Wilms’ tumor: biology, diagnosis and treatment
Introduction
Wilms’ tumor (nephroblastoma), an embryonal type of renal cancer, is one of the most common solid malignant neoplasms in children. It accounts for approximately 90% of all paediatric tumors of the kidney (1-3). The total number of new cases of Wilms’ tumor in the UK is estimated at about 80 cases per year (4). The tumor usually arises in a single kidney. Synchronous bilateral or multifocal tumors occur in approximately 10% of patients and tend to present at an earlier age (5,6). Wilms’ tumor can also be diagnosed in adolescents or adults, but this is extremely rare, representing less than 1% of all renal tumors (7). The usual treatment approach in most patients is a combination of surgery and chemotherapy, with the addition of radiotherapy in high risk patients. Substantial progress in the treatment of Wilms’ tumor over the past few decades has been made by refining risk stratification and by the use of existing chemotherapy schedules. This has improved overall survival (OS) for patients with Wilms’ tumor in high income countries to greater than 90% for localised disease and 75% for metastatic disease (8,9). This excellent outcome results from collaborative efforts among paediatric surgeons, pathologists, radiologists and oncologists. The two largest collaborative groups that have studied the optimal management of Wilms’ tumor are the Children’s Oncology Group (COG) and the International Society of Paediatric Oncology (SIOP). The COG recommends primary surgery before an adjuvant treatment except in specific circumstances such as synchronous bilateral disease. By contrast, the SIOP approach favours pre-operative chemotherapy for all cases except very young infants (<6 months of age) (3,4,10). Clinical outcomes are excellent in both groups, and there is an ongoing debate on the merits of each approach.
The aim of this article is to review the current thoughts on biology, diagnosis, and management recommendations for children with Wilms’ tumor.
Epidemiology
Wilms’ tumor affects one in 10,000 children and accounts for 5% of all childhood cancers (2,5,8,11). More than 80% of children are diagnosed with Wilms’ tumor below the age of five years, and the median age at diagnosis is 3.5 years (12). The tumor is one of the few childhood cancers with a slight female preponderance among Caucasian patients. In contrast, the disease in Asian children has a peak in the second year of life, and a greater incidence among boys than girls has been observed in the East-Asian population (2,13). Although topographic variations in the incidence of this tumor have been shown, the incidence of the tumor varies almost entirely along ethnic groups rather than geographic areas. The highest rates reported are in those of black African descent and the lowest in those of Asian descent. This variation suggests that genetic factors play the most important role in its aetiology (13-15).
Pathology of Wilms’ tumors
In development, the definitive foetal kidney develops from the ureteric bud (forming collecting ducts) and the metanephric mesenchyme/blastema (forming the stroma and through mesenchymal to epithelial transition the proximal tubular structures; glomeruli, proximal and distal tubules and loop of Henle) (5). The blastema has usually disappeared by 36 weeks gestation, however at birth approximately 1% of infants retain residual blastema within their kidney (16,17). These cells are described as nephrogenic rest, which was defined by Beckwith as ‘a focus of abnormally persistent nephrogenic cells, retaining cells that can be induced to form a Wilms’ tumor’ (16).
Wilms’ tumors can be observed to develop within a proportion of nephrogenic rests and in 40% of Wilms’ tumor patients nephrogenic rest can be identified (16,18). Nephrogenic rests are thought to be the precursor lesions of Wilms’ tumors. Rests may have a variety of fates, many will become obsolescent and disappear, however, a proportion will become proliferative and may undergo neoplastic transformation into a Wilms’ tumor. Each stage of progression is thought to result from the acquisition of stable somatic changes, either in the form of genetic mutation or epimutation. Nephrogenic rests are present in 90% of bilateral cases, which is thought to reflect mutations/epimutations either in the germline or occurring very early in the developing embryo (16,18).
Rests are subdivided into two types: intralobar nephrogenic rests (ILNR), found anywhere within the renal lobe and perilobar nephrogenic rests (PLNR), confined to the periphery of the renal lobe, and thought to develop later during embryogenesis (18,19). Nephroblastomatosis is defined as the ‘diffuse or multifocal presence of nephrogenic rests or their recognised derivatives’ (16).
The distribution of nephrogenic rests varies by ethnic group. The largest described cohort is of patients in the North American NWTS 3, 4 and 5 studies in which rests were identified in 42% of 5,934 patients with Wilms’ tumors (20% PLNR, 18% ILNR, 4% PLNR + ILNR) (18). In contrast, Fukuzawa et al. reported PLNR rates of 8% in Asian-Americans and only 2% in Japanese patients compared to 24% in white Americans (13). In a case series of 127 Wilms’ tumors from India, no cases of PLNR were observed, whereas 45% had associated ILNR (20).
Within Wilms’ tumors there are three main types of tumor cells; blastema, resembling the undifferentiated embryonic metanephric mesenchyme, and thought to contain any tumor stem cells, together with epithelium, and stroma, both thought to have differentiated from the blastema. These cell types are distinguished histologically and currently there are no good markers to specifically identify blastema. In the SIOP classification, where histology is assessed after chemotherapy, Wilms’ tumors are sub-classified and risk stratified based on the percentage of each of these types of cells. Survival of a high proportion of blastemal cells is classified as high risk (Tables 1,2) (21). The COG classification, which is based on histological assessment of the chemotherapy-naive tumor, does not take the predominant cell type into account for risk-stratification purposes (Tables 3,4) (21). In both schemes, the presence of diffuse anaplasia, which is defined morphologically, is considered high risk. The two groups differ in how they classify focal anaplasia, which is considered intermediate risk by the SIOP and has recently been placed in the high risk category by the COG. Consequently the SIOP and COG subtypes whilst similarly named are not inter-changeable as the relative presence of certain cell types can be affected by chemotherapy.

