
Multi-modal imaging of the pediatric heart transplant recipient
Background
Almost 6,000 orthotopic heart transplants (OHT) are performed each year, with approximately 700 performed in pediatric patients (1,2). Although there have been significant advances in the care of pediatric OHT recipients, acute rejection, cardiac allograft vasculopathy (CAV), and graft failure continue to be major causes of morbidity and mortality. Between 1 month and 5 years post-transplant, up to 15% of pediatric OHT mortality can be attributed to acute rejection (3). In contrast, CAV is a later finding, resulting in 20% and 24% of OHT mortality at 5–10 years and >10 years post-transplant, respectively (3). Graft failure (which can result from diverse processes) accounts for approximately 40% of pediatric OHT mortality at all timepoints (3,4). Given the potential for graft complications, OHT patients undergo routine screening, largely consisting of serial exams, laboratory assessment, cardiac imaging, and cardiac catheterization with endomyocardial biopsy (EMB) (5). This review will focus on the non-invasive imaging modalities commonly used during routine monitoring of OHT patients, as well as novel non-invasive imaging methods with the potential to modify current clinical practice (Table 1).
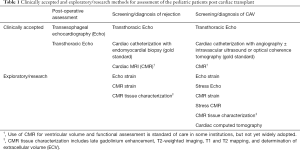
Full table
Pathophysiology of rejection and cardiac allograft vasculopathy
While a complete understanding of the pathophysiology of rejection and CAV is beyond the scope of this review, a limited understanding is necessary to adequately describe the strengths and weakness of various imaging methods. In general, there are two types of rejection, acute cellular rejection (ACR) and antibody mediated rejection (AMR). ACR is classically described as a T-cell mediated process that leads to leukocyte infiltration of the myocardium. This infiltration leads to significant inflammation and results in necrosis and apoptosis (6,7). These areas of inflammation can be either diffuse or patchy in nature (7). AMR, on the other hand, is mediated by B-cells and manifests as intravascular macrophages, interstitial edema, myocyte necrosis, and hemorrhage (8). Both types of rejection are potentially modificable processes that may remain silent until extensive myocardial destruction leads to hemodynamic compromise. In this manuscript, ACR and AMR will be used when studies specifically characterize the type of rejection; for studies without more specific characterizations, we will use the more general term, “rejection.”
Cardiac allograft vasculopathy (CAV) is a gradual process initiated by endothelial dysfunction that may lead to graft hypoperfusion. It results from diffuse intimal thickening of the coronary arteries due to smooth muscle proliferation, inflammation, and deposition of lipids (9). This progressive vasculopathy is thought to be due to a complex interaction between multiple factors, including ischemia/reperfusion, rejection and immune response, immunosuppression, infection, metabolic abnormalities, and genetic predisposition (10).
Current diagnosis of rejection and cardiac allograft vasculopathy
Cardiac catheterization with EMB is the current gold standard for the diagnosis of acute rejection. EMB allows for direct visualization of myocardial tissue for histologic evidence of either ACR or AMR, and also facilitates assessment of complement deposition in the setting of AMR. Cardiac catheterization also provides hemodynamic data (e.g., end diastolic pressures and pulmonary pressures) useful for clinical diagnosis. Unfortunately, EMB location is generally restricted to the RV surface of the interventricular septum, which limits diagnostic yield. Therefore, patchy ACR and/or ACR present in areas other than the septum may elude detection using EMB (7,11). Diagnosis of AMR using EMB can also be difficult, and though detection is improved using immunopathologic staining, these techniques do not provide 100% sensitivity (8,12). These limitations are compounded by significant inter-observer variability in the pathologic grading of rejection (13).
The gold standard of CAV diagnosis is also based on cardiac catheterization, either using standard angiography to detect luminal narrowing or intravascular ultrasound (IVUS).
Unfortunately, in addition to the limitations related to diagnostic yield, cardiac catheterization with EMB is invasive, involves radiation, may have associated complications from anesthesia or sedation, and may result in tricuspid regurgitation, bleeding, cardiac perforation, arrhythmia, and death (14-17). In addition, angiography can lead to renal dysfunction and IVUS has been associated with coronary artery dissection in as many as 1.6% of catheterizations (7,18,19).
