
Tourette’s disorder in children and adolescents
Introduction
Tic disorders are among the most common neurodevelopmental disorders (neuropsychiatric conditions) in the pediatric population (i.e., children, adolescents) (1-6). The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) lists five tic disorders: provisional tic disorder, persistent (chronic) motor or vocal tic disorder, Tourette’s disorder (TD), other specified tics disorders, and unspecified tic disorders (1). This discussion on TD presents basic definitions, epidemiology, etiologic considerations, co-morbidities, clinical features, and management concepts for TD. It begins with basic definitions of the three main tic disorders: provisional tic disorder, persistent (chronic) motor or vocal tic disorder, and TD.
Definitions
Tics (habit spasms) are movements that are sudden, brief, purposeless and involuntary (1,2). The term, stereotypies, refers to movements that more prolonged than tics; they are purposeless, rhythmic, and repetitive. Stereotypies are impulsive, specific to each patient, and usually begin under age 3 years (7).
Tics are typically classified in three types: motor tics, vocal tics and sensory tics. Tic disorders are placed by the American Psychiatric Association’s DSM-5 in the category of Motor Disorders within the Neurodevelopmental Disorders category (1). These tic disorders begin under age 18 years and are not due to other underlying disorders such as substance use disorders (cocaine substance use disorders, others), Huntington’s disease, or post-viral encephalitis (1). The DSM-5 taxonomy removed the term, stereotype, from definition of a tic and also added a new specifier, tic-related, to its obsessive-compulsive disorder (OCD) (1).
Provisional tic disorder is defined in a person who develops single or multiple tics (motor and/or vocal) for less than 1 year before the age of 18 years (1). This condition is not due to substance use disorders or medial disorders as noted before. Also, the criteria for TD or persistent (chronic) motor or vocal tic disorder are not met (as discussed below). The tics can be worsened with stress and they can be voluntarily suppressed for periods of time ranging from minutes to hours. Disappearance of these tics within weeks of onset is the norm.
Persistent (chronic) motor or vocal tic disorder refers to a condition in which there is single or multiple tics that are either motor or vocal but not both types (1,2,4,5). This condition is present for over 1 year and the tics can vary in intensity as well as frequency. The tics are not due to substance use disorder or other medical illness as noted. The DSM-5 criteria for TD are not met.
TD is a neurodevelopmental (neuropsychiatric) disorder
Characterized as a familial condition with multiple motor tics as well as vocal tics (one or more) present for more than 1 year with of varying severity (1-6). As with the other tics, the condition is not due to substance use disorder or medical illnesses noted (i.e., Huntington’s disease, post-viral encephalitis) (1,2).
Tics may be simple or complex tics that involve the head, neck, trunk and/or extremities (upper or lower) (2). Motor tics can involve eye blinking, lip smacking, shoulder shrugging, grimacing, head tossing or other movements. Simplex vocal tics can involve coughing, grunting, shouting, barking, sniffing, throat clearing, or crying. Complex vocal tics can involve swearing (coprolalia), repeating words (echolalia), repeating the last sound (palilalia) and/or nonobscene socially inappropriate behaviors (NOSIBs) (1,2,5).
Epidemiology
Provisional tic disorder (previously called “transient tic disorder” in the 1994 DSM-IV) is found in 4% to 20% of pediatric persons (including young adolescents) (2). In addition to a positive family history there is a 2–3:1 male to female ratio. Persistent (chronic) motor or vocal tic disorder is usually identified in 1% to 2% of the general population often with a positive family history and with a link to (or association with) Tourette’s disease (2,8). Some also note chronic motor tic disorder can be found in 3–50 per 1,000 school children while chronic vocal tic disorder is seen in 2.5 to 9.4 per 1,000 school children (2,8).
Tourette’s disease (Tourette’s syndrome; Gilles de la Tourette syndrome) is classically identified in 5 (3-8) per 1,000; it is ten times more common in children in contrast to adults with a 3–4:1 male to female ratio (1-3,9). The average age of onset is 4–7 years of age with a typical range of 2 to 15 years and the defined final age of onset of 21 years. History taking reveals a positive family history for tic disorder—as persistent (chronic) motor or vocal tic disorder and/or TD.
Etiology
Etiologic underpinnings for Tourette’s disease and chronic motor tic disorder include central nervous system (CNS) dopamine metabolism dysfunction with circuitry abnormalities in CNS structures as the frontal (prefrontal) lobe, striatum, globus pallidus and thalamus; dysfunction occurs with the connections of the basal ganglia and cortical areas. The improvement noted with neuroleptic medication supports the dopaminergic dysfunction theory for tic disorders.
