 80% in standard-risk (104,105), 70% in high-risk (106-108) and 50% in children <3 years with MB (109). Contemporary MB therapy includes maximal surgery, adjuvant radiation [cranial spinal irradiation (CSI) with posterior fossa boost] and chemotherapy. Therapeutic approach in young children focuses on delaying or avoiding radiation to minimize the detrimental effects on the immature CNS through the use of multi-agent chemotherapy.
Advances in the last decade and current clinical trials include:
- Standard-risk MB and age >3 years:
- Post-radiation adjuvant chemotherapy allowed lowering CSI dose from 36 to 23.4 Gy while maintaining 5-year EFS at 83% (n=86) (104) and 81% (n=379) (105);
- Current COG phase III RCT (NCT00085735) asks whether EFS can be maintained with 18 Gy CSI and conformal tumor site radiation with concomitant chemotherapy.
- High-risk MB and age >3 years treated with CSI (36-39.6 Gy):
- Chemoradiotherapy using carboplatin and vincristine (CV) followed by maintenance chemotherapy in a phase I study resulted in 5-year PFS of 71% (n=55) (107);
- Post-operative chemotherapy for eight weeks followed by hyperfractionated accelerated radiotherapy +/– consolidation with myeloablative chemotherapy produced 5-year EFS of 70% (n=33) (106);
- Four courses of post-radiation intensive chemotherapy with hemapoietic stem cell support in SJ-MB-96 study achieved 5-year EFS of 70% (n=48) (108);
- Current COG phase III RCT (NCT00392327) evaluates the addition of carboplatin to chemoradiotherapy and isotretinoin to maintenance chemotherapy.
- MB in young children:
- Histology is an important prognostic factor. In a systematic review of five studies, desmoplastic/nodular (DN) or MB with extensive nodularity (n=108) have significantly better outcome (8-year EFS 55%; OS 76%) than classical MB (n=145; 8-year EFS 27%; OS 42%) in children <5 years (110);
- Thiotepa-based myeloablative therapy eliminated the need of CSI in 50% of young children (<3 years) with non-metastatic MB. 5-year EFS and OS were 52% and 70% (n=21) (111);
- Current COG phase III RCT (NCT00336024) assesses the addition of high-dose methotrexate to thiotepa-based myeloablative therapy in high-risk MB.
- Molecular profiling performed over the last decade has allowed segregation of MB into four distinct groups: WNT, sonic hedgehog (SHH), Group 3 and Group 4 (112-115) (Table 5):
- The WNT tumors, of which only 5% to 10% are metastatic, are found in children >3 years with classical MB, and have the best prognosis (OS 90%) (116). Patients may be candidate for reduced intensity therapy;
- The SHH tumors are bimodal in age distribution (<3 years and adult) and have intermediate prognosis (OS 60-80%). DN MB is exclusive to this group (115). Smoothened (SMO) receptor is a target for SHH (117) and vismodegib (GDC-0449), a SMO inhibitor, is a potential therapeutic (118,119). Phase II trials of vismodegib in refractory (NCT01239316) and newly diagnosed MB (NCT01878617) are ongoing;
- Group 3 comprises non-WNT and non-SHH tumors that are MYC-driven. It has the highest incidence of large cell/anaplasia (LCA) histology and metastases and the worst survival (50%);
- Group 4 tumors, of which 35% are metastatic, are largely classical MB with intermediate outcome (OS 75%).

Full table
Low grade glioma (LGG)
Glioma is a CNS tumor of glial cell origin and can arise anywhere within the CNS. It consists of astrocytomas, oligodendroglioma and mixed gliomas, and can be classified into low grade (WHO grade I and II) and high grade (WHO III and IV). LGG, most frequently located in the cerebellum, is the most common type of brain tumor (30-50%) (4-6). Pilocytic (WHO grade 1) and diffuse fibrillary astrocytoma (WHO grade 2) are the predominant histology in childhood LGG. Children with neurofibromatosis type 1 (NF1) have a high incidence (20%) of optic pathway LGG (OPG) (120) and contributes to 60% of OPG (121). The natural history of LGG is not always predictable.
Surgery remains the first-line treatment for LGG and is considered curative in areas of the brain amenable to complete resection. The 8-year PFS and OS are 90% and 99% following gross total resection (122). Incomplete resection and young age are both poor prognostic factors. Partially resected LGG has a10-year EFS of 18% and OS of 87% (123). Infants with diencephalic syndrome from hypothalamic lesion have the worst outcome (5-year PFS 11%; 10-year OS 43%) (124). Patients <1 year old have EFS of 19%, compared with patients aged >5 years with EFS of 64% (123,125). Radiotherapy can provide long-term control of LGG (123,126), but with significant late cognitive and endocrine sequelae (126,127), especially in young children who are more prone to the late effects of radiation (128). In NF1-associated OPG, the risk of second tumor was found to be three times higher in those who received radiation (129).
In the last decade, the growing trend to manage unresectable, progressive, or recurrent LGG with chemotherapy has successfully deferred or avoided radiation in young children. The two most widely used chemotherapy regimen are CV (130,131) or thioguanine, procarbazine, lomustine and vincristine (TPCV) (132,133). In the German study of CV (n=213), 32% experienced partial response (PR) and 75% did not require subsequent radiation. 10-year PSF and OS was 44% and 88%, respectively (123). However, it remains unclear whether chemotherapy improves visual outcome in OPG (134).
COG conducted a phase III RCT of CV versus TPCV (125). Initial response was similar for both groups (1/3 PR, 1/3 stable disease (SD), 1/3 progressive disease). While 2-year EFS was similar at 60% for both regimens, there were increasingly more events in the CV group after two years, resulting in 5-year EFS of 39% for CV and 52% for TPCV. Due to non-proportionality, log rank test for EFS was not significant (P=0.1); however, by cure model analysis, TPCV was more superior to CV (P=0.007). 5-year OS was equivalent (87% vs. 86%). Toxicity profile was different with more CNS toxicity for TPCV. Allergic reaction was only reported in CV due to carboplatin allergy. CV is the regimen of choice for NF1 patients due to potential increased risk of second malignancy from alkylating agents in TPCV. Other agents that has been tested in phase I/II studies for LGG include vinblastine (135,136), temozolomide (137) and bevacizumab and irinotecan (138).
