Mesenchymal stem cells: potential application in intervertebral disc regeneration
Introduction
Chronic low back pain is a widespread condition which affects a large portion of the population and is the predominant cause of disability, resulting in huge cost implications, economic outlay and health provisions (1). Causing approximately $50 billion of estimated expenditure in the USA alone and with 210,000 Australians developing chronic low back pain annually the costs are increasing, with the direct cost of $1.2 billion in Australia in 2001 (2,3).
Although the exact cause of back pain remains to be defined, degenerative disc disease is considered to be a predominant source of chronic low back pain (4). The intervertebral discs (IVD) act as shock absorbers during compressive loading of the spine. As the disc degenerates, the spinal column motion segment function decreases, with less load-bearing potential. The disc is an immuno-privileged avascular organ with a low cell density and abundant extracellular matrix (ECM). It is well known that a damaged disc has a very limited capacity for self-repair. At the cellular level, the degeneration process is characterized by cellular dysfunction accompanied by reduced synthesis of ECM (5).
Disc degeneration is currently managed by non-surgical, conservative treatments or surgical interventions aimed at symptomatic relief and muscular stabilization with no clinical therapy targeting the reversal of disc degeneration (6). Recent advances in stem cell biology and tissue engineering provide an exciting potential approach to bio-regeneration focusing on the delivery of cells capable of restoring disc function.
Considering the limited regenerative capacity of the IVD, suitable sources of cells for regenerative therapies are highly sought after. Adult mesenchymal stem cells or stromal cells (MSCs) are multipotent (7), mainly residing in the bone marrow but also found in many other tissues. Bone marrow MSCs (BM-MSCs) and adipose tissue derived MSCs (AT-MSCs) are highly accessible, there are no ethical dilemmas for their use, they have the ability to self-renew, proliferate and differentiate into multiple mature cell types, including chondrocytes (8,9) and have been shown to possess immunomodulatory properties (10). Further, they are able to secrete a variety of soluble mediators and to be recruited chemotactically to injured tissues (11).
MCSs may participate in the repair of degenerative disc tissue in several ways: (I) supply the lost or damaged cells by direct differentiation into disc tissue-specific cells which promote the formation of ECM; (II) indirectly enhance tissue regeneration by the secretion of growth factors; and (III) immuno-modulate the inflammatory response (12). MSC based therapy is emerging as an extremely promising cell therapy for degenerative disc repair. This review aims to provide a better understanding of recent advances in MSC technologies for regenerative therapies in disc degeneration disease, their current challenges and future research directions.
Intervertebral disc degeneration (IDD)
Intervertebral disc (IVD)
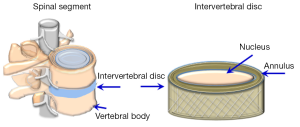
The IVD is located between the vertebral bodies of the spinal column (Figure 1). The properties of the disc allow for spinal mobility and its ability to resist compressive loads, as well as maintaining a uniform stress distribution in the area of the vertebral endplates (EP).
The disc is made up of an inner gelatinous structure, the nucleus pulposus (NP, nucleus) surrounded by a fence-like ring of fibrous laminated collagen, the annulus fibrosus (AF, annulus), sealed superiorly and inferiorly by the cartilaginous EP prior to the vertebral bodies. Each region of the disc has distinct functional structures and complex biological properties (13,14).
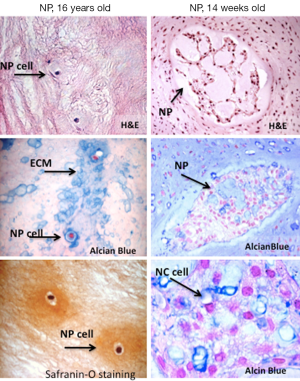
The nucleus pulposus is a hydrated gelatinous structure composed of water, ECM, and cellular elements. In healthy discs, the water content of the nucleus is 80-88% which progressively decreases with age. The NP is derived from the notochord during development. In humans, notochordal cells (Figure 2) disappear in early childhood and differentiate progressively into chondrocyte-like cells (15). The chondrocyte-like cells of the nucleus (Figure 3) are characterised by expression of major ECM components proteoglycans (PG), collagen II, a small amount of elastin fibres (16) and chondrogenic transcription factor SOX9 (17). The main PG within the NP is aggrecan, which contains approximately 30 chains of sulfated glycosaminoglycans (GAGs), contributing to a negative charge that fosters the hyper-hydrated state of the NP (18). NP cells are highly specialized and survive in a hypoxic environment with a high lactic acid concentration as well as relatively high hydrostatic pressure (19).
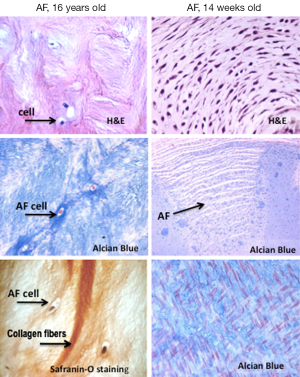
The AF consists of 15-25 concentric rings of collagen fibrils arranged parallel to each other in a lamellar fashion (Figure 3). Each lamella layer consists of parallel bundles of collagen fibers orientated at about 60° from the vertical axis, with the orientation being reversed in successive lamellae (14,20). The lamellar structure of the AF enables the IVD to withstand the high tensile stresses (circumferential, longitudinal and torsional) the spinal segments are subjected to. The collagen content is at its highest in the outer annulus, comprising up to 70% of its dry weight, with collagen type-I the predominant species, decreasing in a gradient towards the centre of the disc (21). The annulus structure is normally delineated into “outer” and “inner” annulus regions. The inner annulus is less laminar and consists of a more poorly organized ECM that contains type II collagen, PG and water, thereafter a thin fibrous tissue between the inner annulus and the central nucleus has been defined as a transitional zone. Smaller quantities of Type III, V, VI, IX and X collagens are present in the outer AF (22,23).
The annulus contains roughly 9×103 cells/mm3 of fibroblast-like cells embedded in the outer annulus forming a parallel pattern. More chondrocyte-like cells which are present in the inner annulus (Figure 2) (24).
The vertebral EP, composed of 0.6 mm thick avascular hyaline cartilage and fibrocartilage, partially calcified at the vertebral body interface (25), overlies the superior and inferior surface of the vertebral body and is strongly attached to the collagen fibers of the disc annulus. The cells of the EP are chondrocytic. In adulthood, the human EP has the typical structure of an epiphyseal growth plate, acting principally as a growth plate for the vertebral bodies (26). The biochemical components of the EP are similar to that of the disc: water, PG and collagen, but with less proteoglycan and water and higher collagen content than the adjacent regions of the disc. The EP serves as a semi-permeable membrane to facilitate diffusion of solutes from the vertebrae to the disc, which plays a vital role in disc nutrition by regulating the passive diffusion of nutrients into the avascular disc (25).
Intervertebral disc degeneration (IDD)
Back pain and discal degeneration
The degeneration of the delicate structures comprising the IVD is a major cause of back pain. Particularly manifested in the nucleus, changes in disc water content can be visualized using magnetic resonance imaging (MRI), with increasing degeneration visible as increased darkness in the disc region (27). Increasing grade of degeneration has been shown to correlate with back pain (28,29) and the extent of degeneration can be estimated using MRI (27,30-32).
A degree of degeneration is attributable to normal aging (28,33,34) and this, coupled with variability in sample sizes, definitions of degeneration and back pain and ethnic genetic diversity are attributed to inconsistent reports of back pain–disc degeneration correlations (35,36).
Certainly the costs related to back pain treatment and lost economic output are reaching the billions globally with the life time prevalence of back pain now approaching 85% (37) and statistics showing it as the main cause of disability worldwide (38).
The biology of IDD
IDD occurs as a consequence of the normal aging process (34) but can also be instigated by injury trauma (39).