Other metabolic derangements have been studied in Tourette syndrome. Pourfar et al. looked at glucose utilization in the brain of patients with Tourette syndrome compared to controls; this report found varying utilization in the basal ganglia, increased activity in the premotor cortex as well as cerebellum and decreased resting activity of the striatum along with the orbitofrontal cortex (10).
Abnormalities in the GABA-ergic system have also been identified in those with Tourette syndrome. These abnormalities lead to a loss of inhibition and a maldistribution of GABA receptors. The areas of the brain most impacted by these changes were the thalamus, amygdala, bilateral ventral striatum and right insula (11). In addition, there is a theory for the action of Group A beta-hemolytic streptococcal infection leading to PANDAS (Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococci) (12,13).
Also, in addition to environmental impacts, there are genetic influences as suggested by research dealing with the SLITRK1 gene and others interacting with various environmental (epigenetic) factors (14-18). The SLITRK1 gene is noted to play a role in dendritic growth. It has been shown to be present in brain areas that have been implicated in Tourette syndrome (19).
Furthermore, the HDC gene, which is responsible for encoding L-histidine decarboxylase, has been identified. It is mostly present in the posterior hypothalamus and has connections to other brain regions. This gene has an autosomal dominant inheritance but is rare and only present in few families (20).
Neuroimaging pathology
The pathology of Tourette syndrome has not been fully elucidated. Though no consistent brain abnormalities have been noted, various studies have shown increased activity in specific brain areas related to the urge to tic and tic action. It has been suggested that the grey matter in the left frontal lobes of those with TS was smaller compared to controls (21). Another study stated that there is reduced thickness of gray matter in the various sulci as pre- and post-central, superior, inferior and internal frontal. These findings are significant because they suggest an abnormality originating in brain development (22). A decrease in caudate volume has also been identified. Furthermore, an inverse relationship between tic severity and sensorimotor cortex volume has been noted (23).
Using imaging modalities such as PET and MRI, the activity of different brain regions in the urge to tic and tic action were evaluated. Increased activity has been noted in neocortical, paralimbic and subcortical regions. During the urge to tic, areas that have revealed increased activity include the insula, cingulate cortex and supplementary cortical areas. Activation in sensorimotor areas, including cerebellum and bilateral superior parietal lobule, have been noted at tic onset. The combined effects of excessive activity in motor pathways and reduced activation in controlling regions of the cortico-striato-thalamo-cortical regions also correlates during the duration of urge to tic to tic onset (24,25).
One specific study observed the importance of the insular cortex and its role in the urge to blink. This finding is supportive on the insula being an integral part of other bodily urges, thoughts and behaviors. This is consistent with findings that have looked at other disorders with abnormal urges, including obsessive compulsive disorder (OCD), which is a known comorbidity of TS (26).
Differential diagnosis and co-morbid conditions
A careful evaluation should be obtained to be sure the person has various tics in contrast to other involuntary muscle movements such as myoclonus, spasm, tremor, chorea, dystonia, athetosis, or ballismus (2). As with the APA DSM-5 guidelines, the tics are not due to medications (i.e., stimulants) or illnesses (i.e., post-viral encephalitis or Huntington’s disease). In Tourette’s disease a wide variety of tics may present over time: motor, simple vocal and/or complex focal tics. A sensory tic can be seen in 3% characterized by an irritating sensation arising over a joint or muscle group that is improved by the tic. The tic may be stopped for a period of time until such unpleasantness (“premonitory sensory urges”) arises that the tic occurs to relieve the negative feeling for a “just-right” perception (18).
A wide variety of conditions are co-morbid with Tourette’s disease that includes 30% to 50% having attention-deficit/hyperactivity disorder (ADHD) and 30% to 60% having OCD (1,2,4,5,27,28). A subtype of OCD with tics has been classified as an OCD subtype (4). Issues of microglial dysregulation in Tourette syndrome (disease), OCD and PANDAS are described in the literature (29). Genetic and phenotypic overlaps between Tourette’s disease, OCD and ADHD are also described (30).
A wide variety of other conditions have been associated with Tourette’s disease that include other anxiety disorders (30–40%), mood disorders (30–40%), learning disorders with or without ADHD (20–30%), substance use disorders, intermittent explosive disorder, “rage attacks”, and autism spectrum disorder (1,2,4). A large prospective study looking at Tourette syndrome and the comorbidities revealed that tic severity decreased throughout the adolescent years.
Furthermore, the comorbid OCD and ADHD severity also decreased over time. It should be noted that subclinical symptoms and co-existing emotional pathologies remained as these patients progressed through adolescence. These conditions need to stay in the mind of clinicians as patients may still require treatment in this regard (31).
