Social and biological factors influencing the outcomes of children with Wilms tumors in Kenya and other Sub-Saharan countries
Wilms tumor (WT) is a common pediatric solid tumor, and the event-free survival rate of patients with this tumor is high in developed countries (1). WT as well as Burkitt lymphoma and Kaposi sarcoma are target cancers in Sub-Saharan Africa, in which limited resources are available (2), because the incidence of WT is high among pediatric cancers in Africa, and a high cure rate may be accomplished if standardized therapy could be more accessible. Epidemiological studies showed that the incidence of WT was high in Africa, low in Asia, and intermediate in Caucasian in North America (3). Furthermore, the incidence of WT in Asians was shown to be approximately one half to two-thirds of that in Caucasians in Hawaii and Britain (4,5), and was higher in Black American than in Caucasians in USA (3). These findings suggest that the different incidence rates among the three populations may be caused by different genetic backgrounds, and not environmental factors. The 5-year event-free survival rates of patients with WT in developed countries has reached 85-90%, whereas those in developing countries were reported to be 50% or less (1,6,7). To overcome these disparities, physicians and investigators in developed and developing countries are currently performing research with the aim of the better management of children with WT in Kenya and other Sub-Saharan countries (8).
Cancer in Africa
The World Health Organization (WHO) has estimated that 70% of cancer deaths occur in low and middle income countries (9). In spite of the fact that cases of cancer are increasing in Africa due to various reasons such as changes to a less healthy lifestyle and an increase in oncogenic viral infections (10-12), the limited resources available for healthcare are used to control more rampant child killers such as diarrhea and pulmonary infections as well as world-focused infections such as HIV, tuberculosis, and malaria (8).
Although pediatric tumors in Africa account for a small proportion of all cancers and receive less attention in health policies in each country, the importance of understanding their epidemiology and clinicopathology is significant for both scientists and health policy makers, considering the impact of the increasing burden of cancer in Africa as well as the importance of genetic and environmental understanding of pediatric cancers in general (13).
Cancer and the health system in Kenya
Axt and colleagues (7) published a study that increased understanding of the clinicopathology of WT in Kenya and also in low resource countries. With the increasing number of cases of cancer in Kenya, greater efforts have been made to create awareness and develop control policies towards cancer, especially in the last ten years. The Ministry of Health, Kenya established the “National Cancer Control Strategy 2011-2016” for the first time in its history to tackle issues impacting the lives of people in Kenya. Although population-based data do not exist in a country with a population of 43,000,000, the annual incidence of cancer has been estimated at approximately 28,000 cases and annual mortality as over 22,000 (National Cancer Control Strategy 2011-2016, the Ministry of Health Kenya). Regarding pediatric cancer, only one in ten children with cancer survives in Kenya while seven in ten survive in developed countries (unpublished data from Kenyatta National Hospital by Jessie Githanga in Feb 2013). Based on these findings, the establishment of the Kenya WT registry is meaningful for epidemiological analyses as well as a more common understanding of WT in Kenya. It could also assist many scientists in developing a more detailed research agenda because only a limited number of reliable scientific studies have been conducted, which has been attributed to patients not presenting to health facilities for a diagnosis and also poor record keeping.
This article revealed several issues caused by the weak health system in Kenya from the point of view of cancer management (7). Some have a negative impact on the production of scientific data. However, others may positively assist policy makers to strengthen the health system. These include poor access to health facilities due to long distances and financial reasons, lower awareness towards cancer among the general public, less specialized health providers, the limited number of health facilities and infrastructures, in which cancer treatment is offered, and the absence of standardized treatment protocols as well as poor record keeping. The National Health Insurance Fund (NHIF) has gradually increased and achieved an enrolment of 12.3 million members and dependents in 2012 (Table 1). Patients enrolled in the NHIF showed the better completion rate of therapy and better event-free survival than those not enrolled, indicating insufficient health coverage for those not enrolled in the NHIF. Social misconception is also a large factor that interferes with proper pediatric cancer management.