Undoubtedly the main issue in IDD is the deterioration of nucleus pulposus tissue architecture (40,41). Changes are characterised by the loss of gelatinous, hydrated ECM into a more fibrous tissue. PG become fragmented and are lost from the tissue (14), leading to reduced hydration (42) and a loss of pressure within the nucleus (43). Discal degenerative changes occur progressively as a result of an altered balance between anabolic and catabolic processes in the disc (24,42,44,45). The loss of complex protein and proteoglycan networks that hold water and contribute to the shock absorbing properties in the disc (46), weaken and destabilise the structure contributing to loss of biomechanical function (19,24,47). Ongoing degeneration can lead to herniation, stenosis, segmental instability, and degenerative scoliosis.
Cellular changes: despite obvious tissue deterioration, viable cells isolated from degenerated discs have shown an ability to proliferate and differentiate in vitro (48), although with limited regeneration potential (49). Indeed, progenitor cells have been isolated from human degenerated discs (50), potentially derived from specific niche regions located near the cartilaginous end plate (51) and migrating into the disc (52).
Other studies have reported viable cells derived from degenerated discs with variable morphology but degenerated tissues had increased numbers of cells with the hallmarks of both necrosis and increased cell senescence and apoptosis (40,53,54). Cells were also found to form clusters in degenerated discs, in particular in the NP and inner AF (40,55) and many clustered cells showed increased production of matrix-degrading enzymes such as the matrix metalloproteinases (MMPs) (33,40).
Inflammatory mediators, cytokines & molecular dysregulation: the disc ECM is maintained by a balance of anabolic and catabolic molecular activities (19). In the degenerated disc, the catabolic activity of MMPs, disintegrins, metalloproteinases with thrombospondin motifs (ADAMTSs), and tissue inhibitors of metalloproteinases (TIMPs) is altered, compared to that of normal disc tissue (33,40,56-59), resulting in enhanced breakdown of ECM proteins. These proteins facilitate tissue remodeling in normal disc tissue and are regulated, at least in part, by biomechanical pressure stimuli (33,60,61). Conversely, expression of key ECM components aggrecan and collagen is reduced in degenerated disc tissues (59,62).
Inflammatory mediators, like metalloproteinases, have potential to act as normal growth signals in the normal disc as well as in pathology, but the regulation and expression of these molecules appears to be aberrant in disc degeneration. Increased expression of inflammatory cytokines such as IL-1, IL-6, IL-17 and TNFα have been detected in degenerated disc tissues (63-66) where expression was found in both invading inflammatory cells and resident cells of the NP (63). Degenerated tissues also displayed altered responses to inflammatory cytokines and changed receptor levels (44,62), with only chemokine CXCL8 mRNA expression found to be significantly up-regulated in degenerate compared to non-degenerate samples (63).
The release of chemokines from degenerating discs promotes the infiltration and activation of immune cells, further amplifying the inflammatory cascade and potentially also involving neurogenic factors capable of inducing and enhancing pain effects in the dorsal root ganglion (67). Leukocyte migration into the IVD is accompanied by the appearance of microvasculature tissue and nerve fibres (67) but there is evidence that such invasion is less likely in DDD compared to herniation (40).
Genetic variation in disc degeneration: there is increasing evidence that discal degeneration may be influenced by multiple inherited genetic factors which influence or predispose an individual to the development of this condition (68,69). Several allelic variations in genes coding for ECM proteins have been identified with linkage to degeneration: asporin, a small leucine-rich repeat proteoglycan (SLRP) family member implicated in several common bone and joint diseases (70,71) has also been identified as a susceptibility gene for discal degeneration (72). Immunohistochemical localization identified asporin in the OAF and less strongly the IAF and showed higher mRNA expression in degenerated discs (73). The ECM protein, cartilage intermediate layer protein (CILP) also showed significant allelic linkage with disc degeneration in a Japanese population (74). CILP is expressed in the disc ECM and levels are elevated in degeneration, increasing as degeneration grade progresses (74,75). Recently an isoform of fibronectin produced by alternative slicing has been linked with degeneration (76) as well as a genetic variant of the sulfotransferase gene (77).
Degenerative disc therapies
Current therapeutic options
There are no currently available treatments that target disc degeneration itself. Therapeutic interventions are for chronic or acute back pain and are determined by the degree, severity and persistence of pain. A variety of conservative treatment options form initial therapies generally involving rest, pain medication, specialist physiotherapy and/or specific back strengthening which aim to help the spine to heal naturally and in many cases such therapies are successful. However, pain will become chronic (present for more than three months) in approximately 20% of patients and about 5% of chronic back pain sufferers progress to surgical options for further treatment.
Surgical disc treatment mostly involves spinal fusion of two or more disc levels, but technological advancements include more minimally invasive disc replacement therapies that aim to preserve the natural motion of the vertebral segment. Motion preserving devices aimed at supporting the vertebrae by mimicking a normal disc are in limited use and under further development (47,78). The long term success of such devices in preserving existing spinal segments and maintaining full function is not yet known. Biodevices restore some function to the motion segment, however, contrary to expectations the risk of adjacent-level degeneration does not appear to have been reduced (79).
Future therapies
The cascade of degeneration being well known and well documented (24,80), it is attractive to envision the early intervention in discal deterioration by supporting and repairing existing disc tissues biologically, potentially an ideal solution to back pain caused by a loss of disc integrity and function.
Growth factors
One approach is the application of naturally occurring growth factors capable of stimulating appropriate protein synthesis directly into the disc, restoring tissue integrity and normal pain-free spinal function (81). Candidate proteins for therapeutic discal regeneration have been focused on the bone morphogenetic protein (BMP) family, several of which have shown promise (81-83). BMP13 is one member of this family that appears to play a role in the normal development of the spinal disc in vertebrates (84) and as a result, may have the potential to be a “best in class” molecule for therapeutic application to IDD. BMP13 has shown promise in early large animal studies of annular injury and disc repair (85). The BMP14 molecule is also being trialed as a potential disc regeneration therapeutic (86,87) and investigations into the potential of BMP7 have shown promise (88,89). Clinical grade proteins are proposed to be delivered directly into the disc space. Retention in the disc following injection may depend on many factors, not the least of which is the degree of degeneration and the integrity of the annular ring, data from animal studies is encouraging (85,88). Indeed purified BMP14 protein is currently under phase I/II clinical investigation for the treatment of early stage lumbar disc degeneration in Australia. These studies will provide important validation for the potential of BMP family members as future therapies.
Resident stem cells
Several studies have identified progenitor cell populations in the IVD which may provide a source of cells to repopulate a degenerated nucleus, data from animal studies is encouraging the correct stimuli (50,51,90,91). BMP13, following direct injection into the disc in a large animal study, showed evidence of having mobilized cells from the EP region, compared to controls (85) and indeed growth factor therapies rely on endogenous cell populations for their success. Some BMPs can function as chemoattractants (92-94) but involvement of BMP13 in inducing cell migration is still under study. Certainly cell migration into the nucleus pulposus has been reported and may be a valid mechanism for inducing the regeneration of this complex tissue (52,90,95).
MACs and their regeneration potential
MSC phenotypes
MSCs are adult stem cells that possess self-renewal ability, multilineage differentiation potential and immunomodulatory properties (7,85,96,97). The term MSCs refers to similar cell populations from multiple tissues that share the basic characteristics of stem cells, but remain heterogeneous cells with variations among individual donors and among different clones from the same donor.
Currently BM-MSCs, followed a close second by AT-MSCs, are major commercial sources of adult MSCs undergoing extensive studies and clinical trials as cell therapies (98-100). MSCs are characterized by the expression of CD73, CD90 and CD105 but lack expression of major lineage markers CD45, CD34, CD14, CD11b, CD79α, CD19 and HLA-DR. They are able to differentiate into multi-lineage specialized cells including osteoblasts, chondrocytes and adipocytes, functions required for a minimal criteria defining these cells (101). Although controversy exists regarding stem cell plasticity (102-104), under appropriate conditions MSCs are not only capable of differentiating into cells of mesenchymal origin (7) but can also trans-differentiate into cells of non-mesodermal lineages such as neuronal cells (104,105) and insulin-producing β-cells (106,107). This has led to the exploration of the therapeutic potential of MSCs for tissue repair in regenerative medicine and as therapeutic tools in immune-mediated diseases (97).