Non-invasive imaging modalities
Given the potential risks associated with cardiac catheterization, as well as the unclear mortality benefit of more frequent surveillance EMB (20), non-invasive modalities are increasingly used for routine screening of pediatric patients after OHT. The primary modalities are echocardiography (Echo) and cardiac magnetic resonance imaging (CMR). Cardiac computed tomography (CT) has also been used in patients with OHT. Echo is a readily available imaging modality used for periodic outpatient screening of myocardial health for patients with OHT. CMR provides reproducible functional imaging and novel tissue characterization. CMR has been adopted by some centers as standard practice for healthy chronic OHT recipients.
Of note, multiple studies using various methodologies have demonstrated that pediatric and adult OHT patients have significant decrements in heart function soon after transplant that resolve, in the absence of rejection, over the first 3–6 months. Presumably, these decrements are due to inflammation and ischemia related to initial procurement of the donor heart, and the slow recovery represents either resolution of inflammation or remodeling. Therefore, we recommend caution in the implementation of non-invasive imaging for screening purposes during this recovery period.
Echocardiography
Echo provides the most readily accessible means of imaging the OHT recipient. It may be the first imaging modality to indicate a clinical issue. Echo can provide clues regarding myocardial health. New valvular regurgitation, pericardial effusion, or global reduction in function may suggest rejection. The initial comprehensive Echo post-transplant, either transesophageal or transthoracic, measures global myocardial function and also visualizes valves and suture lines. Potential areas of obstruction, to be interrogated well by Echo, depend upon the type of anastomosis, usually bicaval or biatrial. Post-operative Echo must ensure no pulmonary artery or aortic stenosis and must evaluate for systemic or pulmonary venous obstruction, especially after bicaval anastomosis.
In a study comparing adult OHT patients to healthy controls, OHT patients demonstrated larger atrial volumes post-transplant (21). They also tended to have thicker myocardial walls, a higher left ventricular (LV) mass, smaller LV end diastolic volumes, and slightly lower LV ejection fraction (LVEF) and fractional shortening (FS) compared to controls. The right side of the heart, on the other hand, tended to be larger, though most measures of right ventricular (RV) function were also lower compared with controls (21). We are unaware of a similar assessment of “normal” values in pediatric patients with OHT, but this work suggests that standard cut-offs may not be appropriate after OHT.
Functional assessment
Global myocardial function, assessed by methods outlined by the American Society of Echocardiography (22), include measures of LV systolic function: FS from a mid-short axis LV image, and fractional area change (FAC) and volumetric LVEF from apical four chamber plane. 2D short-axis imaging, rather than M-mode, is the recommended approach to obtain LV short-axis measurements (22). In contrast to adult studies, which suggest that biplane EF is preferable, pediatric studies demonstrate that 5/6 area length EF is more accurate and reproducible in multiple disease processes (23,24). While this has never been evaluated specifically in pediatric OHT, the general familiarity with this technique in pediatric labs suggests it may be the preferred method. All of these methods of assessing systolic function are affected by loading conditions, but FAC and LVEF are less sensitive to abnormal chamber geometry and regional abnormalities.
For the RV, Echo fares less well. Acoustic windows often limit full visualization of the RV, which lies immediately posterior to the sternum. In clinical practice, qualitative assessment of the RV size, mass, and function is the norm with relatively high inter-observer variability (25). While planimetry can be applied to the RV long-axis area and RV fractional area change has been used as an index of RV systolic function, Echo methods of RV assessment have significant limitations. Simsek evaluated multiple echocardiographic measures of RV function (RV FAC, tricuspid annular plane systolic excursion (TAPSE), RV myocardial performance index (MPI), and RV global longitudinal strain) and their correlation with CMR for RVEF (26). They found that only RV FAC correlated with CMR RVEF, and this correlation was only moderately strong with r=0.75. There are little or no data regarding utility, accuracy, and reproducibility of Echo measures of RV function in children (27). Thus, practitioners have turned to CMR for assessment of RV volumes and function.
Regional wall motion assessment by Echo may be considered as a way to understand myocardial health, which may be altered by acute rejection or hypoperfusion from coronary vasculopathy (28). However, qualitative visualization of wall motion is not robust; furthermore, pediatric providers have more limited experience with this assessment than adult cardiologists, who routinely view imaging studies for patients with coronary disease. Echo derived strain analyses have also been proposed as a means of quantifying the regional myocardial contractility.