Management
Management of Tourette’s disease occurs at various therapeutic levels that includes education about this condition, reassurance as is appropriate, treatment of co-morbid conditions, various types of behavioral therapy, and medications as needed (2,18). Surgical measures are, in rare situations, also provided such as deep brain stimulation (18).
The focus in this section is on the pharmacologic management of Tourette’s disease. These agents typically include alpha agonists (clonidine, guanfacine) and antipsychotics (haloperidol, pimozide, risperidone, others) often as first-line drugs; additional medications include dopamine receptor agonist (i.e., pergolide), muscle relaxer/antispasmodic drug (i.e., baclofen), stimulants (methylphenidate, amphetamines), antidepressants (selective serotonin reuptake inhibitors, or SSRIs), anticonvulsants (i.e., topiramate and levetiracetam), and VMAT-2-inhibitors (2,6). As the clinician chooses a specific anti-tic medication, she/he must consider comorbid diagnoses that may be present and how the child or adolescent responds to various prescribed medications—including adverse effects that may arise (2).
Alpha agonists (clonidine, guanfacine)
These medications are typically the one of the first agents utilized when medication is chosen for Tourette’s disease and include clonidine as well as guanfacine (2,32). Alpha agonists provide reduction in adrenergic outflow from the CNS that may lower tic frequency. They have various medical uses that include being alternative or added medications for ADHD management.
Clonidine (presynaptic, central-acting alpha-2 agonist) is given at an oral dose of 0.05 to 0.3 mg per day and is given at bedtime or can be prescribed two to four times a day. Adverse effects typically seen include dizziness, constipation, dry mouth, drowsiness, and sedation (the latter is a serious limiting factor for many patients). A less common adverse effect seen in children is orthostatic hypotension. As the clinician carefully follows the patient on clonidine, slow build-up and withdrawal of dosages are recommended. Rapid withdrawal of clonidine may induce rebound hypertension.
Baseline and follow-up data include the blood pressure, pulse, blood sugar and an electrocardiogram (EKG). The EKG can be taken every 6 months and anecdotal reports are seen of sudden cardiac deaths in a few pediatric patients on clonidine along with methylphenidate (2,33).
Guanfacine is an alpha2A adrenergic agonist related to clonidine that is also prescribed to lower tics with a daily dose of 0.5 to 1 mg given three times a day. The side effects are usually lower than seen with clonidine. However, some patients may have increased agitation and headaches on guanfacine. Typically, there is less sedation and blood pressure issues in persons taking guanfacine than noted with clonidine.
Antipsychotics
Traditional tic management involves use of behavioral therapy and then alpha agonists if needed. If such initial therapy is not sufficient and/or the tics are severe or bothersome to the person, other medications may be tried (34,35). Typically, at this point, a trial of an antipsychotic medication may be provided to help with tic suppression—either partial or full (33-40). The exact mechanism for tic suppression with use of antipsychotics is not precisely known, but it may be related to dopamine blockade of post-synaptic receptors in the cortico-striato-thalamic circuitry of the CNS.
Antipsychotic medications that have been utilized include haloperidol as well as pimozide and more recently—the atypical antipsychotics (AAs): risperidone, aripiprazole and ziprasidone. Whatever medication is used clinicians should seek a balance between the best tic suppression with adverse effects minimization. Table 1 provides some prescription guidelines for antipsychotics.

Full table
Haloperidol
Studies suggest that approximately 25% of pediatric patients with Tourette’s disease placed on haloperidol will develop a 70% lowering of their tics at a dosage that minimalizes or avoids major adverse effects (2). Approximately half of those with bothersome tics experience tic reduction only at doses inducing major antipsychotic side effects and 25% do not improve with haloperidol at all.
Start with a dose of 0.25 mg orally each day and gradually increase to 2 mg, two times per day as the patient tolerates and the overall tic suppression response. Higher doses may be given (i.e., 5 mg twice a day); however, maximum tic suppression usually occurs at doses below that used for overt psychosis management.
Potential adverse effects include extrapyramidal symptoms (EPS) and neuroleptic syndrome (NMS). Contraindications for haloperidol (as well as fluphenazine) include those with liver disease, subcortical brain damage, blood dyscrasias, and mental obtundation.
Pimozide
If pimozide is used, there is typically a 70% to 80% lowering of tics, usually without major side effects (41). One method of pimozide prescription for tic suppression is to use the patient’s weight as a guide: 0.05–0.2 mg/kg/day—not to go over 10 mg per day. Some clinicians just begin with 1 mg daily orally and gradually increase up to 4 mg two times a day.