Full table

Full table
Full table

Full table
In the SIOP WT2001 study blastemal type Wilms’ tumor was classified as a high risk subtype following earlier studies that showed it had an adverse prognosis (21). There is current interest in using the residual volume of blastema following chemotherapy as a biomarker and the biology of such ‘(chemo) resistant’ blastema is an active area of research.
In addition to histological subtyping of tumors histological features are also used within the staging process (Tables 2,4,5).

Full table
Genetics of Wilms’ tumor
In 1972 Knudson proposed a two hit model of Wilms’ tumorigenesis similar to his earlier model of retinoblastoma (22,23). This has subsequently been found not to be the case with a diverse range of genes and mechanisms implicated in Wilms’ pathogenesis.
Approximately 5% of patients with Wilms’ tumor have an underlying predisposing genetic syndrome and over 50 such syndromes have been described (24). Several syndromes result from a disruption of the WT1 gene, which encodes the transcription factor WT1, crucial for renal and gonadal embryogenesis. Disruption of the WT1 gene typically results in genitourinary abnormalities and predisposition to early Wilms’ tumors (almost always under five years of age), often associated with ILNR and rhabdomyoblastic change within the tumor (4,25). Microdeletion of the WT1, along with the neighbouring PAX6 leads to WAGR syndrome (>50% risk of Wilms’ tumor, aniridia, genitourinary abnormalities and mental retardation). In cases of isolated aniridia, due to PAX6 deletion, but with no deletion of WT1 there is no increased risk of Wilms’ tumor (25). Missense mutations of WT1, typically in the zinc finger domains, result in Denys-Drash syndrome characterised by a greater than 50% risk of developing Wilms’ tumor, genitourinary abnormalities (often severe and including pseudohermaphroditism) and nephropathy. The severity of the renal and genito-urinary abnormalities is thought to represent a dominant negative effect of the mutation (25). Mutation in intron 9 can result in abnormal splicing leading to Frasier syndrome characterised by focal glomerular sclerosis, delayed kidney failure, and complete gonadal dysgenesis (25).
The WT2 locus at 11p15 is an area of imprinting, such that the expression of gene is dependent upon whether it was inherited from the mother or father. This is due to differential methylation of alleles depending on their parent of origin. The genetics of this region are complex, though essentially normally only the paternal allele of the IGF2 gene and maternal allele of the H19 gene are expressed (26). In Beckwith-Wiedemann syndrome this region can be disrupted in a number of ways, most commonly as a result of hypomethylation or uniparental disomy. Analysis of the different genotypes suggests that only those that result in increased expression of IGF2 are associated with increased risk of developing Wilms’ tumor (4,25,27). Changes at this locus are also thought to underlie a subgroup of patients with isolated hemi-hypertrophy who are at an increased risk of developing Wilms’ tumor (4). Tumors in patients with Beckwith-Wiedemann syndrome may occur later, though usually before the age of 7 years, and are often found with associated PLNR (16,18).
Other syndromes and genes implicated in an increased risk of Wilms’ tumor include Simpson-Golabi-Behmel syndrome, an overgrowth syndrome featuring coarse facial features, skeletal, cardiac, renal, and intellectual defects, due to mutation in the GPC3 gene, biallelic BRCA2 mutations/Fanconi anaemia D1, Bloom syndrome, and Li Fraumeni syndrome (4).
Genetic linkage analysis in a large pedigree has also mapped further familial Wilms’ tumor loci, FWT1 on chromosome 17q12-21 and FWT2 on chromosome 19q13. However, neither gene has yet been identified (4).
Children with genetic syndromes associated with an increased risk of Wilms’ tumor are screened with regular ultrasound throughout the period of risk. In the US the National Cancer Institute recommends screening at least to the age of 8 years (28). Whereas, in the UK following a review of the literature in 2006 a multidisciplinary working group recommended that screening should be offered to children at a >5% risk of Wilms’ tumor and be continued until the age of five years in cases due to WT1 mutation, and until the age of seven years in cases associated with overgrowth and in familial cases (24,25).
The genes implicated in these genetic syndromes have also been implicated in the pathogenesis of sporadic Wilms’ tumor. The WT1 gene is inactivated, usually by inactivating deletion or point mutation in 10-20% of sporadic Wilms’ tumors (26,29,30). The IGF2 locus is deregulated in 30-69% of tumors through loss of imprinting resulting in IGF2 expression or somatic loss of the maternal allele and duplication of the paternal allele (copy number neutral loss of heterozygosity (LOH) (26,31-35). Other genes implicated in Wilms’ pathogenesis include the WTX gene which is inactivated in 15-30% of sporadic tumors, FBXW7 and MYCN (29,36-38). Exome and whole geneome sequencing projects in both, the US and Europe, are likely to reveal other Wilms’ tumor genes.
Attempts have been made to sub-classify Wilms’ tumors based on their molecular pathogenesis; so called ‘type 1’ tumors are characterised by a younger age of diagnosis, stromal predominant histology, the presence of intra-lobar nephrogenic rests, inactivation of the WT1 gene and activation of beta catenin (18). In contrast so called ‘type 2’ are characterised by an older age at diagnosis, the presence of perilobar nephrogenic rests and deregulation of IGF2, though in reality such a dichotomy is likely a simplification (18,33). Deregulation of the IGF2 locus has been observed to be rare in the Japanese population and suggested as a reason for the lower prevalence of perilobar nephrogenic rest and earlier peak of diagnosis (13).