Neuroblastoma (NB)
NB, an embryonal tumor of the sympathetic nervous system, is the most common extracranial solid tumor in children. Majority of NB is found in children younger than five years. It is well known for its heterogeneity ranging from spontaneous regression, differentiation to benign ganglioneuroma to aggressive metastatic disease (139).
NB is risk-stratified based on age, disease stage and biologic features (140), with survival >90% for low and intermediate risk (141,142) and 50% for high-risk NB (143). MYCN amplification is a well-established molecular prognostic factor and places patients into the high-risk category irrespective of age and stage. A pre-treatment risk stratification system (International Neuroblastoma Risk Group classification system) was developed in 2008 using 13 potential prognostic factors in a cohort of 8,800 children diagnosed with NB worldwide with an aim to facilitate comparison of clinical trials and development of international collaborative studies (140). Standard therapy for high-risk NB includes intensive chemotherapy, surgery, myeloablative chemotherapy with stem cells rescue, radiation and biological therapy. Low or intermediate NB is managed with surgery with or without chemotherapy, with an aim to appropriately minimize therapy.
The following is an overview of advances in NB treatment in the last decade (Table 6).

Full table
Low and intermediate-risk NB
Surgery alone was found to be adequate in localized resectable (stage 1 and 2) NB (142,144). In infants <12 months old with resectable tumor (145) or in young infants <6 months old with small adrenal mass (146), observation approach at diagnosis did not compromise survival while upfront low dose chemotherapy without anthracyclines maintained excellent outcome in infants with localized unresectable tumor (147). In intermediate-risk NB, reducing chemotherapy to four cycles in tumors with favorable biologic features did not adversely affect survival (141). Furthermore, chromosome 11q loss of heterozygosity (LOH) was found to be a poor prognostic factor (140).
High-risk NB
Immunotherapy has provided the best outcome in the setting of MRD. GD2 is a disialoganglioside highly expressed by NB. Immunotherapy with ch14.18 (chimeric antibody against GD2) in conjunction with cytokines and isotretinoin was evaluated in a phase III RCT (n=226). In patients who have achieved at least a PR after myeloablative therapy, immunotherapy was superior to isotretinoin alone (2-year EFS 66% vs. 46%, P=0.01; OS 86% vs. 75%, P=0.02) (148). Immunocytokine therapy using the humanized 14.18-IL-2 fusion protein has been tested in phase I (149) and II (150) studies in recurrent NB.
Metaiodobenzylguanidine (MIBG) scan is routinely used to diagnose NB. Metastatic response assessed by MIBG scan was found to predict survival (151,152). A semiquantitative MIBG scoring system (Curie score) showed that patients with a score >2 after induction therapy had a significantly worse outcome than those with scores ≤2 (3-year EFS: 15% vs. 44%; P<0.001; n=237) (153).
Targeted radiotherapy
Radiolabeled 131-I-MIBG selectively targets radiation to catecholamine-producing NB cells. Phase I studies of 131-I-MIBG in combination with radiosensitizer (154) or with myeloablative therapy and stem cell rescue (155,156) has demonstrated efficacy (25-65% response) in refractory NB. Studies of 131-I-MIBG followed by myeloablative therapy in newly diagnosed NB are ongoing (NCT01175356, NCT00798148).
NB genome
Whole genome sequencing studies have shown paucity of somatic mutations in the high-risk NB genome and identified ALK and ATRX aberrations in ~20% of high-risk NB (157-159). Crizotinib, an ALK inhibitor, is currently being evaluated alone or with chemotherapy in recurrent NB (NCT00939770, NCT01606878).
Ewing sarcoma (ES)
The incidence of ES is highest in the second decade of life. It most commonly presents as an undifferentiated bone tumor and less frequently as a soft tissue mass (extraosseous ES). The pathognomonic t(11;22)(q24;q12)/EWS-FLI translocation is also found in peripheral primitive neuroectodermal tumor (PNET), a more differentiated tumor of bone or soft tissue. These tumors are collectively referred to as the ES family of tumors (ESFT) (160).
In addition to surgery +/– radiotherapy for local control, doxorubicin-containing chemotherapy regimen is essential for effective eradication of micro-metastases in ES (161). Improvement in survival of localized disease in the 1990’s was attributed to the addition of ifosfamide and etoposide (IE) to the standard three drugs [vincristine, doxorubicin, cyclophosphamide (VDC)] in the US (162,163). However, progress has been marginal in the last 20 years and outcome of metastatic ES remains poor. Currently, survival for localized and metastatic ES is 80% (164) and <35%, respectively (165,166).
For localized ES, one advance of note in the last decade is the effective use of “interval compression” of chemotherapy (as opposed to dose intensification). The COG AEWS001 phase III RCT in 587 patients demonstrated 5-year EFS of 73% and OS 83% in the intensified arm (alternating VDC and IE every two weeks), compared with 5-year EFS of 65% and OS 73% in the standard arm (3-week cycle) (P=0.048) with no worsening toxicity (164).
Topotecan and irinotecan, both topoisomerase I inhibitors, have shown activity in recurrent ES. Response was observed in a third of recurrent ES treated with topotecan and cyclophosphamide in two prospective studies (167,168) and in 60% of patients receiving irinotecan and temozolomide in a retrospective study (169). Based on this evidence, the current COG phase III RCT (NCT01231906) examines the efficacy of adding CVT (cyclophosphamide, vincristine, topotecan) to the intensively timed 5-drug (VDC/IE) regimen in newly diagnosed localized ES.
Large tumours, pelvic site and poor histologic response to induction chemotherapy are known poor prognostic factors in localized disease (170,171). The French EW93 study demonstrated an improved 5-year EFS of 45% in a small number of high-risk patients treated with high-dose busulphan/melphalan and hemapoietic stem cell support (172). Furthermore, high-dose chemotherapy may be beneficial in the setting of isolated pulmonary metastases, which has a better outcome than other types of metastases (170,173). Hence, both the recently completed multi-center EuroEwing 99 (NCT00020566) study and the current EuroEwing 2012 (ISRCTN92192408) (174) phase III RCT examine therapy intensification with high-dose busulphan and melphalan in patients with isolated lung metastases and in those with large (>200 mL volume) or poor histologic response (>10% viability) tumors.
Novel targeted therapy is needed for metastatic and recurrent ES. Inhibition of insulin growth factor-1 receptor (IGF1R) using monoclonal antibody has shown efficacy in relapsed ES. Anti-IGF1R antibody that has been studied in phase I/II studies included figitumumab (CP-751,871) (175,176), ganitumab (AMG-479) (177) and cixutumumab (IMC-A12), either alone or with temsirolimus (mTOR inhibitor) (178,179). These studies reported SD and response between 25-50%. COG is developing a phase II RCT (AEWS1221) to assess the feasibility of adding ganitumab to the interval-compressed regimen (180).