Pimozide is not used for tic disorders other than for Tourette’s syndrome; also, it generally should not be used with stimulant medications if the clinician concludes that the stimulant(s) is causing the tics and not the Tourette’s syndrome. Pimozide can prolong the QTc interval; thus, it should not be prescribed with other drugs that can in combination prolong the QTc interval; such medications include nefazodone, chlorpromazine, thioridazine, citalopram, ziprasidone, fluoxetine, sertraline, and fluvoxamine.
Risperidone
The tic-suppression dosage for the AA risperidone is 0.25 mg daily to 2 mg twice a day orally. Risperidone (along with haloperidol and pimozide) are at risk for causing EPS and NMS. Other adverse effects include cognitive dysfunction (impairment) and lethargy. All the AAs can increase blood glucose and in increasing order, can lead to weight gain: risperidone, quetiapine, and olanzapine. The newer AAs can increase serum prolactin except for the AA aripiprazole and quetiapine.
Baclofen
The muscle relaxant, baclofen, has been utilized with some success to reduce tics in persons with Tourette disease (2,42). Its action may involve inhibitory effects via GABA (gamma-aminobutyric acid) of which this chemical is an analogue. The initial dose of baclofen for Tourette’s disease is 5 mg (three times a day) with a dosage augmentation every 5 days (as tolerated) to a maximum of 20 mg three times a day; the upper limit of the adult dosage is 80 mg per day.
As with all medications, observance for side effects is important. Somnolence is noted in over half of those taking baclofen at first; fortunately, this adverse effect tends to be transient. Other common adverse effects include weakness, confusion, headache, dizziness, fatigue, nausea, and urinary frequency. In some persons on this medication, an increase may occur in blood sugar, alkaline phosphatase and SGOT (serum glutamic-oxaloacetic transaminase; AST: aspartate aminotransferase). Thus, monitoring those on baclofen may include periodic testing for these measures.
Stimulants
As noted, Tourette’s disease may be co-morbid with ADHD and the use of stimulant medications may help both the tics and the ADHD symptomatology (2,27,33,43-50). The use of stimulants (i.e., methylphenidate; amphetamines) augments the dopamine/norepinephrine availability at post-synaptic neurons probably due to action at the pre-synaptic neurons (i.e., increased release; reuptake blockade) (2,27,33).
Persons with Tourette’s syndrome and ADHD may be prescribed stimulant medication and other anti-tic medication (i.e., antipsychotics, others) of bothersome tics develop (43-50). There is no clear research that stimulants are the etiologic agent behind tics and thus, careful evaluation as well as management is needed. Clinicians should follow the patient carefully and provide or stop such medications as the clinical course unfolds or dictates (2,27,33). Anti-ADHD medications that do not worsen tics can be considered, such as alpha-2-agonists or atomoxetine (selective norepinephrine reuptake inhibitor).
Antidepressants
Selective serotonin reuptake inhibitors (SSRIs) have been utilized to ameliorate tics in some persons with Tourette’s syndrome while also potentially improving various Tourette syndrome co-morbidities; these include OCD, generalized anxiety disorder, and phobias. Tics may be worsened with anxiety and thus anti-anxiety treatment (pharmacologic or psychologic) may be helpful in improving the tic disorder.
The mechanisms of action include the augmentation of catecholamine availability via reuptake inhibition, antagonism (pre/post-synaptic) and/or a combination of these pathophysiologic actions (2). Antidepressant medications that have serotonergic activity (not mirtazapine or venlafaxine) are contraindicated with pimozide due to the increase in the adverse effect of prolongation of the QTc interval (as noted previously).
Some clinicians prefer to use citalopram or escitalopram for persons with OCD and TD since this combination is less likely to induce behavior activation and agitation—in contrast to other SSRIs. Maximum dosage of the SSRI may be needed for successful amelioration of the tics and thus, careful monitoring of such combinations is needed. Finally, the combination of risperidone and an SSRI may be needed for severe situations of Tourette disorder (51).
Anticonvulsants
Anticonvulsant medications, particularly topiramate and levetiracetam, have been utilized to reduce tic frequency in persons with Tourette syndrome; carbamazepine and valproic acid have also been utilized (2,52,53). The mechanism of action for improvement is unclear but may be related to such issues as augmentation of GABA (an inhibitory neurotransmitter), stabilization of neuronal membranes and/or inhibition of amino acids (i.e., aspartate, glutamate) that are excitatory. Those with Tourette syndrome also have an increased risk of having epilepsy (54).
Dosages used for topiramate are 50 to 200 mg once per day while dosages for levetiracetam range from 1,000 to 2,000 mg once per day. As with these various anti-tic medications, clinicians should start with a low dose and gradually increase as needed for maximum tic control.