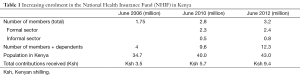
Full table
Study limitations
This study had some limitations due to the retrospective study design that inhibited obtaining exact factors that could improve the treatment outcomes of WT (7). This was also negatively boosted by several social factors. For example, the improved treatment outcomes among the study populations who were enrolled in the NHIF may have been due to the direct benefit of the NHIF; however, the population enrolled in the NHIF may have been already biased by a baseline financial status, stronger health seeking behavior, and a more urban population who are employed. The same could be applied to the tribal proportions of WT cases and may be a genetic issue that many scientists can recognize; however, it could also be influenced by the original locality of tribes, financial status, and also other cultural factors. Therefore, some factors influencing treatment outcome and tribal bias of enrollment in the Kenyan Wilms Tumor Registry (KWTR) raised by the investigators should be analyzed in a prospective study. In addition, better writing and keeping of medical records in hospitals and clinics and improvement of the KWTR system are needed to determine the proper social and biological factors that influence outcome of Kenyan children with WT.
National Health Insurance System and outcomes of patients who received WT treatments
Even though several study limitations were observed, the results obtained indicated that various factors may have improved outcomes of patients that received WT treatments (7). The study revealed the clear benefit of the NHIF. As “Universal Health Coverage” is currently one of the top priority global health agendas since the 58th World Health Assembly of 2005 adopted the resolution on “Sustainable health financing, universal coverage, and social health insurance” (World Health Assembly Resolution 58.33, 2005), the clear benefit of the NHIF shown in this study should encourage the country policy makers to strategize improvements in the enrolment rate. Approximately 20-30% of Kenyan population is estimated to be covered by some forms of health insurance, mostly by the NHIF (the Government of Kenya/NHIF, 2012). This could be improved through different approaches such as compulsory enrolment by the law, improved payment systems, increased awareness of insurance benefits among the general public, improved accountability/integrity of the fund, and better benefit packages.
Strategy to reduce Lost to Follow Up
It is also crucial to determine at which point patients stopped their treatment and why (7). The study proved that the completion of treatment led to the significantly better outcomes of patients with WT. However, it is not easy to specify the timing and reasons for Lost to Follow Up (LTFU) from the findings of the study; the finding that fifty percent of study patients were LTFU indicate large problems both in the study results and also in the completion of treatment. The large number of LTFU may have been due to financial constraints at the individual level, distance to the treatment facility, and cultural beliefs/superstitions including witchcraft and/or misconceptions towards the WT management. In African culture, especially in rural areas, people tend to link medical conditions with religious and cultural beliefs. Therefore, when sick children are not immediately responding to “Western medicine”, the guardians often try to bring them to religious leaders or traditional healers or any other forms of traditional treatment methods, which waste a lot of time and money, and increases the number of LTFU.
This finding could also be attributed to factors on the side of the health services such as inability to obtain central venous access for chemotherapy, as was described in the Discussion section, discouragement due to drugs being out of stock, and other forms of poor services. Strategies to increase the treatment completion rate are essential to improve the outcomes of the treatment for WT. As dropouts were reported during pre- and post-operative chemotherapy, it is also important to consider quality communication and sufficient explanations of the treatment to the families before and after the treatment starts. Irrespective of developed or developing countries, the success of cancer treatment often depends on the relationship between the patient/their family and health care providers and how their social and psychological issues are followed-up by a multi-disciplinary team. Therefore, comprehensive care for cancer should also be included in the strategies to increase treatment completion rates.