Novel therapeutics in pediatric cancer
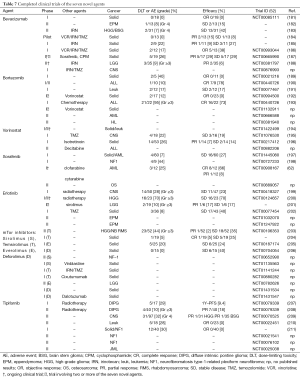
Our increased understanding of the molecular basis of childhood cancer in the last decade has allowed researcher to define molecular targets and evaluate available therapeutics that are in clinical development for adult cancers. We selected seven novel agents or class of drugs that are most frequently studied in clinical trials involving childhood cancer. In most instances, the agent has a track record in adult oncology and has been subjected to safety scrutiny in adult phase I/II trials, in which a maximum tolerated dose was established. Tables 7 and 8 provide a list of completed and ongoing clinical studies that involve pediatric patients. All but one drug have approval from the US Food and Drug Administration (FDA) for specific cancers; however, none are pediatric cancers.
Full table
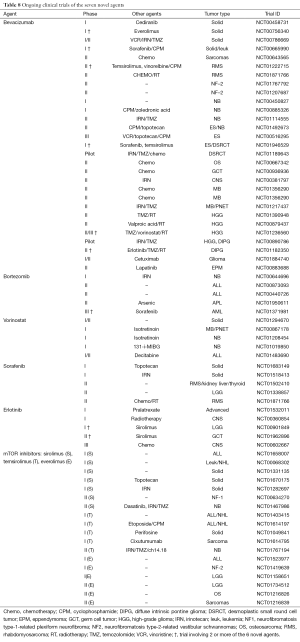
Full table
Bevacizumab (Avastin®)
Vascular endothelial growth factor (VEGF) is a regulator of tumor angiogenesis and is a prerequisite for cancer growth. Bevacizumab, a humanized anti-VEGF monoclonal antibody, sequestrates VEGF (212). Bevacizumab has FDA approval for adult malignancies including colorectal, lung and prostate cancer (213). In the pediatric population, bevacizumab was most effective when combined with irinotecan +/– temozolomide (184-186). Numerous Phase II clinical trials are ongoing to investigate bevacizumab mostly in combination with other agents in a variety of childhood malignancies.
Bortezomib (Velcade®)
The ubiquitin-proteasome pathway regulates the stability of proteins and deregulated proteolysis has been reported in many tumor types. Proteasome inhibition has been found to selectively induce pro-apoptotic proteins in cancer cells (214). Bortezomib is the first FDA approved proteasome inhibitor for the treatment of multiple myeloma and mantle cell lymphoma (215,216). Multiple preclinical and early clinical trials demonstrated its safety and significant anticancer activity towards haematological malignancies in both adults (217) and children (190,193). Bortezomib is currently being evaluated in a COG phase III study in de novo AML and in a phase II study with arsenic in APL.
Vorinostat (Zolinza®)
Histone acetylation is a reversible process where histone acetyltransferases transfer the acetyl moiety from acetyl co-enzyme A to a lysine while histone deacetylases (HDACs) remove this acetyl groups. HDAC inhibition results in accumulation of acetylated proteins and induces growth arrest, apoptosis and reactive oxygen species-mediated cell death (218). Vorinostat is the first HDAC inhibitor approved for advanced cutaneous T-cell lymphoma (219). It has also been tested in phase I trials in pediatric solid tumors with predominantly disease stabilization (195,196).
Sorafenib (Nexavar®)
It is a multi-kinase inhibitor that targets tyrosine kinases VEGFR, PDGFR and FLT3, as well as the RAF/MEK/ERK pathway (220). It is currently approved for the treatment of metastatic renal cell carcinoma and hepatocellular carcinoma (221,222). In pediatric early clinical trials, sorafenib combination therapy resulted in 30% response in solid tumors (187) and 75% response in AML (62). Furthermore, next generation TKIs that have been developed to provide increased target specificity and inhibition have been evaluated in adult clinical trials. Pazopanib (223), axitinib (224) and tivozanib (225) are potent VEGFR inhibitors whereas quizartinib and crenolanib are potent FLT3 inhibitors (226).
Erlotinib (Tarceva®)
Targeting the epidermal growth factor receptor (EGFR) is well established in the treatment of a number of adult malignancies. Erlotinib, a potent EGFR TKI (227), has been approved for the treatment of advanced NSCLC and pancreatic cancer (228,229). In early clinical trials of erlotinib combined with radiation, prolonged SD was observed in pediatric high-grade gliomas (HGG) and relapsed/refractory brain tumors (199,200).
Tipifarnib (Zarnestra®)
Farnesylation is necessary for Ras activation, which triggers activation of the PI3-kinase/AKT and RAF/MEK/ERK pathway, and is implicated in the pathogenesis of solid and hematologic malignancies. Tipifarnib (farnesyl transferase inhibitor) was shown to have anti-leukemic activity in adult phase I/II trials (230,231). There are no ongoing paediatric trials of tipifarnib, most likely due to lack of activity as a single agent in a number of pediatric studies (207-211).
The mammalian target of rapamycin (mTOR) inhibitors
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase and the PI3-kinase/AKT/mTOR pathway plays an important role in the regulation of cell growth, proliferation, motility, survival and transcription (232). mTOR inhibitors include sirolimus (Rapamune®), its analogue deforolimus, and its derivates temsirolimus (Torisel®) and everolimus (Afinitor®). The latter two are approved for the treatment of renal cell carcinoma, subependymal giant cell astrocytoma, pancreatic and breast cancer (233). There are quite a number of ongoing pediatric phase I/II trials of mTOR inhibitors in both hematologic and solid malignancies.
Discussion
Advances in research that evolve into improved standard of care and outcome in childhood cancer is the result of the remarkable effort invested in childhood cancer research. The current 5-year OS of 83% in the US (Table 1) (3) and 79% in Europe (2) represents only a marginal increase over a decade. While cancers that already have an excellent outcome did not show much improvement, 5-year survival of cancers including AML, NB in >1 year, and ALL and ES in 15-19 years old has increased by 10% to 20% in the last ten years (Table 1). Furthermore, progress in oncology is not limited to better survival. Reducing both short and long-term treatment-related complications is as important, given majority of childhood cancer patients will become long-term survivors. This is best achieved by risk-adapted therapeutic approach that has been made possible through identifying clinical and biologic prognostic factors with rigorous research, stratifying patients using these risk factors and modifying therapy according to risk group assignment.