No routine laboratory testing is usually suggested when taking levetiracetam. Topiramate, however, can increase liver function tests (LFTs) and periodic laboratory testing when on this anticonvulsant include LFTs along with a complete blood count (CBC), blood urea nitrogen (BUN) and creatinine. Common adverse effects from these drugs are noted in Table 2.
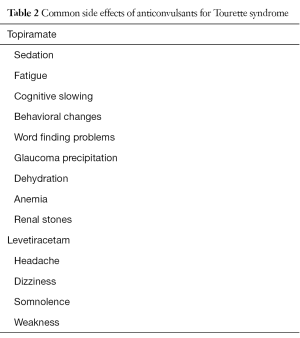
Full table
Miscellaneous drugs
There are a wide variety of old and new (emerging) medications that have been and are being used for various movement disorders in adults as well as occasionally for pediatric patients (2). Caution, however, should be applied in considering these medications and consultation with experts in neurology and psychiatry is recommended. As the 21st century research becomes available, guidelines may develop for the safe application of some of these newer movement disorder medications for severe and/or unique cases of Tourette’s disease.
Pergolide, for example, is a dopamine receptor agonist (ergoline-based) that has been used for management of Parkinson’s disease and neuroleptic-resistant Tourette’s disease (2,55). However, this drug was withdrawn from the United States human pharmacopoeia market in 2007 because of links with valvular heart disease (56).
Benzamides are selective DA-D2 receptor antagonists that have been studied as antipsychotics (i.e., sultopride), analgesics (i.e., salicylamide), antiemetics (i.e., metoclopramide), and others (2,57-60). Tiapride is a benzamide utilized in Europe and other countries for management of tics (59,60). Tetrabenazine is a CNS depressant and VMAT2 (vesicular monoamine transporter 2) inhibitor used to treatment movement disability in Huntington’s disease, other hyperkinetic movement disorders and severe Tourette’s disease (2,6,38,60-62). Other VMAT2 inhibitors are under study as well including deutetrabenazine and valbenazine (53).
A variety of other agents remain under study and include the cannabinoid delta-9-tetrahydrocannabinol, ecopipam (D1 receptor antagonist), clonazepam (benzodiazepine/anxiolytic), n-acetylcysteine (amino acid), omega-3 fatty acids, ningdong granule (complementary and alternative therapy), 5-Ling granule (a proprietary herbal medicine), and others (2,53-68). Botulinum toxin, in addition, has been used to improve motor tics with variable results for about 3 months (69).
Conclusions
Tourette syndrome is a complex neuropsychiatric (neurodevelopmental) disorder with a prevalence of 1% and diagnosed between 4 and 18 years old (up to 21 years of age) (1,2). Classic features include various motor and phonic tics. There can be complex vocal tics with swearing (coprolalia), repeating words (echolalia), repeating the last sound (palilalia) and/or non-obscene socially inappropriate behaviors (NOSIBs) (1,2,5). A variety of co-morbid conditions are described with Tourette syndrome such as ADHD and OCD.
Management of Tourette’s disease occurs at various therapeutic levels that includes education about this condition, reassurance as is appropriate, treatment of co-morbid conditions, various types of behavioral therapy, and medications as needed (2,18,70,71). A variety of pharmacologic agents can be utilized that typically initiate with alpha agonists (clonidine, guanfacine) and then antipsychotics (i.e., risperidone, pimozide, others). If required, a variety of other medications are used that include baclofen (muscle relaxant), stimulants (methylphenidate), antidepressants (SSRIs, others), and anticonvulsants (topiramate, levetiracetam, others).
Other medications are under study and may become increasingly used for Tourette syndrome as the 21st century unfolds. The actual combination of medications is dependent on clinician preference, appearance of bothersome adverse effects and the complications of co-morbid conditions (2,4,5). The benefit of deep brain stimulation remains under research at this time for pediatric and adult patients (6,72-75).
Acknowledgments
The authors thank Department Chair, Professor Dilip R. Patel for supporting this scholarly activity.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5 Edition (DSM-5). Arlington, VA: American Psychiatric Publishing, 2013.
- Greydanus DE. Tic disorders. In: Greydanus DE, Patel R, Pratt HD, et al. editors. Behavioral Pediatrics. 4th Edition. NY: Nova Biomedical Publishers, 2015:321-52.