MSC therapeutic strategies
MSC based therapy aiming to restore damaged tissues relies on not only the ability of MSCs to give rise to specialized lineages, but also on their immunomodulatory and trophic effects. Generally, there are two strategies for the application of MSCs in tissue repair. Firstly, MSCs are used in an undifferentiated state, allowing the cells to undergo differentiation in vivo under the stimulation of local factors. Secondly, MSC differentiation occurs in vitro, prior to transplantation. The disadvantage of the former approach is that unwanted differentiation may occur at the injury site, but prior chondrogenic differentiation can render MSCs phenotypically stable and resistant to trans-differentiation in non-chondrogenic conditions (108). While prior in vitro differentiation ensures MSCs undergo lineage-directed commitment, increasing specificity of the administered cells, the process may affect their immunosuppressive potential (109). In addition, the prolonged cell manipulation procedure may involve increased risks and costs.
The IVD contains ECM derived from chondrocyte-like cells, but with unique properties, particularly in the central nucleus pulposus (24). Initial attempts at cell implantation using NP cells produced favourable results (110) however, given the limited number of disc cells available and the technical difficulties in their harvest, alternate sources such as MSCs are being pursued. Stem cell therapy involves MSC isolation from an appropriate tissue, in vitro expansion, potentially enhanced by additional growth factors or gene transfer to produce chondrocytic differentiation of the MSCs (111,112). Chondrogenic differentiation of MSCs for seven days produced cells that closely resembled the phenotype of native hyaline cartilage when combined with osteogenic cells in a bilayered oligo [poly (ethylene glycol) fumarate] hydrogel composite (113). Further, the respective chondrogenic and osteogenic phenotypes could be maintained in vitro for 28 days.
Effective MSC differentiation
Differentiation of MSCs into disc-like chondrocytic cells requires up-regulation of lineage-specific genes and suppression of genes associated with stem cell plasticity. There is a large body of research focused on the efficient induction of MSCs to differentiate into osteo-chondrocyte lineages. Methods of chondrogenic differentiation can be adopted and modified for differentiation of disc-like cells, with various in vitro differentiation conditions established that favour development of this phenotype along with a specific gene/protein expression profile to monitor the differentiation (114), characterised by up-regulation of SOX9 expression followed by chondrogenic specific matrix gene/protein expression. A three dimensional (3D) culture system and serum-free medium containing transforming growth factor β (TGF-β) and dexamethasone are fundamental conditions for chondrogenic differentiation, with TGF-β3 the most effective compared to other TGF-βs. Some BMPs can enhance TGF-β3-mediated chondrogenic differentiation, but are not effective when used alone (112,115).
MSCs from different individual donors may show various levels of differentiation, even under identical in vitro induction conditions. Epigenetic modification, the process of adding and removing chemical tags such as methyl or acetyl groups on DNA or histones which result in specific gene activation or suppression, is one explanation. Genomic regions that contain a high number of methylated cytosine are usually transcriptionally inactive; the absence of DNA methylation is a prerequisite for transcriptionally active genes (116). Whilst a limited number of studies have been performed, epigenetic regulation mechanisms have been identified in MSC chondrogenesis (117). DNA methylation of CpG-rich promoters associated with chondrocyte-specific genes were largely kept at low levels in human synovium-derived MSCs during in vitro chondrogenic differentiation. Further understanding of epigenetic complexities may provide solutions to donor variability, but at present it represents a known source of inconsistency.
MSC application to IVD repair
Chondrogenic differentiation of MSCs in vitro
MSCs can be induced to differentiate into chondrocytes or disc-like cells in vitro using several strategies: utilising co-culturing techniques, cytokine regulation, gene introduction, and 3D culture.
Co-culture
MSCs have been co-cultured with disc cells or tissues in vitro either in micromass culture or indirectly, to investigate the compatibility of MSCs in the IVD. Our own studies have demonstrated that rat BM-MSCs can differentiate into NP-like cells after co-culture with intact IVD tissue in a transwell membrane system (118), where although MSCs were separated from disc tissues by an insert membrane they showed morphological cell-cell/cell-tissue interactions via the pores of the membrane, through cytoplasmic processes (Figure 4). The presence of the disc tissue was sufficient to influence the MSCs differentiation pathway and cells from the disc may have provided soluble signalling molecules that influenced MSCs differentiation. Several other studies have co-cultured MSCs and NP cells under different conditions and reported similar observations (119-122). Direct cell-to-cell contact in specific ratios was essential for BM-MSC or AT-MSC (123) in monolayer to differentiate into an NP-like phenotype, with enhanced SOX9, aggrecan, collagen II and VI gene expression (111,124). The AT-MSCs may be more capable of differentiating into NP like cells than BM-MSCs (123). Effective chondrogenic differentiation of either umbilical cord blood derived mesenchymal stem cells (hUCB-MSCs) or olfactory stem cells was also achieved by injecting the MSCs into disc tissue in an in vitro culture system (125). Interestingly, even degenerated NP cells in co-culture with BM-MSCs induced increased production of collagen and PG (126).
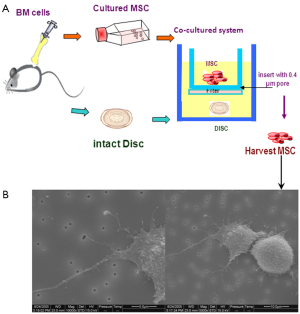
The underlying mechanisms of cell interactions in co-culture systems remain unclear, but overall the data suggest that co-culture systems are a powerful tool for the investigation of MSC potential in IVD regeneration. Evidence of bi-directional transfer of membrane components between the cell types, spontaneous cell fusion and gap-junctional communication have been reported, all potentially viable mechanisms (127,128).
Growth factors
A range of growth factors have been employed to induce chondrogenic differentiation of MSCs including TGF-βs, insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2), and BMPs (129). Of these, TGF-β1 has been most extensively investigated for inducing chondrogenesis in stem cells. An early study first reported that MSCs differentiated towards the chondrogenic lineage upon stimulation with TGF-β1 (130) and several studies have shown TGF-β3 to stimulate MSC to differentiate into chondrocytes (112,129,131) including under hypoxic conditions (131).
Apart from TGF-βs the BMPs, in particular BMP13 (GDF6) and BMP14 (GDF5), have shown MSC chondrogenic differentiation potential. BMP13 stimulation of MSCs in culture induced down-regulation of osteogenic expression markers and promoted higher levels of proteoglycan production (132). Further, undifferentiated MSCs, when treated with BMP13 in vitro, induced cellular proliferation and morphological changes resembling chondrocytes at 120 and 168 hours of culture (133). BMP14 possessed a greater ability to induce differentiation of BM-MSCs into NP-like cells than TGF-β under conditions of hypoxia (134). These data suggest that BMP13, unlike BMP2, may not induce the terminal differentiation of stem cells into bone, an attractive characteristic when considering disc regeneration therapy.
The combination of other growth factors with TGF-β has been examined for potential ability to induce MSCs towards a chondrogenic phenotype for use in IVD repair. BMP2 and TGF-β3 induced chondrogenic differentiation of human BM-MSC when cultured in alginate beads (112), and BM-MSC stimulation with BMP7 and TGF-β3 produced superior chondrocytic proteoglycan accumulation, collagen-II, and SOX9 protein expression in alginate and pellet cultures compared to either factor alone (135). Similarly a combination of TGF-β2 and BMP7 was shown to be the most effective combination in inducing AT-MSCs towards chondrogenic differentiation when compared to other factors (136).
Genetic manipulation
Directing the differentiation of MSCs into specific NP like cells can be achieved by genetic manipulation. SOX9, as an essential transcription factor for the differentiation of the chondrocytic lineage, is required for notochord maintenance and normal vertebral column formation during embryonic development (137). Enhanced chondrogenesis of BM-MSCs was reported both in vitro and in vivo in a mouse model (138) and in human BM-MSCs (139) through over-expression of adenovirus-mediated SOX9. Similarly adenoviral-mediated SOX9 expression induced differentiation into NP-like cells, evident in the expression of chondrogenic genes and proteins (111). More recently BM-MSCs genetically modified with lentiviral-mediated anti-apoptotic GFP-Bcl-2 gene expression showed chondrogenic differentiation in nanofibrous scaffold cultures with TGF-β1 stimulation in hypoxia culture (140). Such studies provide valuable information regarding the appropriate genetic markers and gene regulation required to produce disc cells. However, studies related to disc regeneration are somewhat limited as there are still significant safety concerns regarding the in vivo use of gene therapy (141).