Optimizing delivery of conventional therapeutics has been the driving force behind continuous improvements in pediatric cancer survival in the last 40 years. However, the pediatric oncology field acknowledges that further escalation of conventional therapy is unlikely going to yield improvement in cancers that currently have unacceptably low cure rates. This include AML, high-risk ALL and NB, high-grade brain tumors, and metastatic bone tumors and sarcomas. Advances in medical research technology have led to a rapid increase in our understanding of the genetics of childhood cancer in the last decade and will continue to facilitate identification of molecular targets that can potentially be exploited for therapeutic benefits. As we move into the era of targeted therapeutics, searching for novel agents that target specific genetic lesions in this group of poor prognosis cancer becomes both a priority and a challenge.
Unlike conventional cytotoxic chemotherapies, the premise of effective targeted therapy involves “hitting” the intended target resulting in disruption of specific signalling pathways. Hence clinical trials will need to focus on biologically defined patient subsets, meaning even smaller patient population. It will no longer be feasible to conduct the standard phase III RCT that requires hundreds of patients. The key challenge will be to design trials that can clearly delineate the effect of the new agent under study. Options include single arm study, of which results will be compared with historical control, and phase II randomized trial comparing an active but non-curative cytotoxic chemotherapeutic regimen with or without the new agent. National and international collaborative studies will be required to attain sufficient patients and complete trials in a timely manner. Furthermore, new endpoints that utilize functional imaging or molecular biomarkers can be incorporated into clinical trials as a measure of response and MRD. However, these endpoints need to be properly validated to ensure they accurately reflect clinical benefits.
As we continue into the 21st century, our increased understanding of the molecular and genetic basis of childhood cancer will facilitate further refinement of risk-adapted therapy that utilizes molecular and genetic signatures for risk stratification. The ultimate goal is to cure childhood cancer with the best quality of long-term survivorship.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (2000-2010) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission.
- Gatta G, Corazziari I, Magnani C, et al. Childhood cancer survival in Europe. Ann Oncol 2003;14 Suppl 5:v119-27. [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (1973-2010 varying) - Linked To County Attributes - Total U.S., 1969-2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission.
- Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol 2009;24:1397-408. [PubMed]
- Freeman CR, Farmer JP, Montes J. Low-grade astrocytomas in children: evolving management strategies. Int J Radiat Oncol Biol Phys 1998;41:979-87. [PubMed]
- Pollack IF. Brain tumors in children. N Engl J Med 1994;331:1500-7. [PubMed]
- Ten leading cause of death and injury. Centers for Disease Control and Prevention Available online: http://www.cdc.gov/injury/wisqars/leadingcauses.html
- Birch JM, Marsden HB, Jones PH, et al. Improvements in survival from childhood cancer: results of a population based survey over 30 years. Br Med J (Clin Res Ed) 1988;296:1372-6. [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence-Based Mortality - SEER 18 Regs (Excl Louisiana) Research Data, Nov 2012 Sub (2000-2010) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released June 2013, based on the November 2012 submission.
- Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood 2011;118:243-51. [PubMed]
- Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood 2005;105:948-58. [PubMed]
- Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol 2012;30:3187-93. [PubMed]
- Pui CH, Gaynon PS, Boyett JM, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 2002;359:1909-15. [PubMed]
- Asselin BL, Gaynon P, Whitlock JA. Recent advances in acute lymphoblastic leukemia in children and adolescents: an expert panel discussion. Curr Opin Oncol 2013;25 Suppl 3:S1-13. [PubMed]
- Teachey DT, Hunger SP. Predicting relapse risk in childhood acute lymphoblastic leukaemia. Br J Haematol 2013;162:606-20. [PubMed]
- Karsa M, Dalla Pozza L, Venn NC, et al. Improving the identification of high risk precursor B acute lymphoblastic leukemia patients with earlier quantification of minimal residual disease. PLoS One 2013;8:e76455. [PubMed]
- Mullighan CG. The molecular genetic makeup of acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2012;2012:389-96.
- Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 2009;106:9414-8. [PubMed]
- Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol 2009;27:5175-81. [PubMed]
- Signorovitch J, Ayyagari R, Reichmann WM, et al. Major molecular response during the first year of dasatinib, imatinib or nilotinib treatment for newly diagnosed chronic myeloid leukemia: A network meta-analysis. Cancer Treat Rev 2014;40:285-92. [PubMed]
- Brown P, Levis M, McIntyre E, et al. Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia 2006;20:1368-76. [PubMed]
- Diaz T, Navarro A, Ferrer G, et al. Lestaurtinib inhibition of the Jak/STAT signaling pathway in hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS One 2011;6:e18856. [PubMed]
- Hexner EO, Serdikoff C, Jan M, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood 2008;111:5663-71. [PubMed]
- Dinndorf PA, Gootenberg J, Cohen MH, et al. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist 2007;12:991-8. [PubMed]
- Creutzig U, Zimmermann M, Bourquin JP, et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood 2013;122:37-43. [PubMed]