Inactivation of the TP53 gene is found particularly in anaplastic tumors, and in tumors with focal anaplasia may only be inactivated in anaplastic areas (36,39-41). Genetic changes resulting in loss of TP53 function are also the commonest change observed between primary and relapse tumor samples (42).
In addition to specific genes implicated in Wilms’ tumorigenesis, whole and partial chromosomal gains and losses, as well as LOH are commonly seen in Wilms’ tumors, particularly gains of chromosomes 1q, 2, 7q, 8, 12 & 13 and losses of chromosomes 1p, 7p, 16q and 22q (36,43).
Increasingly such changes are being investigated and used as biomarkers to direct treatment. Analysis of the National Wilms Tumor Study Group (NWTS) 5 trial in the USA identified that in favourable histology Wilms’ tumors if both LOH of chromosome 16p and 1p were present there was an increased risk of relapse and death (44). These molecular markers have now been incorporated into the risk stratification of the current Children’s Oncology Group Wilms’ Tumor risk stratification protocol (Table 3). Similar retrospective analyses of UK and other datasets have identified similar patterns for 16q loss, and analysis of tumors in the European SIOP WT 2001 trial for LOH at 1p and/or 16q is currently ongoing (45,46). However combined 1p and 16q loss is only identified in 2.6-4.6% of cases and so this has limited applicability (44-46).
In contrast, gain of chromosome 1q is more common, observed in approximately 25% of cases. It was initially described as a potential adverse biomarker in the UK (47,48) and this has been subsequently confirmed in other studies in Europe and recently a large retrospective study of the NWTS-4 cohort of favourable histology Wilms’ tumor patients where cases with 1q gain had a lower eight years survival than those without 1q gain [76% (95% CI, 63-85%) vs. 93% (95% CI, 87-96%) (P=0.0024)] (49,50).
Further understanding of the molecular pathology and genetic changes in Wilms’ tumors is hoped to support the development of novel biomarkers to aid diagnosis, risk stratification and monitoring of therapy and relapse. In addition such understanding is hoped to lead to the development of newer molecular targeted therapies to treat the high risk patients who continue to have high relapse and mortality rates and to reduce morbidity and overtreatment of low risk patients.
Presentation
The vast majority of Wilms’ tumors present with an asymptomatic abdominal mass. It is not uncommon for a firm mass in the abdomen to be discovered by a family member during bathing the child or by a healthcare professional who notices a protrusive abdomen. In approximately 20-30% of cases presenting signs and symptoms include abdominal pain, malaise, either microscopic or macroscopic haematuria (51). Associated hypertension, most likely due to increased renin activity, is found in about 25% of children with Wilms’ tumor (51). Hypertension, which may occur as a direct effect of the presence of a renal mass, usually resolves after nephrectomy. However, a severe or prolonged hypertension merits more detailed investigations and consideration of the possibility of an underlying genetic disorder such as Denys-Drash syndrome. Atypical presentations are found in less than 10% of cases and these results from compression of surrounding organs or vascular infiltration. For example, tumor extension into the renal vein or inferior vena cava occurs in less than 4% of patients (51). Presenting symptoms of children with vascular extension include ascites, congestive cardiac failure, hepatomegaly (51). Occasionally, a child may present with an acute abdomen (rapidly enlarging abdominal mass, anaemia, hypertension, pain and fever) due to tumor rupture or for investigation of a varicocele or other genitourinary abnormalities (24,51). Tumor production of hormonal substances may lead to paraneoplastic syndromes, including hypercalcaemia, erythrocytosis and acquired von Willebrand disease (51,52).
Evaluation
An abdominal ultrasound scan is the most useful initial investigation to confirm the presence of a primary intrarenal mass. This study is also used to evaluate tumor extension and involvement of the contralateral kidney. It enables to determine whether there is an evidence of extension into the inferior vena cava or beyond, and to check for the liver metastases. One of the goals of ultrasound imaging should be to identify associated genitourinary malformations and to confirm the presence of a functioning contralateral kidney (24,51,53). It is now considered standard practice to perform a computed tomography (CT) or preferably magnetic resonance imaging (MRI) scan of the abdomen and pelvis in children with a suspected renal tumor (1,12). MRI scan is especially beneficial in children with suspected bilateral renal lesions and enables reduction of exposure to radiation. Additional techniques such as ADC (Apparent Diffusion Coefficient) mapping are also used to give further information about the biology of the tumor (54).
The lungs are the most common site of metastatic spread that occurs in 10-20% of children with Wilms’ tumor at the time of diagnosis. Historically the chest has been assessed using a plain, two-view radiography, however CT of the chest is increasingly being used, though there is a debate about how lesions which are visible only on chest CT and not plain X-ray should be treated (55).
In approximately 11% of children Wilms’ tumor extends intravenously (51). Thrombus extension into the inferior vena cava occurs in around 4% of cases and echocardiography should be considered in the rare circumstances when intracardiac tumor infiltration is expected (51).