Scaffolds
The application of scaffolds in MSC therapy for disc regeneration will be discussed in section Scaffolds for MSC therapy in disc regeneration.
MSC therapy for disc repair in vivo
The regenerative potential of MSCs in vivo for disc regeneration has been studied with a variety of animal models by using allogeneic, autologous or xenogenic transplantation (118,142-146). MSCs alone, together with biomaterials or combined with cytokines have been introduced into the disc to attempt both repopulation and repair of damaged cells for the production of new disc matrix.
Survival of transplanted cells is a crucial step to ensure disc repair. Early studies have shown the survival of translocated MSCs in the special microenvironment of a healthy disc for up to six months, as well as their ability to differentiate into NP-like cells (147,148). Multiple in vivo studies in a wide array of animal models showed that BM-MSCs can survive and proliferate in the damaged disc post-transplantation. Surviving cells also differentiated towards a disc-like phenotype and produced disc matrix, with partial disc height restoration and hydration when compared to control groups (142,149-156). The use of AT-MSCs with a hyaluronic acid (HA) carrier was also successful, with increased disc matrix protein, restoration of disc hydration and MRI T2 signal intensity eight weeks post transplantation compared to controls (157).
Interestingly, several xenogeneic transplantation studies of human MSCs into murine, porcine or rabbit discs have demonstrated MSC survival without immunosuppressant administration (118,154,158,159). We have demonstrated that transplanted human BM CD34– cells survived in rat discs for up six weeks, expressing collagen II and aggrecan after three weeks transplantation (118). The implanted cells had a broad differentiation potential and an ability to develop into disc-like cells, suggesting that CD34– BM-MSCs are a potential source of cells for restoring degenerated discs (118). Notably, surviving xenogeneic cells expressed a high level of Fas-L protein, suggested to be an immunosuppressive factor (118).
Successful xenogeneic transplantation also reflects the immune-privileged nature of the disc and the immunosuppressive nature of MSCs (160). The immune privileged nature of the disc is attributed to the anatomically structured blood-tissue barrier of the NP, and also to the constitutive expression of Fas ligand on disc cells (161). In vivo studies are encouraging and the data consistent with the potential for the future clinical use for MSCs in IVD regeneration. This data is summarised in Table 1.

Full table
Scaffolds for MSC therapy in disc regeneration
Biomaterial scaffolds and hydrogels are three-dimensional matrices. Incorporation of MSCs into a biomaterial scaffold can enhance cell survival after transplantation. The scaffold acts as a carrier protecting MSCs from leaking into other tissues and concomitantly to support the transduction of mechanical loads. They also provide a framework to fertilise proliferation and differentiation of implanted MSCs. To improve the survival of transplanted MSCs, scaffolds need to be non-immunogenic, biodegradable, biocompatible and to withstand the mechanically loaded microenvironment in the IVD (162).
A range of scaffolds have been structured as ideal biomaterials for cell therapy in disc regeneration, including a variety of natural gels and hydrogels based on collagen, HA, GAGs, agarose, gelatine and alginate (163). Hydrogels based on collagen and HA have been the most widely employed in tissue engineering applications since they are hydrophilic polymers that readily allow for incorporation of biological signals and cells to derive into the native matrix (149,152,155,162). HA is the most abundant water absorption molecule in the natural disc, able to dehydrate and rehydrate under a range of mechanical loading parameters. Such scaffolds also ideally degrade slowly to permit the seeded MSCs to differentiate and produce new matrix (135,162). More recently it was demonstrated that AT-MSCs seeded in chitosan-alginate-gel scaffolds are capable of differentiating into NP-like cells under hypoxic conditions (164). Similarly, MSC seeded in an injectable hydrogel differentiated along a chondrogenic lineage under physiological loading conditions (165) or when combined with pentosan polysulphate (166,167).
Several hydrogels constructed from natural components of the ECM including fibrin (168), hyaluronan (169), and collagen (170) and natural biopolymers such as chitosan (171) have been investigated as carriers for cell delivery to the disc. Thermoreversible chitosan-glycerophosphate (C/Gp) hydrogels alone are capable of inducing MSC differentiation in cultures (172), and the PEG-LM111 hydrogel in explant cultures showed significantly higher cell retention properties over two weeks, compared to cells in liquid suspension (173).
The therapeutic potential of injectable scaffolds as cell carriers for disc regeneration has been investigated in vivo in animal models (149,174,175). Thermoreversible Atelocollagen is composed of 0.3% collagen and, like C/Gp gels, is liquid at room temperature and gelatinizes at body temperature. Transplantation of autologous MSCs encapsulated in Atelocollagen gel into the degenerated disc of a rabbit produced deceleration of the degeneration process (149). Similarly, autologous BM-MSCs embedded in HA-derived biodegradable polymers, Hyaff®-120 and Hyadd®-3 and injected into damaged, nucleotomized porcine discs maintained the normal biconvex structure of the NP and contained viable cells forming a matrix at six weeks, compared to the control group undergoing nucleotomy alone (175).
Human clinical trials
MSC transplantation for the treatment of disc degeneration disease in human clinical trials has shown encouraging outcomes. Autologous BM-MSCs have been reported for the first time to treat disc degeneration in two patients with back and leg pain (176), with a collagen sponge containing MSCs transplanted into the degenerated IVD. At two years post-transplantation, pain was reduced and an intradiscal water content increase was observed in both patients. No improvement of disc height was reported.
A pilot phase-1 study in 10 patients (age 35±7 years) with chronic low back pain and lumbar disc degeneration was conducted using autologous BM-MSCs expanded in vitro and injected into the NP of degenerated discs, without major surgery (177). After one year of transplantation, pain relief and an improvement in disc hydration (by T2-weighted sagittal images) were observed in nine patients (9/10). The author concluded that MSC therapy may be a valid alternative treatment for back pain caused by degenerative disc disease. However, disc height was not improved, control groups were not included and evidence of survival of injected cells was also not provided in the trial.
More recently, a multi-centre FDA-approved clinical trial was performed in the United States [2012], including 100 participants with IDD who received a single injection of BM-MSCs encapsulated in a HA carrier, followed for a period of three years.
The outcome of these phase-1 clinical trails is encouraging, suggesting that MSC based therapy is clinically safe and produced relatively effective relief of back pain. However, studies to determine the long term safety and survival of MSCs in the degenerated disc are still needed in order to determine its full potential.
Current challenges in MSC
Although MSCs show great potential in regenerative medicine, issues are emerging regarding quality control and standardization of cells for clinical application. To answer the following questions will be critically important for future safety and efficacy of MSC based therapies.
Obtaining sufficient cell numbers whilst maintaining MSC characteristics
Adult MSCs exist at extremely low frequency. Although human bone marrow is considered one of richest sources, MSCs only account for 0.001-0.01% of total bone marrow mononuclear cells upon isolation. There is no doubt that therapeutic MSCs must be expanded in vitro to provide sufficient cell numbers (>1×106/kg) without losing stem cell characteristics, which can be judged by examining morphology, cell surface marker phenotypes, multiple differentiation potential, cytogenetic stability and their immunosuppressive property. Recent research suggests that human BM-MSCs in culture maintain their morphology and a stable phenotype until passages 6-8, with some individual donor variation (178). With low propensity for spontaneous transformation, they could be safely expanded in vitro without immortalization or development of chromosomal abnormalities (179,180). However, other studies have suggested a risk of cells accumulating genetic and epigenetic abnormalities beyond passage 4 (181). To date, this issue remains a major challenge for stem cell expansion on a clinical scale.