- Rodriguez MA, Pytlik R, Kozak T, et al. Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma: report of the pivotal phase 2 study. Cancer 2009;115:3475-82. [PubMed]
- Bomgaars L, Geyer JR, Franklin J, et al. Phase I trial of intrathecal liposomal cytarabine in children with neoplastic meningitis. J Clin Oncol 2004;22:3916-21. [PubMed]
- Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 2007;109:896-904. [PubMed]
- Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group. J Clin Oncol 2005;23:3376-82. [PubMed]
- Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009;360:2730-41. [PubMed]
- Veerman AJ, Kamps WA, van den Berg H, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004). Lancet Oncol 2009;10:957-66. [PubMed]
- Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 2011;29:551-65. [PubMed]
- Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov 2007;6:149-65. [PubMed]
- Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008;321:974-7. [PubMed]
- Alonso-Camino V, Sánchez-Martín D, Compte M, et al. CARbodies: Human Antibodies Against Cell Surface Tumor Antigens Selected From Repertoires Displayed on T Cell Chimeric Antigen Receptors. Mol Ther Nucleic Acids 2013;2:e93. [PubMed]
- Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725-33. [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [PubMed]
- Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005;106:376-83. [PubMed]
- Triplett B, Handgretinger R, Pui CH, et al. KIR-incompatible hematopoietic-cell transplantation for poor prognosis infant acute lymphoblastic leukemia. Blood 2006;107:1238-9. [PubMed]
- Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood 2012;120:3187-205. [PubMed]
- Zhou GB, Zhang J, Wang ZY, et al. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Philos Trans R Soc Lond B Biol Sci 2007;362:959-71. [PubMed]
- Taub JW, Ge Y. Down syndrome, drug metabolism and chromosome 21. Pediatr Blood Cancer 2005;44:33-9. [PubMed]
- Balgobind BV, Raimondi SC, Harbott J, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood 2009;114:2489-96. [PubMed]
- Meyer C, Kowarz E, Hofmann J, et al. New insights to the MLL recombinome of acute leukemias. Leukemia 2009;23:1490-9. [PubMed]
- Balgobind BV, Zwaan CM, Pieters R, et al. The heterogeneity of pediatric MLL-rearranged acute myeloid leukemia. Leukemia 2011;25:1239-48. [PubMed]
- Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood 2006;108:3654-61. [PubMed]
- Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008;111:2776-84. [PubMed]
- Ortega JJ, Madero L, Martin G, et al. Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA Group. J Clin Oncol 2005;23:7632-40. [PubMed]
- Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood 2005;106:447-53. [PubMed]
- Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 2010;116:3751-7. [PubMed]
- Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 2012;120:1570-80. [PubMed]
- ClinicalTrial.gov registry (US National Institute of Health). Available online: http://clinicaltrials.gov/
- Sorrell AD, Alonzo TA, Hilden JM, et al. Favorable survival maintained in children who have myeloid leukemia associated with Down syndrome using reduced-dose chemotherapy on Children’s Oncology Group trial A2971: a report from the Children’s Oncology Group. Cancer 2012;118:4806-14. [PubMed]
- Abildgaard L, Ellebaek E, Gustafsson G, et al. Optimal treatment intensity in children with Down syndrome and myeloid leukaemia: data from 56 children treated on NOPHO-AML protocols and a review of the literature. Ann Hematol 2006;85:275-80. [PubMed]
- Kudo K, Kojima S, Tabuchi K, et al. Prospective study of a pirarubicin, intermediate-dose cytarabine, and etoposide regimen in children with Down syndrome and acute myeloid leukemia: the Japanese Childhood AML Cooperative Study Group. J Clin Oncol 2007;25:5442-7. [PubMed]
- Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol 2009;27:4007-13. [PubMed]
- Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol 2010;11:543-52. [PubMed]
- Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol 2011;29:310-5. [PubMed]
- Gibson BE, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia 2005;19:2130-8. [PubMed]
- van der Velden VH, van der Sluijs-Geling A, Gibson BE, et al. Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia 2010;24:1599-606. [PubMed]
- Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children’s Oncology Group. Blood 2012;120:1581-8. [PubMed]
- Inaba H, Rubnitz JE, Coustan-Smith E, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol 2011;29:3293-300. [PubMed]
- Zwaan CM, Reinhardt D, Zimmerman M, et al. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: results of a phase II study. Br J Haematol 2010;148:768-76. [PubMed]
- Brethon B, Yakouben K, Oudot C, et al. Efficacy of fractionated gemtuzumab ozogamicin combined with cytarabine in advanced childhood myeloid leukaemia. Br J Haematol 2008;143:541-7. [PubMed]
- Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer 2012;118:761-9. [PubMed]
- Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012;379:1508-16. [PubMed]
- Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 2012;30:3924-31. [PubMed]
- Brunnberg U, Mohr M, Noppeney R, et al. Induction therapy of AML with ara-C plus daunorubicin versus ara-C plus gemtuzumab ozogamicin: a randomized phase II trial in elderly patients. Ann Oncol 2012;23:990-6. [PubMed]
- Amadori S, Suciu S, Stasi R, et al. Sequential Combination of Gemtuzumab Ozogamicin and Standard Chemotherapy in Older Patients With Newly Diagnosed Acute Myeloid Leukemia: Results of a Randomized Phase III Trial by the EORTC and GIMEMA Consortium (AML-17). J Clin Oncol 2013;31:4424-30. [PubMed]
- Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol 2011;29:369-77. [PubMed]
- Magrath I. Malignant Non-Hodgkin Lymphomas in Children. In: Pizzo P, Poplack D. eds. Principles and Practice of Pediatric Oncology. 4th ed. Lippincott Williams & Wilkins, Philadelphia, 2002:661-705.