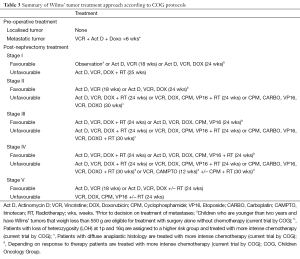
The diagnosis and treatment of Wilm’s tumor evolved with two different approaches taken by the Children’s Oncology Group (COG) and the International Society of Paediatric Oncology (SIOP) (Tables 1,2,3,4,5) (3,4,10). The COG in North America was formed in 2001 and took forward clinical trials run by the National Wilms’ Tumor Study group (NWTS) since 1969. It favours initial surgery (nephrectomy) to endure precise assessment of tumor extent (stage) and histology prior to chemotherapy. The data available provides evidence that this approach is associated with a higher risk of tumor spillage or rupture which then mandates flank radiotherapy for a stage III tumor (8). Hence, the SIOP nephroblastoma group, which commenced its trials in 1971, favours pre-operative chemotherapy to reduce complications of surgery and tumor spillage, at the time of delayed nephrectomy which takes place 4-6 weeks later. There is a long standing controversy regarding the merits and disadvantages of each approach (8,9). The UK group aimed to address this in a randomised clinical trial that ran between 1999 and 2001 (56,57). The results of this trial showed no difference in event free survival or OS between the two arms and led to a change in practice to a routine pre-operative chemotherapy in the UK (56,57). However, it was recognised that the trial was underpowered and that concomitant trials of immediate nephrectomy in North America were giving equally good survival. The COG therefore continues with an immediate nephrectomy approach which they believe gives more accurate staging information for risk adapted post-operative treatment planning (8,9).
The clinical protocols conducted by the SIOP recommend an immediate nephrectomy for infants below the age of six months. Benign tumors, such as mesoblastic nephroma, and high risk malignant rhabdoid tumors of kidney are more common among this age group for which different and still relatively ineffective chemotherapy is used. Children aged ≥6 months receive empirical pre-operative chemotherapy, and histology is determined at the time of delayed nephrectomy. The pathological evaluation of a tumor after pre-operative chemotherapy renders an in vivo test of treatment response, which is merged into the histological risk stratification scheme used by the SIOP investigators, where the persistence of large amounts of resistant blastema defines a new high risk category (24,58). This approach may rise some concerns regarding exposure of non-Wilms’ tumors (such as renal clear cell sarcoma) to the same chemotherapy as that used for localised Wilms’ tumors. However, this management does not endanger event-free survival (EFS) when “high risk” post-operative chemotherapy is delivered (58).
The COG approach favouring immediate surgery, with subsequent chemo- and radiotherapy allows to define carefully pathological staging of a tumor, prior to initiation of chemotherapy, based on assessment of both the extent of tumor spread and the success of surgical resection. However, this approach does not permit the evaluation of histological response to chemotherapy. It is also not possible to factor a tumor response into the risk stratification scheme, apart from the scope of metastatic and some bilateral disease (24,56). The current COG protocols recommend pre-operative chemotherapy for stage V tumors, and the decision for whole-lung radiotherapy in stage IV tumors is based on metastatic tumor response. Although the COG and SIOP philosophies differ, there is no apparent difference in the EFS and OS between contemporaneous approaches adopted for an individual patient. Both approaches result in long-term OS rates of around 90% in localised Wilms’ tumors and above 70% metastatic disease at a population level (4,8-10,56).
The role of biopsy remains controversial. The COG deems that any biopsy renders a tumor stage III and assignment to a treatment with radiotherapy confined to the tumor bed. In the UK, a percutaneous needle biopsy (PCNB) used to be performed to obtain a tissue sample prior to treatment in patients with non-metastatic disease. The UKCCSG Wilms’ tumor study 3 assessed the usefulness and safety of pre-chemotherapy biopsy and compared histologic features of Wilms’ tumor before and after chemotherapy. The morbidity associated with PCNB was small and therefore a needle biopsy of any renal mass was recommended prior to initiation of chemotherapy. However, this type of biopsy was not taken into account for staging purposes and tumor stage was determined on the nephrectomy sample, independent of the timing of the procedure. The results showed that a number of renal tumors can have the correct histologic diagnosis made by a PCNB. Of 188 of suitable cases with Wilms’ tumor, blastema was found in 89% of tumors at biopsy, but in only 50% at nephrectomy (10,56). This trial showed that pre-operative chemotherapy conducted to a more favourable stage distribution in the treatment arm assigned to biopsy, and rates of delayed nephrectomy and EFS did not differ from those in the arm treated by immediate nephrectomy (56). As a result of this trial, a pre-operative chemotherapy approach is now a standard practice in the UK. Although morbidity is low, due to unresolved concerns about a possible risk of biopsy track seeding, diagnostic biopsy prior to pre-operative chemotherapy is permitted but not considered standard practice according to the current SIOP Wilms’ protocol (59).
Treatment
The management of Wilms’ tumor requires multidisciplinary input of paediatric oncologists, specialist surgeons, radiologists, pathologists, and radiation oncologists. The role of surgery in the Wilms’ tumor therapy is critical as a meticulous and well performed procedure reduce the risk of tumor rupture and need for radiotherapy, which can be minimised in more experienced hands (60,61). Therefore children with a suspected renal tumor should be treated in specialist centres that have experience of management more than one case of Wilms’ tumor per year. The SIOP and NWTSG have conducted randomised clinical trials to establish the most efficient combinations of treatment for these children. The primary goals were to maximise cure while minimising toxicity. Pre-operative chemotherapy has been included in the treatment of children with Wilms’ tumor in SIOP protocols since the 1970s (8). It consists of double-agent chemotherapy (Vincristine and Dactinomycin) in children with localised tumors, and additional Doxorubicin in those presenting with metastases. The SIOP surgeons have demonstrated that the overall complication rate for the SIOP patients was significantly lower in comparison to NWTS patients (6.4% vs. 9.8%) (8). Nevertheless, both trial groups agree that specific patient groups seem to benefit from pre-operative chemotherapy. These are patients with extensive, inoperable tumors at presentation, children with synchronous disease in both kidneys, and those with expansive involvement of the inferior vena cava or right atrium (1,8).