Three-dimensional (3D) echocardiographic imaging may also play a role in OHT. 3D TEE guidance of EMB in pediatric OHT patients demonstrated no complications and minimized required fluoroscopy time (29). Parthiban evaluated pediatric OHT patients using 3D echocardiography to calculate 3D LVEF as well as the systolic dyssynchrony index, a measure of dyssynchrony based on segmental differences in timing to reach minimal systolic volume (30). They compared 3D LVEF to 2D measures and demonstrated significant divergence between the two values in OHT, and this divergence increased in those with higher levels of dyssynchrony. Their data suggests that 3D LVEF may be more accurate in pediatric OHT, particularly in those patients with significant dyssynchrony.
Doppler assessment
The velocity of atrioventricular valve (AVV) inflow Doppler signals [peak E wave, peak A wave, A wave duration, deceleration times, isovolumic relaxation time (IVRT)] may be used to characterize diastolic function, but can be ill-affected by rapid heart rates in children. Tissue Doppler assessment of AVV annular motion, using low Nyquist levels, has been suggested in recent years as a means of assessing diastolic function with normative data published for children. For instance, the ratio of the atrioventricular valve early inflow (E wave height) to the lateral annulus e’, can be applied to RV and LV. Tissue Doppler velocities are decreased in transplant patients without a history of rejection while isovolumic acceleration was no different from controls (31). During episodes of rejection, tissue Doppler indices demonstrate a further decrease and may be useful as a screening method for rejection (32-34). However, tissue Doppler is dependent on adequate image quality, is load dependent, and good Doppler alignment is critical (22).
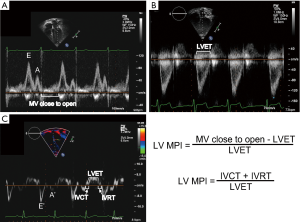
MPI (also known as the Tei index) is a measure of systolic and diastolic function and may be tracked for OHT recipients (35) (Figure 1). Abnormalities in myocardial relaxation, from either systolic or diastolic function, will prolong the MPI. MPI may also be applied to the RV. Investigators have shown that RV MPI normalized during the first year following transplant (36). A few publications discuss the use of serial MPI measures as a way to detect rejection (34,37,38). Flanagan, studying a cohort of 40 pediatric OHT recipients with acute cellular rejection, showed that LV MPI was higher at the time of biopsy-proven ACR (0.59±0.17) than on the preceding baseline echocardiogram performed in the absence of ACR (0.41±0.11; P<0.0001). In addition, LV MPI was higher for patients with ACR as compared to non-ACR matched controls (0.39±0.11; P<0.0001) (39). MPI has been proposed as a sensitive way to detect rejection but, because aging of the transplanted heart may affect the MPI, assessment of serial trends rather than an absolute cut-off may be necessary.
Echo scoring methods
Given the poor sensitivity and invasiveness of cardiac catheterization, many authors have explored Echo scoring methods for either detecting, or ruling out, acute rejection. Of note, when used for screening purposes, a high negative predictive value may be more important than a high sensitivity or positive predictive value, as patients with clinical suspicion can undergo further testing while missing a patient with acute rejection could have significant consequences. Multiple non-invasive scoring methods have been evaluated, with varying sensitivity, specificity, and predictive values (40-47). Dodd and colleagues demonstrated that a scoring system using multiple M-mode parameters had a negative predictive value of over 97% in adult patients with OHT, and a similar scoring method created by the same group had a high sensitivity and specificity for detection of significant rejection in pediatric OHT (43,44). In infants, where EMB can be particularly high risk, Echo screening has high sensitivity and specificity for detection of acute rejection (46). Hernandez created the global left ventricular relaxation index, which included E’ of the medial, lateral, and posterior walls as well as LV posterior wall thinning. When using a cut-off of 0.8, this model demonstrated an area under the ROC curve of 0.99 for diagnosis of rejection and a negative predictive value of 100% (41). Of note, the majority of these studies were performed in small cohorts at single centers, making generalizability unclear.
Stress echo
Dobutamine stress echocardiography (DSE) has been employed for risk stratification in adult OHT recipients (48). Using a gradually increasing concentration of dobutamine (5 to a maximal dose of 40 micrograms/kg body weight per min at 3-min intervals) and a standardized scale [graded from 1 (normal) to 4 (dyskinesia)], the qualitative assessment of regional wall motion abnormalities can be mapped to the 17-segment AHA model. Thus, the corresponding affected coronary can be discerned. DSE correctly identified the corresponding hypoperfused segments in patients with coronary vasculopathy with a sensitivity and specificity of 86% and 91%, respectively (48). Dipchand demonstrated relatively high negative predictive value for DSE with angiography as the gold standard (49). Angiography was also able to detect lower grades of CAV. A recent study by Pahl (50) employed DSE for a small pediatric OHT population (n=46). Echocardiographic wall motion abnormalities during stress correlated with coronary arteriopathy. Larger multi-center trials are needed to show the usefulness of this test for pediatric OHT recipients during childhood. Furthermore, pediatric cardiologists require training and experience to adequately perform DSE.