- Anderson JR, Jenkin RD, Wilson JF, et al. Long-term follow-up of patients treated with COMP or LSA2L2 therapy for childhood non-Hodgkin’s lymphoma: a report of CCG-551 from the Childrens Cancer Group. J Clin Oncol 1993;11:1024-32. [PubMed]
- Anderson JR, Wilson JF, Jenkin DT, et al. Childhood non-Hodgkin’s lymphoma. The results of a randomized therapeutic trial comparing a 4-drug regimen (COMP) with a 10-drug regimen (LSA2-L2). N Engl J Med 1983;308:559-65. [PubMed]
- Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood 2000;95:416-21. [PubMed]
- Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood 2005;105:948-58. [PubMed]
- Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 2007;109:2736-43. [PubMed]
- Gerrard M, Cairo MS, Weston C, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Br J Haematol 2008;141:840-7. [PubMed]
- Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood 2007;109:2773-80. [PubMed]
- Patte C, Auperin A, Michon J, et al. The Societe Francaise d’Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood 2001;97:3370-9. [PubMed]
- Gerrard M, Waxman IM, Sposto R, et al. Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy. Blood 2013;121:278-85. [PubMed]
- Le Deley MC, Reiter A, Williams D, et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood 2008;111:1560-6. [PubMed]
- Lowe EJ, Sposto R, Perkins SL, et al. Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: final results of Children’s Cancer Group Study 5941. Pediatr Blood Cancer 2009;52:335-9. [PubMed]
- Asselin BL, Devidas M, Wang C, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children’s Oncology Group (POG 9404). Blood 2011;118:874-83. [PubMed]
- Ducassou S, Ferlay C, Bergeron C, et al. Clinical presentation, evolution, and prognosis of precursor B-cell lymphoblastic lymphoma in trials LMT96, EORTC 58881, and EORTC 58951. Br J Haematol 2011;152:441-51. [PubMed]
- Sandlund JT, Pui CH, Zhou Y, et al. Effective treatment of advanced-stage childhood lymphoblastic lymphoma without prophylactic cranial irradiation: results of St Jude NHL13 study. Leukemia 2009;23:1127-30. [PubMed]
- Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379-91. [PubMed]
- Meinhardt A, Burkhardt B, Zimmermann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol 2010;28:3115-21. [PubMed]
- Goldman S, Smith L, Anderson JR, et al. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia 2013;27:1174-7. [PubMed]
- Windebank K, Walwyn T, Kirk R, et al. Post cardiac transplantation lymphoproliferative disorder presenting as t(8;14) Burkitt leukaemia/lymphoma treated with low intensity chemotherapy and rituximab. Pediatr Blood Cancer 2009;53:392-6. [PubMed]
- Gross TG, Orjuela MA, Perkins SL, et al. Low-dose chemotherapy and rituximab for posttransplant lymphoproliferative disease (PTLD): a Children’s Oncology Group Report. Am J Transplant 2012;12:3069-75. [PubMed]
- Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 2012;30:2190-6. [PubMed]
- Mossé YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol 2013;14:472-80. [PubMed]
- Devita VT Jr, Serpick AA, Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Ann Intern Med 1970;73:881-95. [PubMed]
- Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood 2011;117:1806-16. [PubMed]
- Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2012;59:1259-65. [PubMed]
- Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood 2009;114:2051-9. [PubMed]
- Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma. JAMA 2012;307:2609-16. [PubMed]
- Dörffel W, Rühl U, Lüders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: final results of the multinational trial GPOH-HD95. J Clin Oncol 2013;31:1562-8. [PubMed]
- Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol 2010;28:3680-6. [PubMed]
- Kelly KM, Sposto R, Hutchinson R, et al. BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children’s Oncology Group. Blood 2011;117:2596-603. [PubMed]
- Gallamini A, Kostakoglu L. Interim FDG-PET in Hodgkin lymphoma: a compass for a safe navigation in clinical trials? Blood 2012;120:4913-20. [PubMed]
- EU Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu
- Gottardo NG, Gajjar A. Current therapy for medulloblastoma. Curr Treat Options Neurol 2006;8:319-34. [PubMed]
- Merchant TE, Kun LE, Krasin MJ, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys 2008;70:782-7. [PubMed]
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 2006;24:4202-8. [PubMed]
- Gandola L, Massimino M, Cefalo G, et al. Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. J Clin Oncol 2009;27:566-71. [PubMed]
- Jakacki RI, Burger PC, Zhou T, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children’s Oncology Group Phase I/II study. J Clin Oncol 2012;30:2648-53. [PubMed]
- Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 2006;7:813-20. [PubMed]
- Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol 2005;23:7621-31. [PubMed]
- Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol 2010;28:4961-8. [PubMed]
- Dhall G, Grodman H, Ji L, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer 2008;50:1169-75. [PubMed]
- Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer 2012;12:818-34. [PubMed]
- Northcott PA, Korshunov A, Pfister SM, et al. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol 2012;8:340-51. [PubMed]
- Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012;123:465-72. [PubMed]
- Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 2011;121:381-96. [PubMed]
- Ellison DW, Onilude OE, Lindsey JC, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J Clin Oncol 2005;23:7951-7. [PubMed]
- Chen JK, Taipale J, Young KE, et al. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A 2002;99:14071-6. [PubMed]
- Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol 2010;28:5321-6. [PubMed]
- De Smaele E, Ferretti E, Gulino A. Vismodegib, a small-molecule inhibitor of the hedgehog pathway for the treatment of advanced cancers. Curr Opin Investig Drugs 2010;11:707-18. [PubMed]
- Listernick R, Ferner RE, Liu GT, et al. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol 2007;61:189-98. [PubMed]
- Nicolin G, Parkin P, Mabbott D, et al. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer 2009;53:1231-7. [PubMed]
- Wisoff JH, Sanford RA, Heier LA, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery 2011;68:1548-54. [PubMed]
- Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol 2012;14:1265-84. [PubMed]
- Mirow C, Pietsch T, Berkefeld S, et al. Children <1 year show an inferior outcome when treated according to the traditional LGG treatment strategy: a report from the German multicenter trial HIT-LGG 1996 for children with low grade glioma (LGG). Pediatr Blood Cancer 2014;61:457-63. [PubMed]
- Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:2641-7. [PubMed]
- Pierce SM, Barnes PD, Loeffler JS, et al. Definitive radiation therapy in the management of symptomatic patients with optic glioma. Survival and long-term effects. Cancer 1990;65:45-52. [PubMed]
- Pollack IF, Claassen D, al-Shboul Q, et al. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg 1995;82:536-47. [PubMed]
- Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist 2004;10:293-310. [PubMed]
- Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol 2006;24:2570-5. [PubMed]