In resource-limited countries, one of the challenges is late presentation with advanced disease (stage III or IV). Here, the SIOP approach favouring pre-operative chemotherapy makes surgery safer (10,59). Israëls et al. demonstrated that it is feasible and efficacious to give pre-operative chemotherapy (SIOP approach) for patients with Wilms’ tumor in Malawi, a country with very limited resources (62). In 2012 a multidisciplinary group of clinicians produced clinical recommendations for the management of children with Wilms’ tumor in a low income setting based on available evidence and personal experience (59). The group recommends pre-operative chemotherapy which is a logical strategy for patients with large tumors, in a setting where supportive care is restricted and radiotherapy may not be available (59).
Surgery maintains an important role in the treatment of Wilms’ tumor despite the fact that the improved outcome for this malignancy during the last century is assigned mainly to advances in chemotherapy. Careful removal of the tumor without rupture or spill is imperative because these patients have a six-fold increased risk of local abdominal relapse (12). Therefore such a precise and well performed procedure enables to avoid the need for postoperative irradiation. Transperitoneal radical nephrectomy, that ensures thorough exploration of the abdominal cavity, is the preferred operative procedure for unilateral Wilms’ tumor. If pre-operative imaging technique (CT or MRI) demonstrates normal liver and contralateral kidney, intraoperative inspection of these organs is no longer required in view of the high accuracy of current imaging modalities (1,12). However, a review of lymph node sampling demonstrated a false negative rate of more than 30% on surgical assessments (1). Hence although formal lymph node dissection is not needed, lymph node sampling is critically important during the surgical procedure regardless of the absence of abnormal nodes on pre-operative imaging or surgically benign looking nodes. Similarly, enlarged lymph nodes seen on pre-operative imaging do not require lymph node clearance, as these are often simply ‘reactive’ and there is no evidence that lymphadenectomy improves survival and it has considerable potential for side effects. Also, any case with histological evidence of lymph node involvement should be treated with radiotherapy to the entire para-aortic lymph node area. The absence of node sampling may result in understaging and undertreatment of the tumor as reported by the NWTS group in 2005. This could increase the relative risk of local recurrence (1,3,4). Surrounding structures are infrequently invaded by Wilms’ tumors. The exclusive indication for an excision of the tumor with closely adherent structures is when the tumor cannot be cleanly separated from adjacent parts, e.g., hepatic invasion (1).
Partial nephrectomy or wedge excision of the tumor is advocated for suitable cases of children with synchronous disease in both kidneys, who account for approximately 5% of all patients with Wilms’ tumor and for those with syndromes that predispose to late renal failure such as Denys-Drash syndrome (63). These techniques should not be considered as a standard approach for unilateral Wilms’ tumor due to the increased risk of positive surgical margins and local tumor recurrence (10). The risk of renal failure is a concern for patients with bilateral Wilms’ tumor (1). The incidence of end-stage renal disease is approximately 15% at 15 years post-surgery but varies according to genetic aetiology (63). Because surgery is a crucial element of Wilms’ tumor treatment, achieving a high cure rate while maintaining adequate long-term renal function can be very challenging in the management of these patients. The vast majority of tumors at initial presentation are too large for a partial nephrectomy, hence making it hard to obtain negative margins to decrease recurrence. Apart from that, there are inherent risks involved with surgical removal of a large renal mass in a small child. The most common complication during surgery is bleeding while the most common complication post-surgery is small bowel obstruction that occurs in more than 5% of children. Therefore, a pre-treatment chemotherapy can be used to facilitate tumor burden to a size which is susceptible to renal-sparing surgery (12).
Management of a child with bilateral Wilms’ tumor is very challenging and requires planning according to individualised patient needs, careful monitoring of response to chemotherapy, together with an understanding of the underlying histology and biology. For example, chemotherapy is not beneficial for stromal-predominant Wilms’ tumor with rhabdomyoblastic features, while other histological subtypes may benefit from additional chemotherapy. Therefore the surgical approach for each kidney has to be considered individually. In case of discordant histology, chemotherapy is given as appropriate to the higher risk lesion (24).
Late effects of Wilms’ tumor treatment
Over the last few decades the treatment of Wilms’ tumor has undergone incremental improvement in survival rates despite a general trend to reduced therapy for the majority. This risk adaptation of therapy has been driven by the well-established recognition of the ‘cost of cure’ for those children treated with doxorubicin and radiotherapy. Long-term survivors of Wilms’ tumor are at increased risk of treatment related morbidity and mortality (64). The most common complications are cardiotoxicity (4.4%), musculoskeletal problems (3%), and the development of secondary malignant neoplasms (1%) (12,51,65). Patients treated with anthracyclines, such as Doxorubicin, may present with congestive heart failure occurring many years after treatment. The most important risk factor of cardiac dysfunction is total cumulative dose of Doxorubicin, female sex and left flank irradiation, but any amount of anthracycline exposure may lead to myocardial injury (12). Radiation therapy can have an effect on growing and developing tissues. Significant musculoskeletal conditions have been reported among children receiving radiation treatment in early NWTSG trials. These side effects were dependent on total radiation dose, age at the time of treatment, fractionation and field (12). The doses that are currently recommended should not cause significant height sequelae. However, there are potential long-term hazards of radiotherapy to the lungs, i.e., pulmonary fibrosis (66). Long-term survivors of Wilms’ tumor have also been noted to have an increased risk of developing subsequent secondary malignant neoplasms (6.7% at 40 years from diagnosis) (65). Secondary malignancies include bone and soft-tissue sarcomas, breast cancer, lymphoma, leukaemia, melanoma (12). Therefore children who were treated with radiation therapy or received chemotherapeutic drugs with the potential for causing significant organ dysfunction require additional counselling and monitoring. There is a concern with regards to late occurrence of renal dysfunction following nephrectomy. The long-term risk of renal failure following treatment for unilateral Wilms’ tumor is low (0.25%) and usually associated with congenital disorders such as Denys-Drash and WAGR syndromes (63). Nevertheless one of the aims of long-term follow up is monitoring of renal function. In 2005 the UK Late Effects Group published follow-up guidelines that recommend that Wilms’ tumor survivors should have both, blood pressure and an early morning urine test for protein/creatinine ratio measured annually, and serum creatinine measurement every five years for life (67). Ongoing care for long-term survivors can be challenging as Wilms’ tumor survivors often lack knowledge about their diagnosis, therapy, and risk of late effects. Therefore it is imperative that survivors receive an appropriate and individualised education and screening so that late effects can be recognised at the earliest and most treatable stage. The increased risk of late effects are directly associated with the aggressiveness of treatment for high stage Wilms’ tumor. Hence current treatment protocols focus on reduction in the aggressive therapy while decreasing morbidity especially for low stage disease.