The use of growth factors or cytokines in addition to foetal bovine serum (FBS), such as epidermal growth factor (EGF) (182), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF) or ascorbic acid, may boost MSC proliferation and suppress spontaneous differentiation during in vitro expansion (183). Importantly, such treatment does not appear to bias future differentiation and immunosuppressive properties of the cells. Our own unpublished data suggests that supplementation of growth factors or cytokines can increase MSC proliferation yielding more than 10-fold higher cell numbers than basic cultures. However, overloading of factors aimed at promoting MSC proliferation may alter cell characteristics.
Most laboratories culture MSCs under normoxia (20% O2) conditions. Hypoxic (2-9% O2) environments can greatly improve growth kinetics of MSC, multi-lineage differentiation capacity, genetic stability and chemokines receptor expression during in vitro expansion (184,185). Optimal formulation of culture medium and conditions for in vitro MSC expansion will require further study to ensure optimal yields.
Standardization of methods for MSC isolation, expansion and characterization
The huge potential of MSC therapy in regenerative medicine has attracted tremendous interest for MSC research. Study methodology, however, lacks coherence in methods of isolation, expansion and characterization making outcomes comparisons difficult, despite the publication of minimal criteria to define human MSCs (101). Variability is largely derived from the following factors.
- Donor variation. Huge variability in proliferative capacity and life span has been noted among donors (179), which is associated with individual genetic and epigenetic variations, age, gender, and health status.
- Tissue variation. MSCs derived from bone marrow, adipose or other tissues are similar, but not identical.
- Isolation methods. Ficoll-Paque gradient centrifugation (1.077 g/mL) is included in most MSC isolation methods to obtain mononuclear cells, followed by further enrichment by plastic adherence for three days. In addition to this basic procedure extra purification steps may be added, such as immune-depletion or positive selection using monoclonal antibodies.
- Cell culture media. Basic MSC culture medium contains 10% FBS but can be up to 20%. In addition, the source and lot number of FBS provides increased variability in cell growth, reflecting considerable variations in composition of growth factors and other trophic factors in the serum.
- Oxygen pressure. MSCs are mostly cultured in ambient normoxia conditions. Currently though, more and more laboratories are realising the advantages of culturing MSCs under hypoxia (2-5% O2), conditions which influence cellular aging and composition leading to altered MSC functions.
Donor variation in MSCs is unavoidable but variability can perhaps be minimised by determining standard selection criteria and MSC expansion methodology. Such measures are extremely necessary in order to ensure reproducibility of MSC preparations and a high level of therapeutic efficacy.
How pure should the MSC population be?
No matter which method is applied to isolate MSCs, they are by nature a heterogeneous cell population containing a small percentage of stem cells and many other differently committed progenitors. These cells present heterogeneity in morphology, phenotype and multipotentiality. It remains unclear whether heterogeneous MSC populations are good enough or whether pure stem cells are required for clinical use. It is conceivable that heterogeneity contributes to their various therapeutic effects (186), However, clonogenic MSC subpopulations have been studied, showing that a CD73+/CD39+ human synovial MSCs subset displayed greater chondro-osteogenic potency (187).
Low engraftment rate, disadvantage or advantage?
Although the fate of MSCs after clinical infusion is not fully understood, MSCs have a relatively short life in the recipient and engraftment rates are low. To improve therapeutic efficacy, should we focus on increasing engraftment rates or improving MSC quality? In wound repair studies, although MSC differentiation occurred at injury sites, most of the therapeutic effects were attributed to soluble factors released by the administered cells which regulate local cellular responses to cutaneous injury (188). It was also observed in a clinical study that there was no correlation between MSC engraftment and treatment response, with MSCs appearing to mediate their function through a “hit and run” mechanism. Fortunately, the lack of sustained engraftment limits the long-term risks of malignant transformation and ectopic tissue formation during MSC therapy (189).
It remains to be established whether MSCs can differentiate into disc NP cells, with their precise phenotype and chondrocytic markers. Current evidence regarding MSC therapy for IVD repair is from small animal studies which may not resemble human disc degeneration.
Summary
MSC therapy for disc repair has made significant progress over the last several years with regards to further understanding of stem cell biology and the different applications of MSCs for the treatment of low back pain. MSCs have been reported to be effective in vitro for the differentiation of disc like cells. In addition, in vivo transplantation of MSCs has been shown to have regenerative potential in several animal models for disc degeneration. Overall, the recent advances in MSC therapies suggest a promising future for such therapies in disc repair.
Notably it has been suggested that injection of chondrocytes into the NP resulted in the formation of hyaline cartilage but not a gelatinous matrix (190), emphasizing that the direction of the MSCs towards NP cell fates is essential for effective disc repair. Moreover, the disc has unique anatomical characteristics and biological properties and much remains unknown about the molecular mechanisms and interactions in the degenerated disc, as well as the mechanisms of action behind the therapeutic effects of MSCs. In this regard, it may be beneficial for more efforts to focus on further investigating the survival of transplanted cells in the damaged disc niche, the molecular regulators and signalling pathways controlling proliferation and cell fates following transplantation, and the appropriate integration of the transplanted cells within their surrounding microenvironment
Although the current clinical literature involves only small sample sizes, phase-1 clinical trails of MSC therapy for low pack pain have provided certain positive outcomes for disc repair. These cases are valuable studies for creating a foundation to direct future experimental and clinical investigations. Importantly, safety remains one of the main concerns, particularly in view of the necessary in vitro manipulation of MSCs, such as for cell expansion. Thus, before any clinical application can be recommended, improved knowledge from basic studies and large scale randomized trials with controlled implementation will be necessary to determine the true safety and efficacy of cell therapies in IVD regeneration.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mathewa J, Singha SB, Garis S, et al. Backing up the stories: The psychological and social costs of chronic low-back pain. The International Journal of Spine Surgery 2013;7:e29-e38.
- Walker BF, Muller R, Grant WD. Low back pain in Australian adults: the economic burden. Asia Pac J Public Health 2003;15:79-87. [PubMed]
- Editorial. 2000-2001 Australian Bureau of Statistics. MJA 2000,175.