- Rampello L, Alvano A, Battaglia G, et al. Tic disorders: From pathophysiology to treatment. J Neurol 2006;253:1-15. [Crossref] [PubMed]
- Martino D, Ganos C, Pringsheim TM. Tourette syndrome and chronic tic disorders: The clinical spectrum beyond tics. Int Rev Neurobiol 2017;134:1461-90. [Crossref] [PubMed]
- Martino D, Madhusudan N, Zis P, et al. An introduction to the clinical phenomenology of Tourette syndrome. Int Rev Neurobiol 2013;112:1-33. [Crossref] [PubMed]
- Martino D, Pringsheim TM. Tourette syndrome and other chronic tic disorders: an update on clinical management. Expert Rev Neurother 2018;18:125-37. [Crossref] [PubMed]
- Eirís-Puñal J. Motor developments in neurodevelopmental disorders. Tics and stereotypies. Rev Neurol 2014;58:S77-82. [PubMed]
- Scahill L, Specht M, Page C. The prevalence of tic disorders and clinical characteristics in children. J Obsessive Compuls Relat Disord 2014;3:394-400. [Crossref] [PubMed]
- Delgado MR, Albright AL. Movement disorders in children: Definitions, classifications and grading systems. J Child Neurol 2003;18:S1-8. [Crossref] [PubMed]
- Pourfar M, Feigin A, Tang CC, et al. Abnormal metabolic brain networks in Tourette syndrome. Neurology 2011;76:944-52. [Crossref] [PubMed]
- Lerner A, Bagic JM, Simmons JM, et al. Widespread abnormality of the γ-aminobutyric acid-ergic system in Tourette syndrome. Brain 2012;135:1926-36. [Crossref] [PubMed]
- Swedo SE. Pediatric autoimmune neuropsychiatric disorders: associated with streptococcal infections (PANDAS). Mol Psychiatry 2002;7:S24-5. [Crossref] [PubMed]
- Gilbert DL. Inflammation in tic disorders and obsessive-compulsive disorder: Are PANS and PANDAS a path forward? J Child Neurol 2019;34:598-611. [Crossref] [PubMed]
- Paschou P. The genetic basis of Gilles de la Tourette Syndrome. Neurosci Biobehav Rev 2013;37:1026-39. [Crossref] [PubMed]
- Dietrich A, Fernandez TV, King RA, et al. The Tourette International Collaborative Genetics (TIC Genetics) study, finding the genes causing Tourette syndrome: objectives and methods. Eur Child Adolesc Psychiatry 2015;24:141-51. [Crossref] [PubMed]
- Sun N, Nasello C, Deng L, et al. A The PNKD gene is associated with Tourette disease or tic disorder in a multiplex family. Mol Psychiatry 2018;23:1487-95. [Crossref] [PubMed]
- Fernandez TV, State MW, Pittenger C. Tourette disorder and other tic disorders. Handb Clin Neurol 2018;147:343-54. [Crossref] [PubMed]
- Serajee FJ, Mahbubul Hug AH. Advances in Tourette syndrome: diagnoses and treatment. Pediatr Clin North Am 2015;62:687-701. [Crossref] [PubMed]
- Abelson JF, Kwan KY, O’Roak BJ, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005;310:317-20. [Crossref] [PubMed]
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, et al. L-Histidine decarboxylase and Tourette’s syndrome. N Engl J Med 2010;362:1901-8. [Crossref] [PubMed]
- Fredericksen KA, Cutting LE, Kate WR, et al. Disproportionate increases of white matter in right frontal love in Tourette syndrome. Neurology 2002;58:85-9. [Crossref] [PubMed]
- Muellner J, Delmaire C, Valabrégue R, et al. Altered structure of cortical sulci in Gilles de la Tourette syndrome: Further support for abnormal brain development. Mov Disord 2015;30:655-61. [Crossref] [PubMed]
- Plessen KJ, Bansal R, Peterson BS. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J Psychosom Res 2009;67:559-73. [Crossref] [PubMed]
- Cavanna AE, Black KJ, Hallett M, et al. Neurobiology of the premonitory urge in Tourette’s syndrome: Pathophysiology and treatment implications. J Neuropsychiatry Clin Neurosci 2017;29:95-104. [Crossref] [PubMed]
- Lerner A, Bagic A, Hanakawa T, et al. Involvement of insula and cingulate cortices in control and suppression of natural urges. Cereb Cortex 2009;19:218-23. [Crossref] [PubMed]
- Berman BD, Horovitz SG, Morel B, et al. Neural correlates of blink suppression and the buildup of a natural bodily urge. Neuroimage 2012;59:1441-50. [Crossref] [PubMed]