Adult Wilms’ tumor
Wilms’ tumor is extremely rare in adults, accounting for less than 1% of renal tumors in this age group (7). Only 70 new cases of this tumor are diagnosed in Europe each year (68). The diagnosis is often unexpected and made after nephrectomy for presumed renal cell carcinoma, which is the most frequent adult kidney cancer. Outcome for adults is inferior compared with children as there is often a delay in initiating chemotherapy while diagnostic review is undertaken by adult oncologists and pathologists. In 2006, Mitry et al. reported the 5-year survival rate of adults with Wilms’ tumor 73.7%, 47.5%, and 14.7% for those with localized tumors, regional extension, and metastatic tumors, respectively (68). Hence in 2011 European and US paediatric oncologists proposed a standardised approach to the diagnosis, staging, and treatment of adults with Wilms’ tumor based on international consensus (7). A pathological review by a paediatric pathologist expert in Wilms’ tumors is recommended. One should also be alert for severe neurotoxicity secondary to Vincristine and hepatotoxicity due to Actinomycin D that are more frequent in adults than children. Although open partial nephrectomy has become the gold standard for a single small tumor of kidney in adults, the international consensus recommends total nephrectomy as per adult nephrectomy guidelines for any renal cancer, when the diagnosis of Wilms’ tumor has been made before nephrectomy (7,69). Previously published data reported worse survival for adults than children, but adults treated according to recent paediatric protocols may have somewhat better outcomes. The international consensus encourages register patients in paediatric clinical trials where possible (7).
Concluding remarks
Over the past five decades, the multidisciplinary approach to Wilms’ tumor management has become an example of the success stories of paediatric oncology. Successful management of this malignancy requires meticulous attention to the correct staging of the tumor and good communication between members of a multidisciplinary team. New treatment protocols are designed on the basis of risk assignment to minimise toxicity for low risk patients and improve the outcome for children with high risk disease. Advances in the treatment of Wilms’ tumor have come from detailed analysis of treatment based outcomes, molecular biology and genetics of the tumor. Future efforts will concentrate on unlocking the molecular mechanisms of metastasis while clinical endeavours will remain focused on minimising toxicity and improving outcomes for children with unfavourable histology and recurrent disease. In contrast, survival in low income settings remains much lower, ranging from 11% to 50% in sub-Saharan Africa. A treatment guideline adapted to local circumstances is one of the keys to improving results (58). Different settings require different strategies to be able to provide locally optimal management to children with Wilms’ tumor.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Davidoff AM. Wilms’ tumor. Curr Opin Pediatr 2009;21:357-64. [PubMed]
- Chu A, Heck JE, Ribeiro KB, et al. Wilms’ tumor: a systematic review of risk factors and meta-analysis. Paediatr Perinat Epidemiol 2010;24:449-69. [PubMed]
- Stiller CA, Allen MB, Eatock EM. Childhood cancer in Britain: the National Registry of Childhood Tumors and incidence rates 1978-1987. Eur J Cancer 1995;31A:2028-34. [PubMed]
- Scott RH, Walker L, Olsen ØE, et al. Surveillance for Wilms tumor in at-risk children: pragmatic recommendations for best practice. Arch Dis Child 2006;91:995-9. [PubMed]
- Rivera MN, Haber DA. Wilms’ tumor: connecting tumorigenesis and organ development in the kidney. Nat Rev Cancer 2005;5:699-712. [PubMed]
- Buckley KS. Pediatric genitourinary tumors. Curr Opin Oncol 2011;23:297-302. [PubMed]
- Segers H, van den Heuvel-Eibrink MM, Pritchard-Jones K, et al. SIOP-RTSG and the COG-Renal Tumor Committee. Management of adults with Wilms’ tumor: recommendations based on international consensus. Expert Rev Anticancer Ther 2011;11:1105-13. [PubMed]
- Spreafico F, Bellani FF. Wilms’ tumor: past, present and (possibly) future. Expert Rev Anticancer Ther 2006;6:249-58. [PubMed]
- Pritchard-Jones K. Controversies and advances in the management of Wilms’ tumor. Arch Dis Child 2002;87:241-4. [PubMed]
- Wu HY, Snyder HM 3rd, D’Angio GJ. Wilms’ tumor management. Curr Opin Urol 2005;15:273-6. [PubMed]
- Available online: http://www.cancerresearchuk.org/cancerinfo/cancerstats/childhoodcancer/incidence/#Renal
- Ko EY, Ritchey ML. Current management of Wilms’ tumor in children. J Pediatr Urol 2009;5:56-65. [PubMed]
- Fukuzawa R, Breslow NE, Morison IM, et al. Epigenetic differences between Wilms’ tumors in white and east-Asian children. Lancet 2004;363:446-51. [PubMed]