- Luoma K, Riihimaki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487-92. [PubMed]
- Chung SA, Khan SN, Diwan AD. The molecular basis of intervertebral disk degeneration. Orthop Clin North Am 2003;34:209-19. [PubMed]
- Kishen TJ, Diwan AD. Fusion versus disk replacement for degenerative conditions of the lumbar and cervical spine: quid est testimonium? Orthop Clin North Am 2010;41:167-81. [PubMed]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-7. [PubMed]
- Fortier LA. Stem cells: classifications, controversies, and clinical applications. Vet Surg 2005;34:415-23. [PubMed]
- Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol 1976;47:327-59. [PubMed]
- Uccelli A, Mancardi G, Chiesa S. Is there a role for mesenchymal stem cells in autoimmune diseases? Autoimmunity 2008;41:592-5. [PubMed]
- Hwang NS, Zhang C, Hwang YS, et al. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med 2009;1:97-106. [PubMed]
- Fierabracci A, Del Fattore A, Luciano R, et al. Recent advances in mesenchymal stem cell immunomodulation. the role of microvesicles. Cell Transplant 2013. [Epub ahead of print]. [PubMed]
- Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther 2003;5:120-30. [PubMed]
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine 2004;29:2691-9. [PubMed]
- Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng 2003;9:667-77. [PubMed]
- Bibby SR, Jones DA, Lee RB, et al. The pathophysiology of the intervertebral disc. Joint Bone Spine 2001;68:537-42. [PubMed]
- Gruber HE, Norton HJ, Ingram JA, et al. The SOX9 transcription factor in the human disc: decreased immunolocalization with age and disc degeneration. Spine (Phila Pa 1976) 2005;30:625-30. [PubMed]
- Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5-10. [PubMed]
- Kepler CK, Ponnappan RK, Tannoury CA, et al. The molecular basis of intervertebral disc degeneration. Spine J 2013;13:318-30. [PubMed]
- Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract 2008;8:18-44. [PubMed]
- Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J 2008;17 Suppl 4:480-91. [PubMed]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307-14. [PubMed]
- Eyre DR, Matsui Y, Wu JJ. Collagen polymorphisms of the intervertebral disc. Biochem Soc Trans 2002;30:844-8. [PubMed]
- Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 2006;88 Suppl 2:10-4. [PubMed]
- Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine 1989;14:166-74. [PubMed]
- Urban JP, Winlove CP. Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging 2007;25:419-32. [PubMed]
- Borthakur A, Maurer PM, Fenty M, et al. T1rho magnetic resonance imaging and discography pressure as novel biomarkers for disc degeneration and low back pain. Spine (Phila Pa 1976) 2011;36:2190-6. [PubMed]
- Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009;34:934-40. [PubMed]
- Zuo J, Joseph GB, Li X, et al. In vivo intervertebral disc characterization using magnetic resonance spectroscopy and T1rho imaging: association with discography and Oswestry Disability Index and Short Form-36 Health Survey. Spine (Phila Pa 1976) 2012;37:214-21. [PubMed]
- Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001;26:1873-8. [PubMed]
- Kjaer P, Leboeuf-Yde C, Korsholm L, et al. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976) 2005;30:1173-80. [PubMed]
- Antoniou J, Epure LM, Michalek AJ, et al. Analysis of quantitative magnetic resonance imaging and biomechanical parameters on human discs with different grades of degeneration. J Magn Reson Imaging 2013;38:1402-14. [PubMed]
- Vo NV, Hartman RA, Yurube T, et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J 2013;13:331-41. [PubMed]
- Singh K, Masuda K, Thonar EJ, et al. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976) 2009;34:10-6. [PubMed]
- Kovacs FM, Arana E, Royuela A, et al. Disc degeneration and chronic low back pain: an association which becomes nonsignificant when endplate changes and disc contour are taken into account. Neuroradiology 2014;56:25-33. [PubMed]
- Chou D, Samartzis D, Bellabarba C, et al. Degenerative magnetic resonance imaging changes in patients with chronic low back pain: a systematic review. Spine (Phila Pa 1976) 2011;36:S43-53. [PubMed]
- Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord 2000;13:205-17. [PubMed]
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163-96. [PubMed]
- Inoue N, Orias AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am 2011;42:487-99. vii. [PubMed]
- Lama P, Le Maitre CL, Dolan P, et al. Do intervertebral discs degenerate before they herniate, or after? Bone Joint J 2013;95-B:1127-33. [PubMed]
- Haefeli M, Kalberer F, Saegesser D, et al. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine (Phila Pa 1976) 2006;31:1522-31. [PubMed]
- Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc. Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing and degeneration. J Clin Invest 1996;98:996-1003. [PubMed]
- Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine (Phila Pa 1976) 1999;24:2468-74. [PubMed]
- Millward-Sadler SJ, Costello PW, Freemont AJ, et al. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther 2009;11:R65. [PubMed]
- Le Maitre CL, Richardson SM, Baird P, et al. Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc. J Pathol 2005;207:445-52. [PubMed]
- Iatridis JC, MacLean JJ, O’Brien M, et al. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila Pa 1976) 2007;32:1493-7. [PubMed]
- Iatridis JC, Nicoll SB, Michalek AJ, et al. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J 2013;13:243-62. [PubMed]
- Mizrahi O, Sheyn D, Tawackoli W, et al. Nucleus pulposus degeneration alters properties of resident progenitor cells. Spine J 2013;13:803-14. [PubMed]
- Hegewald AA, Endres M, Abbushi A, et al. Adequacy of herniated disc tissue as a cell source for nucleus pulposus regeneration. J Neurosurg Spine 2011;14:273-80. [PubMed]
- Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate intervertebral disc. Spine 2007;32:2537-44. [PubMed]
- Brisby H, Papadimitriou N, Brantsing C, et al. The presence of local mesenchymal progenitor cells in human degenerated intervertebral discs and possibilities to influence these in vitro: a descriptive study in humans. Stem Cells Dev 2013;22:804-14. [PubMed]
- Kim KW, Ha KY, Lee JS, et al. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine J 2009;9:323-9. [PubMed]
- Zhang L, Niu T, Yang SY, et al. The occurrence and regional distribution of DR4 on herniated disc cells: a potential apoptosis pathway in lumbar intervertebral disc. Spine (Phila Pa 1976) 2008;33:422-7. [PubMed]
- Chen YF, Zhang YZ, Zhang WL, et al. Insights into the hallmarks of human nucleus pulposus cells with particular reference to cell viability, phagocytic potential and long process formation. Int J Med Sci 2013;10:1805-16. [PubMed]
- Ciapetti G, Granchi D, Devescovi V, et al. Ex vivo observation of human intervertebral disc tissue and cells isolated from degenerated intervertebral discs. Eur Spine J 2012;21 Suppl 1:S10-9. [PubMed]
- Pockert AJ, Richardson SM, Le Maitre CL, et al. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum 2009;60:482-91. [PubMed]
- Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol 2004;204:47-54. [PubMed]
- Weiler C, Nerlich AG, Zipperer J, et al. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J 2002;11:308-20. [PubMed]
- Mern DS, Fontana J, Beierfuss A, et al. A combinatorial relative mass value evaluation of endogenous bioactive proteins in three-dimensional cultured nucleus pulposus cells of herniated intervertebral discs: identification of potential target proteins for gene therapeutic approaches. PLoS One 2013;8:e81467. [PubMed]
- Sowa GA, Coelho JP, Bell KM, et al. Alterations in gene expression in response to compression of nucleus pulposus cells. Spine J 2011;11:36-43. [PubMed]
- Korecki CL, MacLean JJ, Iatridis JC. Dynamic compression effects on intervertebral disc mechanics and biology. Spine (Phila Pa 1976) 2008;33:1403-9. [PubMed]
- Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 2005;7:R732-45. [PubMed]
- Phillips KL, Chiverton N, Michael AL, et al. The cytokine and chemokine expression profile of nucleus pulposus cells: implications for degeneration and regeneration of the intervertebral disc. Arthritis Res Ther 2013;15:R213. [PubMed]
- Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809-14. [PubMed]
- Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther 2007;9:R77. [PubMed]
- Studer RK, Vo N, Sowa G, et al. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-alpha. Spine (Phila Pa 1976) 2011;36:593-9. [PubMed]
- Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014;10:44-56. [PubMed]
- Kalichman L, Hunter DJ. The genetics of intervertebral disc degeneration. Familial predisposition and heritability estimation. Joint Bone Spine 2008;75:383-7. [PubMed]
- J Eskola P, Männikkö M, Samartzis D, et al. Genome-wide association studies of lumbar disc degeneration-are we there yet? Spine J 2014;14:479-82. [PubMed]
- Ikegawa S. Expression, regulation and function of asporin, a susceptibility gene in common bone and joint diseases. Curr Med Chem 2008;15:724-8. [PubMed]
- Kizawa H, Kou I, Iida A, et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet 2005;37:138-44. [PubMed]
- Song YQ, Cheung KM, Ho DW, et al. Association of the asporin D14 allele with lumbar-disc degeneration in Asians. Am J Hum Genet 2008;82:744-7. [PubMed]
- Gruber HE, Ingram JA, Hoelscher GL, et al. Asporin, a susceptibility gene in osteoarthritis, is expressed at higher levels in the more degenerate human intervertebral disc. Arthritis Res Ther 2009;11:R47. [PubMed]
- Seki S, Kawaguchi Y, Chiba K, et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet 2005;37:607-12. [PubMed]
- Virtanen IM, Song YQ, Cheung KM, et al. Phenotypic and population differences in the association between CILP and lumbar disc disease. J Med Genet 2007;44:285-8. [PubMed]
- Anderson DG, Markova D, Adams SL, et al. Fibronectin splicing variants in human intervertebral disc and association with disc degeneration. Spine (Phila Pa 1976) 2010;35:1581-8. [PubMed]
- Song YQ, Karasugi T, Cheung KM, et al. Lumbar disc degeneration is linked to a carbohydrate sulfotransferase 3 variant. J Clin Invest 2013;123:4909-17. [PubMed]
- Bae WC, Masuda K. Emerging technologies for molecular therapy for intervertebral disk degeneration. Orthop Clin North Am 2011;42:585-601. ix. [PubMed]
- Costi JJ, Freeman BJ, Elliott DM. Intervertebral disc properties: challenges for biodevices. Expert Rev Med Devices 2011;8:357-76. [PubMed]
- Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631-44. [PubMed]
- Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J 2008;17 Suppl 4:441-51. [PubMed]
- Than KD, Rahman SU, Vanaman MJ, et al. Bone morphogenetic proteins and degenerative disk disease. Neurosurgery 2012;70:996-1002. [PubMed]
- Yoon ST. Molecular therapy of the intervertebral disc. The Spine Journal 2005;5:280S-286S. [PubMed]
- Tassabehji M, Fang ZM, Hilton EN, et al. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Human Mutation 2008;29:1017-27. [PubMed]
- Wei A, Williams LA, Bhargav D, et al. BMP13 prevents the effects of annular injury in an ovine model. Int J Biol Sci 2009;5:388-96. [PubMed]
- Lee C, Attawia M, Holy C, et al. In vitro effects of recombinant human GDF-5 on human and rabbit intervertebral disc cells. The Spine Journal 2007;7:134S-135S.