Myocardial strain
The ability of myocardial strain to detect subclinical myocardial abnormalities, including subtle wall motion abnormalities, has led many to explore strain as an early predictor of rejection. Of note, strain is mathematically increased (less negative) in the setting of worsening function. Echo strain may be limited by image quality, especially for the RV when acoustic windows are limited. However, a study by Wisotzkey demonstrated that strain analysis of the LV in pediatric OHT is feasible in the majority of patients (51). Understanding strain changes over time in “healthy” OHT patients without rejection is an important step before applying these methods for screening purposes. Analysis of pediatric OHT patients without rejection demonstrates a worsening of global longitudinal strain (GLS) compared to controls with a concomitant improvement in global circumferential strain (GCS) early post-transplant (52,53). In some studies, the LV GLS values remained decreased compared with controls at 1 year (54,55), while other pediatric and adult studies have suggested a “normalization” of both GLS and GCS by 1 year of age with stable values up to 5 years post-transplant (52,53). Many authors surmise that the decreased GLS, and preserved or compensatory improvement in GCS, is due to subendocardial ischemia at the time of transplant and the fact that the subendocardium is made up of predominately longitudinal muscle fibers. RV global and free wall strain are also decreased soon after OHT, but while values improve through the first year, they do not “normalize” when compared with controls (36). Right atrial strain is also decreased in OHT compared with controls (56). Of note, patients with biatrial anastomoses had worse RA strain compared with patients who underwent bicaval anastomoses, suggesting caution for the use of RA strain in a cohort that includes both surgical approaches.
Multiple studies have assessed the utility of strain for diagnosing rejection. In adult OHT, LV and RV strain and strain rate predict presence of rejection and have a high negative predictive value, though the cut-offs vary significantly between studies (57-59). Podrouzkova evaluated change in strain from baseline and demonstrated that OHT patients with rejection had a significant worsening in 4 chamber longitudinal strain, 2 chamber longitudinal strain, and 2 chamber longitudinal strain rate; other Echo parameters were unchanged and patients without rejection had no change in strain values from baseline (60). Of note, boxplots demonstrated a significant variability in strain in these patients, suggesting that change from baseline may be a better method for diagnosing rejection. In one study of pediatric OHT early post-transplant, strain appears suboptimal for distinguishing between patients with and without rejection, with only the mid-septum reaching statistical significance (61). However, patients were only 3 months post OHT, suggesting that timing may have affected the analysis. In contrast, Sehgal demonstrated a worsening of GLS, GCS, and global radial strain in patients with rejection, as well as an increased LV mass during rejection (62). They found no differences in fractional shortening or measures of diastolic function. Godown demonstrated significant decrements in GLS, GCS, and systolic and diastolic longitudinal and circumferential strain rate at the time of rejection (63). A longitudinal analysis of all Echos preceding a rejection episode was performed, with the first changes noted 45 days prior to rejection; however, changes in deformation occurred concurrently with changes in LVEF, suggesting that deformation may not improve the early detection of rejection compared with standard measures (63).
Strain has also been used to predict CAV. In adult patients, GLS is worse in patients with CAV compared to those without CAV and controls (64). GLS also appears to worsen depending on severity of CAV. However, the specificity and negative predictive value for GLS predicting CAV were suboptimal. Multiple studies have evaluated the relationship between strain and CAV in pediatric OHT (65). Zoeller evaluated strain at the time of CAV diagnosis as well as 2 years prior and demonstrated that GLS, longitudinal systolic strain rate, and longitudinal diastolic strain rate were decreased both at time of diagnosis and 2 years prior to diagnosis (66). Modeling suggested that GLS on Echo performed 2 years prior to diagnosis could predict development of CAV, though the sensitivity was poor and the negative predictive value was not reported. The same group demonstrated that decrements in GLS as soon as 1-year post transplant could identify patients at risk of developing CAV (67).
Strain has also been used to predict adverse events and mortality. Clemmensen demonstrated that LV GLS predicted major adverse cardiac events (including rejection, admission for heart failure, coronary event) and mortality in adult OHT (68). The combination of RV free wall strain and RV FAC predict mortality, CAV, and rejection in adult OHT patients (69). In pediatric patients, LV GLS predicts late graft failure (70).