- Packer RJ, Lange B, Ater J, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol 1993;11:850-6. [PubMed]
- Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg 1997;86:747-54. [PubMed]
- Prados MD, Edwards MS, Rabbitt J, et al. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol 1997;32:235-41. [PubMed]
- Petronio J, Edwards MS, Prados M, et al. Management of chiasmal and hypothalamic gliomas of infancy and childhood with chemotherapy. J Neurosurg 1991;74:701-8. [PubMed]
- Moreno L, Bautista F, Ashley S, et al. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer 2010;46:2253-9. [PubMed]
- Jakacki RI, Bouffet E, Adamson PC, et al. A phase 1 study of vinblastine in combination with carboplatin for children with low-grade gliomas: a Children’s Oncology Group phase 1 consortium study. Neuro Oncol 2011;13:910-5. [PubMed]
- Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol 2012;30:1358-63. [PubMed]
- Khaw SL, Coleman LT, Downie PA, et al. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer 2007;49:808-11. [PubMed]
- Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer 2009;52:791-5. [PubMed]
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 2003;3:203-16. [PubMed]
- Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009;27:289-97. [PubMed]
- Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 2010;363:1313-23. [PubMed]
- Strother DR, London WB, Schmidt ML, et al. Outcome After Surgery Alone or With Restricted Use of Chemotherapy for Patients With Low-Risk Neuroblastoma: Results of Children’s Oncology Group Study P9641. J Clin Oncol 2012;30:1842-8. [PubMed]
- Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol 2013;14:999-1008. [PubMed]
- De Bernardi B, Mosseri V, Rubie H, et al. Treatment of localised resectable neuroblastoma. Results of the LNESG1 study by the SIOP Europe Neuroblastoma Group. Br J Cancer 2008;99:1027-33. [PubMed]
- Hero B, Simon T, Spitz R, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol 2008;26:1504-10. [PubMed]
- Nuchtern JG, London WB, Barnewolt CE, et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children’s Oncology Group study. Ann Surg 2012;256:573-80. [PubMed]
- Rubie H, De Bernardi B, Gerrard M, et al. Excellent outcome with reduced treatment in infants with nonmetastatic and unresectable neuroblastoma without MYCN amplification: results of the prospective INES 99.1. J Clin Oncol 2011;29:449-55. [PubMed]
- Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324-34. [PubMed]
- Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res 2006;12:1750-9. [PubMed]
- Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol 2010;28:4969-75. [PubMed]
- Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol 2003;21:2486-91. [PubMed]
- Schmidt M, Simon T, Hero B, et al. The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: results of the German Neuroblastoma Trial NB97. Eur J Cancer 2008;44:1552-8. [PubMed]
- Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children’s oncology group. J Nucl Med 2013;54:541-8. [PubMed]
- DuBois SG, Chesler L, Groshen S, et al. Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: a new approaches to neuroblastoma therapy trial. Clin Cancer Res 2012;18:2679-86. [PubMed]
- Yanik GA, Levine JE, Matthay KK, et al. Pilot study of iodine-131-metaiodobenzylguanidine in combination with myeloablative chemotherapy and autologous stem-cell support for the treatment of neuroblastoma. J Clin Oncol 2002;20:2142-9. [PubMed]
- Matthay KK, Tan JC, Villablanca JG, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: a new approaches to Neuroblastoma Therapy Consortium Study. J Clin Oncol 2006;24:500-6. [PubMed]
- Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet 2013;45:279-84. [PubMed]
- Molenaar JJ, Koster J, Zwijnenburg DA, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 2012;483:589-93. [PubMed]
- Cheung NK, Zhang J, Lu C, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA 2012;307:1062-71. [PubMed]
- Potratz J, Dirksen U, Jurgens H, et al. Ewing sarcoma: clinical state-of-the-art. Pediatr Hematol Oncol 2012;29:1-11. [PubMed]
- Nesbit ME Jr, Gehan EA, Burgert EO Jr, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol 1990;8:1664-74. [PubMed]
- Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694-701. [PubMed]
- Wexler LH, DeLaney TF, Tsokos M, et al. Ifosfamide and etoposide plus vincristine, doxorubicin, and cyclophosphamide for newly diagnosed Ewing’s sarcoma family of tumors. Cancer 1996;78:901-11. [PubMed]
- Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:4148-54. [PubMed]
- Miser JS, Goldsby RE, Chen Z, et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: evaluation of increasing the dose intensity of chemotherapy--a report from the Children’s Oncology Group. Pediatr Blood Cancer 2007;49:894-900. [PubMed]
- Ladenstein R, Potschger U, Le Deley MC, et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol 2010;28:3284-91. [PubMed]
- Saylors RL 3rd, Stine KC, Sullivan J, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol 2001;19:3463-9. [PubMed]
- Hunold A, Weddeling N, Paulussen M, et al. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer 2006;47:795-800. [PubMed]
- Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer 2009;53:1029-34. [PubMed]
- Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol 2000;18:3108-14. [PubMed]
- Bacci G, Ferrari S, Bertoni F, et al. Prognostic factors in nonmetastatic Ewing’s sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol 2000;18:4-11. [PubMed]
- Gaspar N, Rey A, Berard PM, et al. Risk adapted chemotherapy for localised Ewing’s sarcoma of bone: the French EW93 study. Eur J Cancer 2012;48:1376-85. [PubMed]
- Paulussen M, Ahrens S, Burdach S, et al. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann Oncol 1998;9:275-81. [PubMed]
- International Standard Randomised Controlled Trial Number Register. Available online: http://controlled-trials.com/ISRCTN92192408
- Juergens H, Daw NC, Geoerger B, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol 2011;29:4534-40. [PubMed]
- Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol 2010;11:129-35. [PubMed]
- Tap WD, Demetri G, Barnette P, et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol 2012;30:1849-56. [PubMed]
- Malempati S, Weigel B, Ingle AM, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:256-62. [PubMed]
- Naing A, LoRusso P, Fu S, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res 2012;18:2625-31. [PubMed]
- Gorlick R, Janeway K, Lessnick S, et al. Children’s Oncology Group’s 2013 blueprint for research: bone tumors. Pediatr Blood Cancer 2013;60:1009-15. [PubMed]
- Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol 2008;26:399-405. [PubMed]
- Gururangan S, Fangusaro J, Young Poussaint T, et al. Lack of efficacy of bevacizumab + irinotecan in cases of pediatric recurrent ependymoma--a Pediatric Brain Tumor Consortium study. Neuro Oncol 2012;14:1404-12. [PubMed]
- Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol 2010;28:3069-75. [PubMed]
- Wagner L, Turpin B, Nagarajan R, et al. Pilot study of vincristine, oral irinotecan, and temozolomide (VOIT regimen) combined with bevacizumab in pediatric patients with recurrent solid tumors or brain tumors. Pediatr Blood Cancer 2013;60:1447-51. [PubMed]