- Lee C, Tonomura H, Asanuma K, et al. Effects of low doses of recombinant human growth and differentiation factor-5 on human intervertebral disc. The Spine Journal 2007;7:3S-4S.
- Masuda K, Imai Y, Okuma M, et al. Osteogenic protein-1 (OP-1) injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit annular puncture model Spine 2006;31:742-54. [PubMed]
- Miyamoto K, Masuda K, Kim JG, et al. Intradiscal injections of osteogenic protein-1 (OP-1) restore the visceoelastic properties of degenerated intervertebral discs. The Spine Journal 2006;6:692-703. [PubMed]
- Henriksson HB, Svala E, Skioldebrand E, et al. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the new zealand white rabbit. Spine (Phila Pa 1976) 2012;37:722-32. [PubMed]
- Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 2009;34:2278-87. [PubMed]
- Fiedler J, Roderer G, Gunther KP, et al. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem 2002;87:305-12. [PubMed]
- Lee DH, Park BJ, Lee MS, et al. Chemotactic migration of human mesenchymal stem cells and MC3T3-E1 osteoblast-like cells induced by COS-7 cell line expressing rhBMP-7. Tissue Eng 2006;12:1577-86. [PubMed]
- Li G, Cui Y, McIlmurray L, et al. rhBMP-2, rhVEGF(165), rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J Orthop Res 2005;23:680-5. [PubMed]
- Kim KW, Ha KY, Park JB, et al. Expressions of membrane-type I matrix metalloproteinase, Ki-67 protein, and type II collagen by chondrocytes migrating from cartilage endplate into nucleus pulposus in rat intervertebral discs: a cartilage endplate-fracture model using an intervertebral disc organ culture. Spine (Phila Pa 1976) 2005;30:1373-8. [PubMed]
- Dorronsoro A, Fernández-Rueda J, Fechter K, et al. Human Mesenchymal Stromal Cell-Mediated Immunoregulation: Mechanisms of Action and Clinical Applications. Bone Marrow Res 2013;2013:203643.
- De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 2012;12:574-91. [PubMed]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007;100:1249-60. [PubMed]
- Jung S, Panchalingam KM, Wuerth RD, et al. Large-scale production of human mesenchymal stem cells for clinical applications. Biotechnol Appl Biochem 2012;59:106-20. [PubMed]
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211-28. [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [PubMed]
- Ying QL, Nichols J, Evans EP, et al. Changing potency by spontaneous fusion. Nature 2002;416:545-8. [PubMed]
- Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004;428:668-73. [PubMed]
- Neirinckx V, Coste C, Rogister B, et al. Concise review: adult mesenchymal stem cells, adult neural crest stem cells, and therapy of neurological pathologies: a state of play. Stem Cells Transl Med 2013;2:284-96. [PubMed]
- Scuteri A, Miloso M, Foudah D, et al. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther 2011;6:82-92. [PubMed]
- Domínguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C. Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med 2012;1:59-63. [PubMed]
- Guo J, Wu G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev 2012;23:61-7. [PubMed]
- Mehlhorn AT, Schmal H, Kaiser S, et al. Mesenchymal stem cells maintain TGF-beta-mediated chondrogenic phenotype in alginate bead culture. Tissue Eng 2006;12:1393-403. [PubMed]
- Ryan AE, Lohan P, O’Flynn L, et al. Chondrogenic differentiation increases antidonor immune response to allogeneic mesenchymal stem cell transplantation. Mol Ther 2014;22:655-67. [PubMed]
- Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng 2007;24:5-21. [PubMed]
- Richardson SM, Curran JM, Chen R, et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials 2006;27:4069-78. [PubMed]
- Shen B, Wei A, Tao H, et al. BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A 2009;15:1311-20. [PubMed]
- Lam J, Lu S, Meretoja VV, et al. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater 2014;10:1112-23. [PubMed]
- Puetzer JL, Petitte JN, Loboa EG. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev 2010;16:435-44. [PubMed]
- Sekiya I, Koopman P, Tsuji K, et al. Dexamethasone enhances SOX9 expression in chondrocytes. J Endocrinol 2001;169:573-9. [PubMed]
- Eslaminejad MB, Fani N, Shahhoseini M. Epigenetic regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells in culture. Cell J 2013;15:1-10. [PubMed]
- Ezura Y, Sekiya I, Koga H, et al. Methylation status of CpG islands in the promoter regions of signature genes during chondrogenesis of human synovium-derived mesenchymal stem cells. Arthritis Rheum 2009;60:1416-26. [PubMed]
- Wei A, Tao H, Chung SA, et al. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J Orthop Res 2009;27:374-9. [PubMed]
- Watanabe T, Sakai D, Yamamoto Y, et al. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res 2010;28:623-30. [PubMed]
- Vadalà G, Studer RK, Sowa G, et al. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine (Phila Pa 1976) 2008;33:870-6. [PubMed]
- Chen WH, Liu HY, Lo WC, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials 2009;30:5523-33. [PubMed]
- Yuan M, Yeung CW, Li YY, et al. Effects of nucleus pulposus cell-derived acellular matrix on the differentiation of mesenchymal stem cells. Biomaterials 2013;34:3948-61. [PubMed]
- Sun Z, Liu ZH, Zhao XH, et al. Impact of direct cell co-cultures on human adipose-derived stromal cells and nucleus pulposus cells. J Orthop Res 2013;31:1804-13. [PubMed]
- Strassburg S, Richardson SM, Freemont AJ, et al. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med 2010;5:701-11. [PubMed]
- Murrell W, Sanford E, Anderberg L, et al. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J 2009;9:585-94. [PubMed]
- Svanvik T, Henriksson HB, Karlsson C, et al. Human disk cells from degenerated disks and mesenchymal stem cells in co-culture result in increased matrix production. Cells Tissues Organs 2010;191:2-11. [PubMed]
- Strassburg S, Hodson NW, Hill PI, et al. Bi-directional exchange of membrane components occurs during co-culture of mesenchymal stem cells and nucleus pulposus cells. PLoS One 2012;7:e33739. [PubMed]
- Allon AA, Butcher K, Schneider RA, et al. Structured coculture of mesenchymal stem cells and disc cells enhances differentiation and proliferation. Cells Tissues Organs 2012;196:99-106. [PubMed]
- Steck E, Bertram H, Abel R, et al. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells 2005;23:403-11. [PubMed]
- Johnstone B, Hering TM, Caplan AI, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 1998;238:265-72. [PubMed]
- Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine (Phila Pa 1976) 2004;29:2627-32. [PubMed]
- Shen B, Bhargav D, Wei A, et al. BMP-13 emerges as a potential inhibitor of bone formation. Int J Biol Sci 2009;5:192-200. [PubMed]
- Dorman LJ, Tucci M, Benghuzzi H. In vitro effects of bmp-2, bmp-7, and bmp-13 on proliferation and differentation of mouse mesenchymal stem cells. Biomed Sci Instrum 2012;48:81-7. [PubMed]
- Stoyanov JV, Gantenbein-Ritter B, Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater 2011;21:533-47. [PubMed]
- Shen B, Wei A, Whittaker S, et al. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem 2010;109:406-16. [PubMed]
- Kim HJ, Im GI. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A 2009;15:1543-51. [PubMed]
- Asou Y, Nifuji A, Tsuji K, et al. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. J Orthop Res 2002;20:827-33. [PubMed]
- Tsuchiya H, Kitoh H, Sugiura F, et al. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 2003;301:338-43. [PubMed]