Although many studies have demonstrated the utility of strain for assessment of rejection or CAV, it must be noted that the cut-offs are different for each study. This may be partially related to methodologic differences (different analysis packages result in different strain values) but also suggests caution in interpreting retrospective analyses performed in single institutions on small cohorts. To some extent, evaluating a change in strain from baseline obviates these issues. While standardization has been attempted in strain analysis, knowledge of the differences between software packages is critical if attempting to screen OHT patients using strain. Moreover, most of these studies compared patients with rejection to those without rejection or patients with CAV to those without CAV. As there are multiple causes of decreased function in OHT patients (rejection, CAV, hypertension, infection, etc.), the specificity of decreased strain is unclear. This suggests that the best use of strain may be for screening purposes to determine a need for further testing (i.e., EMB).
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging (CMR) may be used to assess pediatric patients with OHT. Volumetric assessment, from planimetry of a full short axis stack of images, allows quantification of left and right ventricular volumes, mass, and systolic function with good reproducibility (71,72). Atrioventricular valve inflow pattern, as with Echo, may give a suggestion of ventricular diastolic function. In a study comparing pediatric OHT patients to controls, Latus demonstrated comparable LVEF between groups, but OHT patients had significantly decreased end diastolic and systolic volumes and significantly increased mass to volume ratios (for both the RV and LV) (73). These data suggest that standard normal CMR values cannot be adapted to pediatric OHT patients.
Tissue characterization
Tissue characterization is one of the advantages of CMR as compared with echo. T2-weighted imaging has been used in multiple disease processes, including myocarditis, to assess for myocardial edema (74). After the administration of gadolinium contrast, both early enhancement and late gadolinium enhancement (LGE) can be evaluated. Early enhancement evaluates for hyperemia. LGE represents extracellular matrix expansion due to fibrosis, necrosis, or edema/inflammation.
As with other cardiovascular diseases, multiple studies in adult OHT patients demonstrate that presence and severity of LGE predicts long-term outcomes such as mortality and major adverse cardiac events (MACE), even when corrected for other cardiovascular risk factors (75-77). Pedrotti noted that 22 of 26 patients with LGE had an infarct atypical pattern (77). However, the prognostic value of LGE has not been confirmed in pediatric OHT patients.
Tissue characterization can also be used to assess for rejection or CAV. Krieghoff evaluated a combination of T2-weighted imaging, early enhancement, and LGE, the same methods used to diagnose myocarditis, and demonstrated that a combination of T2-weighted imaging and early enhancement had a relatively high negative predictive value but low positive predictive value for rejection (78). LGE did not improve discrimination. Taylor demonstrated similar findings (79), and multiple other adult studies have demonstrated that LGE is a poor predictor of acute rejection (80,81). In the only pediatric study of which we are aware, LGE was not useful for determining presence or absence of rejection, nor were any of the other CMR parameters, though this study was limited by a small number of patients with rejection (82).
LGE has also been used to assess for CAV, though with limited success. Adult patients with higher grades of CAV are more likely to have infarct-typical patterns of LGE, but this was not the case in all patients with high grade CAV and multiple patients without CAV also had this pattern (83). Interestingly, assessment of coronary LGE may hold promise for detection of CAV in pediatric patients (84,85). Hussain calculated the mean enhancement area, diameter, and index (enhancement area/coronary cross-sectional area) in the left anterior descending artery (85). These measurements were compared to IVUS measures and demonstrated a strong correlation between enhancement diameter and maximal intimal thickness (r=0.8) and enhancement index and mean intimal index (r=0.92). While both of these cohorts were small, these methods hold promise for non-invasive assessment of CAV.
CMR parametric mapping techniques provide a novel method of tissue characterization. Parametric mapping of the longitudinal relaxation constant, or T1 time, can be performed prior to gadolinium contrast (native T1) or after contrast (post contrast) (Figure 2). Each tissue has its own native T1, with different T1 values obtained at different magnetic field strengths and from different sequences [e.g., single-shot acquisition (SASHA), modified Look-Locker inversion recovery (MOLLI)] complicating generalizability. Given this variability, local normative values are required (87). Post contrast T1 values are affected by many variables including contrast agent, contrast dose, time elapsed from contrast to T1 scan, renal clearance, vendor’s T1 sequence, and magnetic field strength. This variability limits the clinical utility of post-contrast T1.