- Greydanus DE, Pratt HD. Attention-deficit/hyperactivity disorder in children and adolescents: Interventions for a complex costly clinical conundrum. Pediatr Clin North Am 2003;50:1049-92. [Crossref] [PubMed]
- Leckman JF. Tourette’s syndrome. Lancet 2002;360:1577-86. [Crossref] [PubMed]
- Frick L, Pittenger C. Microglia dysregulation in OCD, Tourette Syndrome, and PANDAS. J Immunol Res 2016;2016:8606057. [Crossref] [PubMed]
- Hirschtritt ME, Darrow SM, Illmann C, et al. Genetic and phenotypic overlap of specific obsessive-compulsive and attention-deficit/hyperactive subtypes with Tourette syndrome. Psychol Med 2018;48:279-93. [Crossref] [PubMed]
- Groth C, Debes NM, Rask CU, et al. Course of Tourette syndrome and comorbidities in a large prospective clinical study. J Am Acad Child Adolesc Psychiatry 2017;56:304-12. [Crossref] [PubMed]
- Zinner SH, Coffey BJ. Developmental and behavioral disorders grown up: Tourette’s disorder. J Dev Behav Pediatr 2009;30:560-73. [Crossref] [PubMed]
- Greydanus DE, Pratt HD, Patel DR. Attention deficit hyperactivity disorder across the lifespan. Dis Mon 2007;53:70-131. [Crossref] [PubMed]
- Shaw ZA, Coffey BJ. Tics and Tourette syndrome. Psychiatr Clin North Am 2014;37:269-86. [Crossref] [PubMed]
- Malaty IA, Akhar U. Updates in medical and surgical therapies for Tourette syndrome. Curr Neurol Neurosci Rep 2014;14:458. [Crossref] [PubMed]
- Budman CL. The role of atypical antipsychotics for treatment of Tourette’s syndrome: an overview. Drugs 2014;74:1177-93. [Crossref] [PubMed]
- Stern JS. Tourette’s syndrome and its borderland. Pract Neurol 2018;18:262-70. [Crossref] [PubMed]
- Roth J. The colorful spectrum of Tourette syndrome and its medical, surgical and behavioral therapies. Parkinsonism Relat Disord 2018;46 Suppl 1:S75-9. [Crossref] [PubMed]
- Wenzel C, Kleimann A, Bokemeyer S, et al. Aripiprazole for the treatment of Tourette syndrome: a case series of 100 patients. J Clin Psychopharmacol 2012;32:548-50. [Crossref] [PubMed]
- Janik P, Szeiko N. Aripiprazole in treatment of Gilles de la Tourette syndrome--New therapeutic option. Neurol Neurochir Pol 2018;52:84-7. [Crossref] [PubMed]
- Pringsheim T, Marras C. Pimozide for tics in Tourette’s syndrome. Cochrane Database Syst Rev 2009.CD006996. [PubMed]
- Singer HS, Wendlandt J, Krieger M, et al. Baclofen treatment in Tourette syndrome: a double-blind, placebo-controlled, crossover trial. Neurology 2001;56:599-604. [Crossref] [PubMed]
- Takács Á, Shilon Y, Janacsek K, et al. Procedural learning in Tourette syndrome, ADHD, and comorbid Tourette-ADHD: Evidence from a probabilistic sequence learning task. Brain Cogn 2017;117:33-40. [Crossref] [PubMed]
- Shephard E, Jackson GM, Groom MJ. Electrophysiological correlates of reinforcement in young people with Tourette syndrome with and without co-occurring ADHD symptoms. Int J Dev Neurosci 2016;51:17-27. [Crossref] [PubMed]
- Shephard E, Groom MJ, Jackson GM. Implicit sequence learning in young people with Tourette syndrome with and without co-occurring attention-deficit/hyperactivity disorder. J Neuropsychol 2019;13:529-49. [Crossref] [PubMed]
- Rizzo R, Gulisano M. Clinical pharmacology of comorbid attention deficit hyperactivity disorder in Tourette syndrome. Int Rev Neurobiol 2013;112:415-44. [Crossref] [PubMed]
- Tourette Syndrome Study Group. Treatment of ADHD children with tics: a randomized controlled trial. Neurology 2002;58:527-36. [Crossref] [PubMed]
- Erenberg G. The relationship between Tourette syndrome, attention deficit hyperactivity disorder, and stimulant medication: a critical review. Semin Pediatr Neurol 2005;12:217-21. [Crossref] [PubMed]
- Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev 2011.CD007990. [PubMed]
- Osland ST, Steeves TD, Pringsheim T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev 2018;6:CD007990. [PubMed]