Treatment protocol and the outcomes of patients with WT
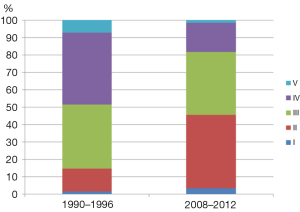
As was described in the study (7), the development of a standardized treatment protocol for WT is also an urgent agenda. Children in Kenya with WT are mainly treated with one of two protocols established by the Children’s Oncology Group (COG) or Société Internationale d’Oncologie Pédiatrique (SIOP). The findings of this study showed that many patients dropped out during chemotherapy; therefore, using the COG protocols, in which up-front resection is performed prior to chemotherapy appears to be more appropriate so that all patients could benefit from resection, which is often very essential for the management of solid tumors. However, it is also important to consider that the majority of WT cases in Kenya already presented in advanced stages at the first visit. The findings of this study showed that over 50% of WT patients in Kenya were diagnosed at stage III and IV (Figure 1), which is markedly different from data obtained in developed countries, in which the majority of patients were diagnosed at stage I or at most II. This could make resection without preoperative chemotherapy less successful. However, the stage distribution of WT in Kenya has been changing over the last few decades. Abdallah et al. reported in 2001 that 78% of WT patients in Kenya were stage III and IV (6) as opposed to the 52.8% reported in Axt and colleagues’ study (7) (Figure 1). This indicates that the clinical features of WT have markedly changed. Therefore, continuous observations and studies are required to determine a standardized treatment protocol that provides a better outcome for Kenyan children with WT.
Blood transfusion and WT treatment outcomes
Blood transfusion received outside of the operating theatre was associated with a poor outcome (7). Almost all patients received a blood transfusion during their time in an operating theatre, and approximately 20% received on outside the theatre, both of which were markedly different from developed countries in which blood transfusions are not always common practice both inside and outside of the operating theatre during the management of WT. Although the reasons for the blood transfusions were not stated or analyzed, it may be attributed to extra complications during the operation because of the advanced stage of tumors, infections due to poor hygiene, the overuse of blood transfusions due to poor risk management, and preconditions such as HIV infections, malnutrition, and sickle cell diseases. Further research is needed to identify the reasons for these transfusions in order to improve the outcomes of patients with WT.
Biological differences in WT among African, Caucasian, and Asian children
Murphy and colleagues studied the molecular characteristics of 15 Kenyan WTs, age-matched North American WT controls, and found an increased mortality, higher incidence of nuclear unrest, and increased proportion of epithelial nuclear β-catenin in Kenyan WTs than in the North American counterparts (14). Anaplastic histology with intense p53 immunostaining was detected in two (13%) of the 15 Kenyan WTs, which was consistent with the incidence of NWTS (10.8%) and appears to be higher than that of anaplastic histology in Japanese WTs (3.5%) (15,16). They demonstrated that the African WT specimens expressed markers of adverse clinical behavior and treatment resistance and may require more intensive treatment protocols.
WT1 is a multifunctional protein that acts as a transcriptional activator or repressor, is predominantly expressed in the embryonic kidney, and plays a pivotal role in its development (17). We reported that if only sporadic tumors were included, the frequencies of WT with WT1 abnormalities (22.8%) would be similar between Japanese and Caucasian populations; however, an exact comparison is difficult because of the absence of data on the population-based incidence of WT1 alterations in WT (18). The study on 15 Kenyan WTs only detected one tumor with a WT1 mutation (6.7%) (14), which indicated that the higher incidence of African WT may be caused by the increased incidence of WT1-wild-type WTs.
IGF2, insulin-like growth factor II, is an imprinted gene expressed from the paternal allele, and encodes a fetal polypeptide growth factor (19). We and other studies previously reported that loss of IGF2 imprinting was markedly lower in Japanese children than in their Caucasian counterparts, and showed that the lower incidence of WT with the loss of IGF2 imprinting may be implicated in the lower incidence of WT in Japan (18,20). Unfortunately, no studies have examined the IGF2 status in African WTs. Thus, studies of the molecular characteristics of African WTs have just begun, and future studies will clarify whether genetic and epigenetic differences correlate with the different incidence rates of WT among different ethnic populations.