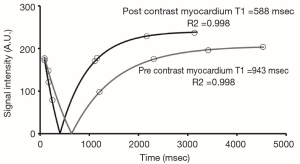
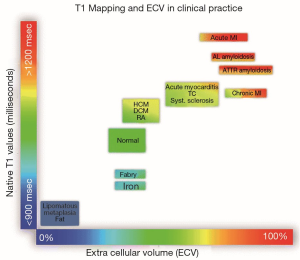
From native and post contrast T1 data from planes that can be co-registered, using either the patient’s hematocrit or a synthetic hematocrit, extracellular volume (ECV) can be calculated (Figure 3). As ECV is a ratio of native and post contrast T1 of blood and myocardium, it is independent of the many variables that affect post contrast T1, and thus can be used across institutions for multi-center study of myocardial disease. ECV is an approximation of extracellular matrix (ECM) expansion. The ECM may be expanded by infiltrative processes (e.g., amyloid, sarcoid) and by microfibrosis associated with ischemia and infarct. Increases in myocardial free water content, as occurs in acute myocardial edema and inflammation, also prolong T1 and T2 relaxation times (87,89,90). Thus, T1, T2, and ECV mapping may be useful for characterizing the transplanted myocardium, which may be subject to inflammation from rejection and from fibrosis from developing coronary vasculopathy (Figure 4).
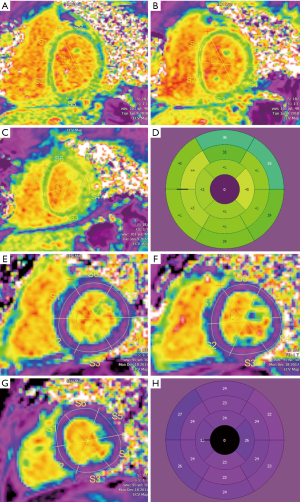
In a CMR study of adult OHT recipients (1.5 T), T1 values were significantly higher in the cohort with rejection (grade 2R, 3R, clinically diagnosed rejection, antibody-mediated rejection) (1,066±78 ms) versus healthy controls (984±42 ms; P=0.0001) and versus healthy transplant recipients without rejection (1,001±54 ms; P=0.001). Using a T1 cutoff value of 1,029 ms, the sensitivity, specificity, and negative predictive value were 93%, 79%, and 99%, respectively (91). Focusing on T2 mapping, Vermes performed CMR scans (1.5 T) and EMBs for 7 adults with OHT having acute rejection and 13 without rejection. T2 signal was higher in those with rejection. The sensitivity, specificity and diagnostic accuracy for basal T2 (cut off: 57.7 ms) were 71%, 96% and 90% respectively. Compared with normal values for ECV (25.3%±3.5%) (88) in this small cohort, a basal ECV cut off 32% had sensitivity, specificity, and diagnostic accuracy for rejection of 86%, 85% and 85% respectively (92).
Several small studies have shown the feasibility of CMR for pediatric OHT evaluation. An early pilot study from Toronto noted that CMR was not able to reliably detect rejection, as no significant differences in functional data (volumetric data or ejection fraction), native T1 times, and ECV fraction were seen between patients with <2R and ≥2R ISHLT rejection. Greenway acknowledge that only 5 of 30 subjects had >2R rejection and the novel T2 mapping technique to assess for edema was not available. Given the potential of novel parametric imaging techniques, the pathobiology of rejection, and the downside of endomyocardial biopsy, the authors recommend continued evaluation of OHT patients with CMR to better understand the role that this modality has for myocardial characterization in this vulnerable population (82).
A prospective CMR study (1.5T) with histologic analyses of endomyocardial biopsy specimens from twenty-five OHT recipients (7.0±6.3 years at transplant and 10.7±6.5 years post-transplant), performed by Feingold, demonstrated that for this healthy OHT population with normal ejection fraction and no symptoms, ECV was similar to healthy controls (25.1%±3.0% vs. 23.7%±2.0%, P=0.09). ECV, though, was moderately correlated with cardiovascular fibrosis (CVF) on biopsy (r=0.47; P=0.019) (93). Another small prospective study, by Ide, of 20 pediatric OHT patients (9.9±6.2 years of age; 9 girls) enrolled a median of 1.3 years (0.02–12.6 years) post OHT showed higher septal native T1 times and ECV in OHT patients compared to healthy controls (1,008±32 vs. 979±24 ms, P<0.005 and 0.30±0.03 vs. 0.22±0.03, P<0.0001, respectively). CVF showed a moderate correlation with native T1 (r=0.53, P<0.05), as well as ECV (r=0.46, P<0.05). Native T1 time, but not ECV and CVF, correlated with ischemia time (r=0.46, P<0.05) (94).