- Roessner V, Schoenefeld K, Buse J, et al. Pharmacological treatment of tic disorders and Tourette syndrome. Neuropharmacology 2013;68:143-9. [Crossref] [PubMed]
- Cavanna AE, Nani A. Antiepileptic drugs and Tourette syndrome. Int Rev Neurobiol 2013;112:373-89. [Crossref] [PubMed]
- Quezada J, Coffman KA. Current approaches and new developments in the pharmacological management of Tourette syndrome. CNS Drugs 2018;32:33-45. [Crossref] [PubMed]
- Weng WC, Huang HL, Wong LC, et al. Increased risks of tic disorders in children with epilepsy: A nation-wide population-based case-control study in Taiwan. Res Dev Disabil 2016;51-52:173-80. [Crossref] [PubMed]
- Cianchetti C, Fratta A, Pisano T, et al. Pergolide improvement in neuroleptic-resistant Tourette cases: various mechanisms causing tics. Neurol Sci 2005;26:137-9. [Crossref] [PubMed]
- Schade R, Andersohn F, Suissa S, et al. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med 2007;356:29-38. [Crossref] [PubMed]
- Abd Ellah NH, Ahmed EA, Abd-Ellatief RB, et al. Metoclopramide nanoparticles modulate immune response in a diabetic rat model: association with regulatory T cells and proinflammatory cytokines. Int J Nanomedicine 2019;14:2383-95. [Crossref] [PubMed]
- Takano A, Suhara T, Yasuno F, et al. The antipsychotic sultopride is overdosed-a PET study of drug-induced receptor occupancy in comparison with sulpiride. Int J Neuropsychopharmacol 2006;9:539-45. [Crossref] [PubMed]
- Mogwitz S, Buse J, Ehrlich S, et al. Clinical pharmacology of dopamine-modulating agents in Tourette’s syndrome. Int Rev Neurobiol 2013;112:281-349. [Crossref] [PubMed]
- Mogwitz S, Buse J, Wolff N, et al. Update on the pharmacological treatment of tics with dopamine-modulating agents. ACS Chem Neurosci 2018;9:651-72. [Crossref] [PubMed]
- Niemann N, Jankovic J. Real-world experience with VMAT2 inhibitors. Clin Neuropharmacol 2019;42:37-41. [Crossref] [PubMed]
- Tarakad A, Jimenez-Shahed J. VMAT2 inhibiors in neuropsychiatric disorders. CNS Drugs 2018;32:1131-44. [Crossref] [PubMed]
- Müller-Vahl KR, Schneider U, Prevedel H, et al. Delta-9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry 2003;64:459-65. [Crossref] [PubMed]
- Müller-Vahl KR. Cannabinoids reduce symptoms of Tourette’s syndrome. Expert Opin Pharmacother 2003;4:1717-25. [Crossref] [PubMed]
- Gonçalves J, Rosado T, Soares S, et al. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines (Basel) 2019. [Crossref] [PubMed]
- Gilbert DL, Budman CL, Singer HS, et al. A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome. Clin Neuropharmacol 2014;37:26-30. [PubMed]
- Li JJ, Li ZW, Wang SZ, et al. Ningdong granule: a complementary and alternative therapy in the treatment of attention deficit/hyperactivity disorder. Psychopharmacology (Berl) 2011;216:501-9. [Crossref] [PubMed]
- Zheng Y, Zhang ZJ, Han XM, et al. A proprietary herbal medicine (5-Ling granule) for Tourette syndrome: a randomized controlled trial. J Child Psychol Psychiatry 2016;57:74-83. [Crossref] [PubMed]
- Pandey S, Srivanitchanpoom P, Kirubakaran R, et al. Botulinum toxin for motor and phonic tics in Tourette’s syndrome. Cochrane Database Syst Rev 2018;1:CD012285. [PubMed]
- Muth CC. Tics and Tourette syndrome. JAMA 2017;317:1592. [Crossref] [PubMed]
- Dale RC. Tics and Tourette: a clinical, pathophysiological and etiologic review. Curr Opin Pediatr 2017;29:665-73. [Crossref] [PubMed]
- Cernera S, Okun MS, Gunduz A. A review of cognitive outcomes across movement disorder patients undergoing deep brain stimulation. Front Neurol 2019;10:419. [Crossref] [PubMed]
- Brito M, Teixeira MJ, Mendes MM, et al. Exploring the clinical outcomes after deep brain stimulation in Tourette syndrome. J Neurol Sci 2019;402:48-51. [Crossref] [PubMed]
- Singer HS. Tics and Tourette syndrome. Continuum (Minneap Minn) 2019;25:936-58. [Crossref] [PubMed]
- Muller-Vahl KR. Deep brain stimulation in Tourette syndrome: the known and the unknown. J Neurol Neurosurg Psychiatry 2019;90:1076-7. [Crossref] [PubMed]