Conquering the disparities in the outcomes of children with WT between developed and developing countries
As described earlier, limited resources are used for common diseases such as diarrhea, pulmonary infection, HIV, tuberculosis, and malaria. However, disparities in the outcomes of children with pediatric cancer such as WT between developed and developing countries cannot be ignored. Axt and colleagues described the present medical situation for treating WT in Kenya, and made recommendations to accomplish better treatment outcomes (7). Researchers in developed countries examine the biology of WT, and believe that this research will improve the outcomes of subgroups of patients with WT who fail to respond to the present standardized regimens. Physicians and other health providers in Kenya take care of children with WT as well as common, but possibly life-threatening diseases. We hope that both these groups can work together in future to conquer disparities in the outcomes of children with WT between developed and developing countries. Further updated studies from both groups are essential for obtaining this goal.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- D’Angio GJ. The National Wilms Tumor Study: a 40 year perspective. Lifetime Data Anal 2007;13:463-70. [PubMed]
- Israels T, Ribeiro RC, Molyneux EM. Strategies to improve care for children with cancer in Sub-Saharan Africa. Eur J Cancer 2010;46:1960-6. [PubMed]
- Parkin DM, Stiller CA, Draper GJ, et al. The international incidence of childhood cancer. Int J Cancer 1988;42:511-20. [PubMed]
- Goodman MT, Yoshizawa CN, Kolonel LN. Ethnic patterns of childhood cancer in Hawaii between 1960 and 1984. Cancer 1989;64:1758-63. [PubMed]
- Stiller CA, McKinney PA, Bunch KJ, et al. Childhood cancer and ethnic group in Britain: a United Kingdom children’s Cancer Study Group (UKCCSG) study. Br J Cancer 1991;64:543-8. [PubMed]
- Abdallah FK, Macharia WM. Clinical presentation and treatment outcome in children with nephroblastoma in Kenya. East Afr Med J 2001;78:S43-7. [PubMed]
- Axt J, Abdallah F, Axt M, et al. Wilms tumor survival in Kenya. J Pediatr Surg 2013;48:1254-62. [PubMed]
- Harif M, Traoré F, Hessissen L, et al. Challenges for paediatric oncology in Africa. Lancet Oncol 2013;14:279-81. [PubMed]
- WHO Cancer Fact Sheet, January 2013. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/index.html
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [PubMed]
- Hadley LG, Rouma BS, Saad-Eldin Y. Challenge of pediatric oncology in Africa. Semin Pediatr Surg 2012;21:136-41. [PubMed]
- Jedy-Agba E, Curado MP, Ogunbiyi O, et al. Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol 2012;36:e271-8. [PubMed]
- Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer 2008;112:461-72. [PubMed]
- Murphy AJ, Axt JR, de Caestecker C, et al. Molecular characterization of Wilms’ tumor from a resource-constrained region of sub-Saharan Africa. Int J Cancer 2012;131:E983-94. [PubMed]
- Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms’ tumor: results from the fifth National Wilms’ Tumor Study. J Clin Oncol 2006;24:2352-8. [PubMed]
- Oue T, Fukuzawa M, Okita H, et al. Outcome of pediatric renal tumor treated using the Japan Wilms Tumor Study-1 (JWiTS-1) protocol: a report from the JWiTS group. Pediatr Surg Int 2009;25:923-9. [PubMed]
- Huff V. Wilms’ tumours: about tumour suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer 2011;11:111-21. [PubMed]
- Haruta M, Arai Y, Watanabe N, et al. Different incidences of epigenetic but not genetic abnormalities between Wilms tumors in Japanese and Caucasian children. Cancer Sci 2012;103:1129-35. [PubMed]
- Foulstone E, Prince S, Zaccheo O, et al. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol 2005;205:145-53. [PubMed]
- Fukuzawa R, Breslow NE, Morison IM, et al. Epigenetic differences between Wilms’ tumours in white and east-Asian children. Lancet 2004;363:446-51. [PubMed]