Stress CMR
While coronary imaging by CMR is possible using a respiratory navigated, 3-dimensional sequence, the resolution of CMR is not sufficient to discern the presence of coronary narrowing. Thus, indirect evidence of vasculopathy is used as a surrogate for the presence of coronary narrowing. Traditionally, resting first pass perfusion imaging can be undertaken using 3 short axis LV planes imaged while the patient receives a bolus dose of gadolinium. Normal myocardial perfusion exists if there is rapid and even uptake of the contrast agent. Myocardial regions downstream from a significantly narrowed coronary will appear dark (95).
Pharmacologic stress imaging using vasodilator agents (e.g., adenosine, dipyridamole, and regadenoson) induces coronary vasodilation with simultaneous infusion of gadolinium contrast, leading to a greater increase in the perfusion of myocardium supplied by normal coronary arteries compared with myocardium supplied by stenotic coronary arteries (72). Adenosine perfusion CMR has better diagnostic performance in adults than perfusion SPECT with superior sensitivity (86.5% vs. 66.5%) and negative predictive value (90.5% vs. 79.1%) (96,97).
Limited experience exists for the use of adenosine perfusion CMR for cardiac transplant recipients, with this group comprising only 12.5% of patients in a multi-center pediatric study of adenosine stress perfusion imaging (98). For the transplant recipient, pharmacologic stress with adenosine has some unique considerations—adenosine poses the risk of prolonged asystole and atrioventricular block in a denervated heart (99). Multiple studies have demonstrated safety of adenosine in adult patients after OHT; while not all studies reported side-effects, those that did reported shortness of breath and chest pain but very few patients requiring discontinuation of the testing (100-104). A recent study by Flyer evaluated the safety of adenosine for treatment of supraventricular tachycardia in pediatric OHT patients (105). In their study, no patients required rescue pacing, and though the majority of patients reported symptoms (shortness of breath, discomfort, and chest pain were most common), none required discontinuation of testing. Of note, the primary outcome measure was induction of AV block, so dosages were likely higher (but also of much shorter duration) than that required for perfusion imaging. In addition, the two patients with CAV were excluded (105). While regadenoson, a more selective activator of the A2A receptor, may have fewer side effects than adenosine, limited experience exists in children or OHT recipients (106-109). Anecdotal experience suggests that pediatric OHT patients tolerate regadenoson without difficulties, but publications evaluating the safety and efficacy of regadenoson perfusion imaging for pediatric OHT recipients will be needed before this becomes standard of care.
Multiple studies in adults have demonstrated that use of indexed myocardial perfusion reserve (MPRi), either alone or in combination with strain, can detect CAV and predict adverse events (100,102,104,109). Primarily a research tool, MPRi can be determined from a region of interest by looking at the upslope of the curve for gadolinium (Gd) uptake (signal intensity, SI) at peak hyperemia, compared to the Gd myocardial signal curve at rest; MPRi can be calculated as MPRi = upslope of SI during hyperemia/upslope of SI at rest (110). Myocardial blush, calculated as the ratio of the plateau of mean grey level pixel intensity divided by the time to maximal intensity, may also provide prognostic information for adults after OHT (101,103). The utility of quantitative perfusion assessment in pediatric OHT is unclear.
Dobutamine stress perfusion CMR imaging is advocated by some centers as a means of understanding the increased myocardial demands posed by exercise. Regional wall motion assessment, using the AHA 17 segment model, can be visualized with short axis cine imaging performed prior to each incremental increase in dose. The addition of first-pass myocardial perfusion imaging to wall motion assessments during peak-dose dobutamine stress CMR, first reported by Lubbers (111) and Gebker (112), has improved the sensitivity for the diagnosis of coronary artery disease. Myocardial perfusion at peak stress can be visualized qualitatively or quantitatively. Limited use of dobutamine stress CMR for allograft evaluation appears in the literature with a small pilot study of adult OHT recipients showing MPRi derived from a dobutamine stress CMR as a reliable technique for noninvasive detection of microcirculatory coronary disease associated with CAV (113). Once again, more studies in pediatric patients are necessary.