- Reeve AE, Becroft DM, Morison IM, et al. Insulin-like growth factor-II imprinting in cancer. Lancet 2002;359:2050-1. [PubMed]
- Pastore G, Znaor A, Spreafico F, et al. Malignant renal tumors incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:2103-14. [PubMed]
- Beckwith JB, Kiviat NB, Bonadio JF. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms’ tumor. Pediatr Pathol 1990;10:1-36. [PubMed]
- Breslow N, Olshan A, Beckwith JB, et al. Epidemiology of Wilms-Tumor. Med Pediatr Oncol 1993;21:172-81. [PubMed]
- Breslow NE, Beckwith JB, Perlman EJ, et al. Age distributions, birth weights, nephrogenic rests, and heterogeneity in the pathogenesis of Wilms tumor. Pediatr Blood Cancer 2006;47:260-7. [PubMed]
- Beckwith JB. Nephrogenic rests and the pathogenesis of Wilms tumor: developmental and clinical considerations. Am J Med Genet 1998;79:268-73. [PubMed]
- Mishra K, Mathur M, Logani KB, et al. Precursor lesions of Wilms’ tumor in Indian children - A multiinstitutional study. Cancer 1998;83:2228-32. [PubMed]
- Vujanić GM, Sandstedt B, Harms D, et al. Revised International Society of Paediatric Oncology (SIOP) working classification of renal tumors of childhood. Med Pediatr Oncol 2002;38:79-82. [PubMed]
- Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A 1971;68:820-3. [PubMed]
- Knudson AG, Strong LC. Mutation and cancer: a model for Wilms’ tumor of the kidney. J Natl Cancer Inst 1972;48:313-24. [PubMed]
- Szychot E, Brodkiewicz A, Pritchard-Jones K. Review of current approaches to the management of Wilms’ tumor. Int J Clin Rev 2012;10:07. doi: [PubMed]
- Scott RH, Stiller CA, Walker L, et al. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumor. J Med Genet 2006;43:705-15. [PubMed]
- Pritchard-Jone K, Vujani GM. Recent Developments in the Molecular Pathology of Paediatric Renal Tumors. The Open Pathology Journal 2010;4:32-9.
- DeBaun MR, Niemitz EL, McNeil DE, et al. Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Widemann syndrome with cancer and birth defect Am J Hum Genet 2002;70:604-611. [PubMed]
- Available online: http://www.cancer.gov/cancertopics/pdq/treatment/wilms/Patient#Keypoint2
- Williams RD, Al-Saadi R, Chagtai T, et al. Children’s Cancer and Leukaemia Group; SIOP Wilms’ Tumor Biology Group. Subtype-specific FBXW7 mutation and MYCN copy number gain in Wilms’ tumor. Clin Cancer Res 2010;16:2036-45. [PubMed]
- Ruteshouser EC, Robinson SM, Huff V. Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer 2008;47:461-70. [PubMed]
- Ogawa O, Eccles MR, Szeto J, et al. Relaxation of Insulin-Like Growth Factor-Ii Gene Imprinting Implicated in Wilms-Tumor. Nature 1993;362:749-51. [PubMed]
- Ravenel JD, Broman KW, Perlman EJ, et al. Loss of imprinting of insulin-like growth factor-II (IGF2) gene in distinguishing specific biologic subtypes of Wilms tumor. J Natl Cancer Inst 2001;93:1698-703. [PubMed]
- Fukuzawa R, Anaka MR, Heathcott RW, et al. Wilms tumor histology is determined by distinct types of precursor lesions and not epigenetic changes. J Pathol 2008;215:377-87. [PubMed]
- Scott RH, Murray A, Baskcomb L, et al. Stratification of Wilms tumor by genetic and epigenetic analysis. Oncotarget 2012;3:327-35. [PubMed]
- Bliek J, Gicquel C, Maas S, et al. Epigenotyping as a tool for the prediction of tumor risk and tumor type in patients with Beckwith-Wiedemann syndrome (BWS). J Pediatr 2004;145:796-9. [PubMed]
- Williams RD, Al-Saadi R, Natrajan R, et al. Molecular profiling reveals frequent gain of MYCN and anaplasia-specific loss of 4q and 14q in Wilms tumor. Genes Chromosomes Cancer 2011;50:982-95. [PubMed]
- Wegert J, Wittmann S, Leuschner I, et al. WTX inactivation is a frequent, but late event in Wilms tumors without apparent clinical impact. Genes Chromosomes Cancer 2009;48:1102-11. [PubMed]
- Rivera MN, Kim WJ, Wells J, et al. An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science 2007;315:642-5. [PubMed]
- Bardeesy N, Beckwith JB, Pelletier J. Clonal Expansion and Attenuated Apoptosis in Wilms-Tumors Are Associated with P53 Gene-Mutations. Cancer Res 1995;55:215-9. [PubMed]
- Bardeesy N, Falkoff D, Petruzzi MJ, et al. Anaplastic Wilms-Tumor, a Subtype Displaying Poor-Prognosis, Harbors P53 Gene-Mutations. Nat Genet 1994;7:91-7. [PubMed]
- Popov SD, Vujanic GM, Sebire NJ, et al. Bilateral Wilms tumor with TP53-related anaplasia. Pediatr Dev Pathol 2013;16:217-23. [PubMed]
- Natrajan R, Little SE, Sodha N, et al. Analysis by array CGH of genomic changes associated with the progression or relapse of Wilms’ tumor. J Pathol 2007;211:52-9. [PubMed]
- Höglund M, Gisselsson D, Hansen GB, et al. Wilms tumors develop through two distinct karyotypic pathways. Cancer Genet Cytogenet 2004;150:9-15. [PubMed]