- Okada K, Yamasaki K, Tanaka C, et al. Phase I study of bevacizumab plus irinotecan in pediatric patients with recurrent/refractory solid tumors. Jpn J Clin Oncol 2013;43:1073-9. [PubMed]
- Venkatramani R, Malogolowkin M, Davidson TB, et al. A phase I study of vincristine, irinotecan, temozolomide and bevacizumab (vitb) in pediatric patients with relapsed solid tumors. PLoS One 2013;8:e68416. [PubMed]
- Navid F, Baker SD, McCarville MB, et al. Phase I and clinical pharmacology study of bevacizumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin Cancer Res 2013;19:236-46. [PubMed]
- Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol 2014;16:310-7. [PubMed]
- Blaney SM, Bernstein M, Neville K, et al. Phase I study of the proteasome inhibitor bortezomib in pediatric patients with refractory solid tumors: a Children’s Oncology Group study (ADVL0015). J Clin Oncol 2004;22:4804-9. [PubMed]
- Messinger Y, Gaynon P, Raetz E, et al. Phase I study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia (TACL) consortium. Pediatr Blood Cancer 2010;55:254-9. [PubMed]
- Horton TM, Pati D, Plon SE, et al. A phase 1 study of the proteasome inhibitor bortezomib in pediatric patients with refractory leukemia: a Children’s Oncology Group study. Clin Cancer Res 2007;13:1516-22. [PubMed]
- Muscal JA, Thompson PA, Horton TM, et al. A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: a Children’s Oncology Group phase I consortium study (ADVL0916). Pediatr Blood Cancer 2013;60:390-5. [PubMed]
- Messinger YH, Gaynon PS, Sposto R, et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood 2012;120:285-90. [PubMed]
- Witt O, Milde T, Deubzer HE, et al. Phase I/II intra-patient dose escalation study of vorinostat in children with relapsed solid tumor, lymphoma or leukemia. Klin Padiatr 2012;224:398-403. [PubMed]
- Hummel TR, Wagner L, Ahern C, et al. A pediatric phase 1 trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: a Children’s Oncology Group phase 1 consortium study. Pediatr Blood Cancer 2013;60:1452-7. [PubMed]
- Fouladi M, Park JR, Stewart CF, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children’s Oncology Group phase I consortium report. J Clin Oncol 2010;28:3623-9. [PubMed]
- Widemann BC, Kim A, Fox E, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children’s Oncology Group Phase I Consortium report. Clin Cancer Res 2012;18:6011-22. [PubMed]
- Kim A, Dombi E, Tepas K, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr Blood Cancer 2013;60:396-401. [PubMed]
- Geoerger B, Hargrave D, Thomas F, et al. Innovative Therapies for Children with Cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol 2011;13:109-18. [PubMed]
- Broniscer A, Baker SJ, Stewart CF, et al. Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clin Cancer Res 2009;15:701-7. [PubMed]
- Yalon M, Rood B, MacDonald TJ, et al. A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG). Pediatr Blood Cancer 2013;60:71-6. [PubMed]
- Jakacki RI, Hamilton M, Gilbertson RJ, et al. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children’s Oncology Group Phase I Consortium Study. J Clin Oncol 2008;26:4921-7. [PubMed]
- Geoerger B, Kieran MW, Grupp S, et al. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur J Cancer 2012;48:253-62. [PubMed]
- Spunt SL, Grupp SA, Vik TA, et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol 2011;29:2933-40. [PubMed]
- Fouladi M, Laningham F, Wu J, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol 2007;25:4806-12. [PubMed]
- Gore L, Trippett TM, Katzenstein HM, et al. A multicenter, first-in-pediatrics, phase 1, pharmacokinetic and pharmacodynamic study of ridaforolimus in patients with refractory solid tumors. Clin Cancer Res 2013;19:3649-58. [PubMed]
- Haas-Kogan DA, Banerjee A, Kocak M, et al. Phase I trial of tipifarnib in children with newly diagnosed intrinsic diffuse brainstem glioma. Neuro Oncol 2008;10:341-7. [PubMed]
- Haas-Kogan DA, Banerjee A, Poussaint TY, et al. Phase II trial of tipifarnib and radiation in children with newly diagnosed diffuse intrinsic pontine gliomas. Neuro Oncol 2011;13:298-306. [PubMed]
- Fouladi M, Nicholson HS, Zhou T, et al. A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high-grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: a Children’s Oncology Group study. Cancer 2007;110:2535-41. [PubMed]
- Widemann BC, Arceci RJ, Jayaprakash N, et al. Phase 1 trial and pharmacokinetic study of the farnesyl transferase inhibitor tipifarnib in children and adolescents with refractory leukemias: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2011;56:226-33. [PubMed]
- Widemann BC, Salzer WL, Arceci RJ, et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol 2006;24:507-16. [PubMed]
- Culy C. Bevacizumab: antiangiogenic cancer therapy. Drugs Today (Barc) 2005;41:23-36. [PubMed]
- Kerr DJ. Targeting angiogenesis in cancer: clinical development of bevacizumab. Nat Clin Pract Oncol 2004;1:39-43. [PubMed]
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer 2004;4:349-60. [PubMed]
- Kane RC, Bross PF, Farrell AT, et al. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist 2003;8:508-13. [PubMed]
- Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res 2007;13:5291-4. [PubMed]
- Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol 2002;20:4420-7. [PubMed]
- Marks PA, Xu WS. Histone deacetylase inhibitors: Potential in cancer therapy. J Cell Biochem 2009;107:600-8. [PubMed]
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 2007;25:84-90. [PubMed]
- Iyer R, Fetterly G, Lugade A, et al. Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother 2010;11:1943-55. [PubMed]
- Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res 2006;12:7271-8. [PubMed]
- Kane RC, Farrell AT, Madabushi R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 2009;14:95-100. [PubMed]
- Bukowski RM, Yasothan U, Kirkpatrick P. Pazopanib. Nat Rev Drug Discov 2010;9:17-8. [PubMed]
- van Geel RM, Beijnen JH, Schellens JH. Concise drug review: pazopanib and axitinib. Oncologist 2012;17:1081-9. [PubMed]
- Hepgur M, Sadeghi S, Dorff TB, et al. Tivozanib in the treatment of renal cell carcinoma. Biologics 2013;7:139-48. [PubMed]
- Grunwald MR, Levis MJ. FLT3 inhibitors for acute myeloid leukemia: a review of their efficacy and mechanisms of resistance. Int J Hematol 2013;97:683-94. [PubMed]
- Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res 1997;57:4838-48. [PubMed]
- Johnson JR, Cohen M, Sridhara R, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res 2005;11:6414-21. [PubMed]
- Senderowicz AM, Johnson JR, Sridhara R, et al. Erlotinib/gemcitabine for first-line treatment of locally advanced or metastatic adenocarcinoma of the pancreas. Oncology (Williston Park) 2007;21:1696-706; discussion 1706-9, 1712, 1715.
- Tsimberidou AM, Chandhasin C, Kurzrock R. Farnesyltransferase inhibitors: where are we now? Expert Opin Investig Drugs 2010;19:1569-80. [PubMed]
- Jabbour E, Kantarjian H, Cortes J. Clinical activity of tipifarnib in hematologic malignancies. Expert Opin Investig Drugs 2007;16:381-92. [PubMed]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004;18:1926-45. [PubMed]
- Hassan B, Akcakanat A, Holder AM, et al. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg Oncol Clin N Am 2013;22:641-64. [PubMed]