- Babister JC, Tare RS, Green DW, et al. Genetic manipulation of human mesenchymal progenitors to promote chondrogenesis using “bead-in-bead” polysaccharide capsules. Biomaterials 2008;29:58-65. [PubMed]
- Fang Z, Yang Q, Luo W, et al. Differentiation of GFP-Bcl-2-engineered mesenchymal stem cells towards a nucleus pulposus-like phenotype under hypoxia in vitro. Biochem Biophys Res Commun 2013;432:444-50. [PubMed]
- Woods BI, Vo N, Sowa G, et al. Gene therapy for intervertebral disk degeneration. Orthop Clin North Am 2011;42:563-74. ix. [PubMed]
- Sakai D, Mochida J, Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30:2379-87. [PubMed]
- Yang H, Wu J, Liu J, et al. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J 2010;10:802-10. [PubMed]
- Bendtsen M, Bunger CE, Zou X, et al. Autologous stem cell therapy maintains vertebral blood flow and contrast diffusion through the endplate in experimental intervertebral disc degeneration. Spine (Phila Pa 1976) 2011;36:E373-379. [PubMed]
- Acosta FL Jr, Metz L, Adkisson HD, et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A 2011;17:3045-55. [PubMed]
- Yang F, Leung VY, Luk KD, et al. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther 2009;17:1959-66. [PubMed]
- Crevensten G, Walsh AJ, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 2004;32:430-4. [PubMed]
- Zhang YG, Guo X, Xu P, et al. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res 2005.219-26. [PubMed]
- Sakai D, Moichida J, Yamamoto Y, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials 2003;24:3531-41. [PubMed]
- Hiyama A, Mochida J, Iwashina T, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res 2008;26:589-600. [PubMed]
- Hohaus C, Ganey TM, Minkus Y, et al. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J 2008;17 Suppl 4:492-503. [PubMed]
- Liang CZ, Li H, Tao YQ, et al. Dual release of dexamethasone and TGF-beta3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater 2013;9:9423-33. [PubMed]
- Miyamoto T, Muneta T, Tabuchi T, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther 2010;12:R206. [PubMed]
- Henriksson HB, Svanvik T, Jonsson M, et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) 2009;34:141-8. [PubMed]
- Sakai D, Mochida J, Iwashina T, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 2006;27:335-45. [PubMed]
- Barczewska M, Wojtkiewicz J, Habich A, et al. MR monitoring of minimally invasive delivery of mesenchymal stem cells into the porcine intervertebral disc. PLoS One 2013;8:e74658. [PubMed]
- Gang EJ, Darabi R, Bosnakovski D, et al. Engraftment of mesenchymal stem cells into dystrophin-deficient mice is not accompanied by functional recovery. Exp Cell Res 2009;315:2624-36. [PubMed]
- Sheikh H, Zakharian K, De La Torre RP, et al. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine 2009;10:265-72. [PubMed]
- Chun HJ, Kim YS, Kim BK, et al. Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg 2012;78:364-71. [PubMed]
- Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res 2012;35:213-21. [PubMed]
- Liu ZH, Sun Z, Wang HQ, et al. FasL expression on human nucleus pulposus cells contributes to the immune privilege of intervertebral disc by interacting with immunocytes. Int J Med Sci 2013;10:1053-60. [PubMed]
- Iatridis JC, Nicoll SB, Michalek AJ, et al. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J 2013;13:243-62. [PubMed]
- Vinatier C, Bordenave L, Guicheux J, et al. Stem cells for osteoarticular and vascular tissue engineering. Med Sci (Paris) 2011;27:289-96. [PubMed]
- Zhang Z, Li F, Tian H, et al. Differentiation of adipose-derived stem cells toward nucleus pulposus-like cells induced by hypoxia and a three-dimensional chitosan-alginate gel scaffold in vitro. Chin Med J (Engl) 2014;127:314-21. [PubMed]
- Smith LJ, Gorth DJ, Showalter BL, et al. In Vitro Characterization of a Stem Cell-Seeded Triple Interpenetrating Network Hydrogel for Functional Regeneration of the Nucleus Pulposus. Tissue Eng Part A 2014. [Epub ahead of print]. [PubMed]
- Frith JE, Cameron AR, Menzies DJ, et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 2013;34:9430-40. [PubMed]
- Frith JE, Menzies DJ, Cameron AR, et al. Effects of bound versus soluble pentosan polysulphate in PEG/HA-based hydrogels tailored for intervertebral disc regeneration. Biomaterials 2014;35:1150-62. [PubMed]
- Saldanha KJ, Piper SL, Ainslie KM, et al. Magnetic resonance imaging of iron oxide labelled stem cells: applications to tissue engineering based regeneration of the intervertebral disc. Eur Cell Mater 2008;16:17-25. [PubMed]
- Peroglio M, Grad S, Mortisen D, et al. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J 2012;21 Suppl 6:S839-849. [PubMed]
- Collin EC, Grad S, Zeugolis DI, et al. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials 2011;32:2862-70. [PubMed]
- Roughley P, Hoemann C, DesRosiers E, et al. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006;27:388-96. [PubMed]
- Richardson SM, Hughes N, Hunt JA, et al. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials 2008;29:85-93. [PubMed]
- Francisco AT, Mancino RJ, Bowles RD, et al. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials 2013;34:7381-8. [PubMed]
- Reitmaier S, Kreja L, Gruchenberg K, et al. In vivo biofunctional evaluation of hydrogels for disc regeneration. Eur Spine J 2014;23:19-26. [PubMed]
- Revell PA, Damien E, Di Silvio L, et al. Tissue engineered intervertebral disc repair in the pig using injectable polymers. J Mater Sci Mater Med 2007;18:303-8. [PubMed]
- Yoshikawa T, Ueda Y, Miyazaki K, et al. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976) 2010;35:E475-480. [PubMed]
- Orozco L, Soler R, Morera C, et al. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 2011;92:822-8. [PubMed]
- Khoo ML, Shen B, Tao H, et al. Long-term serial passage and neuronal differentiation capability of human bone marrow mesenchymal stem cells. Stem Cells Dev 2008;17:883-96. [PubMed]
- Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 2007;67:9142-9. [PubMed]
- Tarte K, Gaillard J, Lataillade JJ, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 2010;115:1549-53. [PubMed]
- Binato R, de Souza Fernandez T, Lazzarotto-Silva C, et al. Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy. Cell Prolif 2013;46:10-22. [PubMed]
- Tamama K, Kawasaki H, Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol 2010;2010:795385.
- Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med 2012;1:771-82. [PubMed]
- Haque N, Rahman MT, Abu Kasim NH, et al. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. ScientificWorldJournal 2013;2013:632972.
- Das R, Jahr H, van Osch GJ, et al. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 2010;16:159-68. [PubMed]
- Schellenberg A, Stiehl T, Horn P, et al. Population dynamics of mesenchymal stromal cells during culture expansion. Cytotherapy 2012;14:401-11. [PubMed]
- Gullo F, De Bari C. Prospective purification of a subpopulation of human synovial mesenchymal stem cells with enhanced chondro-osteogenic potency. Rheumatology (Oxford) 2013;52:1758-68. [PubMed]
- Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213-9. [PubMed]
- von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012;30:1575-8. [PubMed]
- Gorensek M, Jaksimovic C, Kregar-Velikonja N, et al. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett 2004;9:363-73. [PubMed]