- Grundy PE, Breslow NE, Li S, et al. National Wilms Tumor Study Group. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 2005;23:7312-21. [PubMed]
- Messahel B, Williams R, Ridolfi A, et al. Allele loss at 16q defines poorer prognosis Wilms tumor irrespective of treatment approach in the UKW1-3 clinical trials: A Children’s Cancer and Leukaemia Group (CCLG) study. Eur J Cancer 2009;45:819-26. [PubMed]
- Wittmann S, Zirn B, Alkassar M, et al. Loss of 11q and 16q in Wilms tumors is associated with anaplasia, tumor recurrence, and poor prognosis. Genes Chromosomes Cancer 2007;46:163-70. [PubMed]
- Hing S, Lu YJ, Summersgill B, et al. Gain of 1q is associated with adverse outcome in favorable histology Wilms’ tumors. Am J Pathol 2001;158:393-8. [PubMed]
- Lu YJ, Hing S, Williams R, et al. Chromosome 1q expression profiling and relapse in Wilms’ tumor. Lancet 2002;360:385-6. [PubMed]
- Gratias EJ, Jennings LJ, Anderson JR, et al. Gain of 1q is associated with inferior event-free and overall survival in patients with favorable histology Wilms tumor: a report from the Children’s Oncology Group. Cancer 2013;119:3887-94. [PubMed]
- Perotti D, Spreafico F, Torri F, et al. Genomic profiling by whole-genome single nucleotide polymorphism arrays in Wilms tumor and association with relapse. Genes Chromosomes Cancer 2012;51:644-53. [PubMed]
- Davidoff AM. Wilms tumor. Adv Pediatr 2012;59:247-67. [PubMed]
- Baxter PA, Nuchtern JG, Guillerman RP, et al. Acquired von Willebrand syndrome and Wilms tumor: not always benign. Pediatr Blood Cancer 2009;52:392-4. [PubMed]
- Fuchs J, Szavay P, Luithle T, et al. Surgical implications for liver metastases in ephroblastoma--data from the SIOP/GPOH study. Surg Oncol 2008;17:33-40. [PubMed]
- McDonald K, Sebire NJ, Anderson J, et al. Patterns of shift in ADC distributions in abdominal tumors during chemotherapy-feasibility study. Pediatr Radiol 2011;41:99-106. [PubMed]
- Pritchard-Jones K, Moroz V, Vujanic G, et al. Treatment and outcome of Wilms’ tumour patients: an analysis of all cases registered in the UKW3 trial. Ann Oncol 2012;23:2457-63. [PubMed]
- Mitchell C, Pritchard-Jones K, Shannon R, et al. United Kingdom Cancer Study Group. Immediate nephrectomy versus pre-operative chemotherapy in the management of non-metastatic Wilms’ tumor: results of a randomised trial (UKW3) by the UK Children’s Cancer Study Group. Eur J Cancer 2006;42:2554-62. [PubMed]
- Pritchard-Jones K, Moroz V, Vujanic G, et al. Children’s Cancer and Leukaemia Group (CCLG) Renal Tumors Group. Treatment and outcome of Wilms’ tumor patients: an analysis of all cases registered in the UKW3 trial. Ann Oncol 2012;23:2457-63. [PubMed]
- Gooskens SL, Furtwängler R, Vujanic GM, et al. Clear cell sarcoma of the kidney: a review. Eur J Cancer 2012;48:2219-26. [PubMed]
- Israels T, Moreira C, Scanlan T, et al. SIOP PODC: clinical guidelines for the management of children with Wilms tumor in a low income setting. Pediatr Blood Cancer 2013;60:5-11. [PubMed]
- Fuchs J, Kienecker K, Furtwängler R, et al. Surgical aspects in the treatment of patients with unilateral Wilms tumor: a report from the SIOP 93-01/German Society of Pediatric Oncology and Hematology. Ann Surg 2009;249:666-71. [PubMed]
- Godziński J, Weirich A, Tournade MF, et al. Primary nephrectomy for emergency: a rare event in the International Society of Paediatric Oncology Nephroblastoma Trial and Study no. 9. Eur J Pediatr Surg 2001;11:36-9. [PubMed]
- Israëls T, Molyneux EM, Caron HN, et al. Pre-operative chemotherapy for patients with Wilms tumor in Malawi is feasible and efficacious. Pediatr Blood Cancer 2009;53:584-9. [PubMed]
- Ritchey ML, Green DM, Thomas PR, et al. Renal failure in Wilms’ tumor patients: a report from the National Wilms’ Tumor Study Group. Med Pediatr Oncol 1996;26:75-80. [PubMed]
- Termuhlen AM, Tersak JM, Liu Q, et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2011;57:1210-6. [PubMed]
- Breslow NE, Lange JM, Friedman DL, et al. Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int J Cancer 2010;127:657-66. [PubMed]
- van Dijk IW, Oldenburger F, Cardous-Ubbink MC, et al. Evaluation of late adverse events in long-term Wilms’ tumor survivors. Int J Radiat Oncol Biol Phys 2010;78:370-8. [PubMed]
- United Kingdom Children’s Cancer Study Group, Late Effects Group. Skinner R, Wallace WH, et al. eds. Therapy Based Long Term Follow Up. Practice Statement (2005). Available online: http://www.cclg.org.uk/dynamic_files/LTFU-full.pdf, accessed August 2012.
- Mitry E, Ciccolallo L, Coleman MP, et al. Incidence of and survival from Wilms’ tumor in adults in Europe: data from the EUROCARE study. Eur J Cancer 2006;42:2363-8. [PubMed]
- Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 2007;178:41-6. [PubMed]