Myocardial strain
Strain analysis by CMR has also been used in patients after OHT. Of note, the temporal resolution of Echo strain analysis may be higher than that achieved by CMR, which could affect results (114). Initial pediatric OHT CMR studies using grid tags demonstrated abnormalities in twist in OHT patients as compared to controls (115). A more recent analysis by Latus using feature tracking demonstrated findings similar to Echo, with decrements in GLS and maintained GCS and global radial strain (73). Miller evaluated adult OHT patients early post-transplant (within 6 months) and found a significant difference between circumferential strain in patients with and without ACR but also noted significant overlap between the two patient populations (116). The poor discrimination may have been partly related to the early time period after transplant. Strain encoding has been evaluated by multiple authors as a method of assessing CAV. In adult patients, Korosoglou demonstrated reduced systolic strain and strain rate in patients with severe CAV (>50% stenosis) while mean diastolic strain rate was progressively reduced in patients with no CAV, mild CAV (<50% stenosis), and severe CAV as compared with controls (100). They found that diastolic strain rate had the best area under the curve for detection of severe CAV of the three (0.93), though this was lower than that of MPRi (0.95) and neither performed as well for detection of mild CAV. Dedieu evaluated strain calculated using feature tracking in pediatric OHT. They found that CMR was better able to detect wall motion abnormalities than Echo. In addition, GLS was able to quantitatively detect additional patients with wall motion abnormalities. Patients with quantitative wall motion abnormalities had marginally higher grade stenosis (23% vs. 17%) and worse outcomes (28).
Limitations of CMR in OHT
There are some important limitations of CMR in the transplant population. Patients with retained pacemaker leads and non-MRI safe pacemakers require special consideration prior to scanning and must have a stable underlying rhythm (117). Currently, most ICDs are a contraindication for CMR. In addition, it is not uncommon for OHT patients to have renal dysfunction, which would preclude the administration of gadolinium contrast agents. Verifying acceptable glomerular filtration rate (GFR) prior to gadolinium contrast administration if there is a question about renal function is standard practice. Finally, current CMR sequences require patients to remain still and hold their breath during acquisitions, meaning that younger children and those with developmental delay may require sedation or anesthesia in order to obtain adequate imaging. While free-breathing imaging with multiple averages can be undertaken, imaging may not be optimal. Given the small numbers of pediatric OHT patients at any one center and the rarity of rejection (grade 2R or more), multi-center collaborative studies will be necessary to determine the ultimate value of CMR for transplanted myocardium. Limitations posed by poor echocardiographic acoustic windows and by the invasive nature of cardiac catheterization with EMB continue to provide the impetus to explore novel uses for CMR for the OHT population.
Cardiac CT
Until recently, cardiac CT has been used less frequently in pediatric patients due to the risks of radiation exposure. However, the wider availability of dual source scanners has allowed for a significant decrease in radiation dose (118). While studies of cardiac CT in OHT are limited, data suggest that quantification of volumes and function are comparable to CMR and better than Echo, though use of this modality for volumetric assessment is likely only pertinent to patients with contraindications to CMR (119). The superior resolution of CT angiography for coronary artery imaging may allow for more detailed assessment of CAV. Calcium scoring has shown promise for detecting (or ruling out) significant CAV, with relatively high negative predictive values for moderate CAV (120,121). However, these articles compared CT imaging to angiography, not IVUS; studies comparing CT to IVUS have demonstrated poorer results for detecting more subtle CAV (122). Perfusion CT has also been performed and correlates well with perfusion CMR (123). The utility of cardiac CT in children, who often have faster heart rates and smaller coronary arteries, requires further study.
Conclusions
The potential advantages of non-invasive imaging for assessment of pediatric patients after OHT are indisputable. Unfortunately, the vast majority of studies have only assessed these methods in adult OHT populations or small pediatric cohorts. In order to realize their potential, these methods must be evaluated in larger, multi-center pediatric studies.
Acknowledgments
Funding: JH Soslow is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number 1R56HL141248 (Bethesda, MD) and the Enduring Hearts Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1155-68. [Crossref] [PubMed]
- Rossano JW, Cherikh WS, Chambers DC, et al. The Registry of the International Society for Heart and Lung Transplantation: Twentieth Pediatric Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1060-9. [Crossref] [PubMed]
- Rossano JW, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-first pediatric heart transplantation report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1184-95. [Crossref] [PubMed]
- Marchant DJ, Boyd JH, Lin DC, et al. Inflammation in myocardial diseases. Circ Res 2012;110:126-44. [Crossref] [PubMed]
- Estep JD, Shah DJ, Nagueh SF, et al. The role of multimodality cardiac imaging in the transplanted heart. JACC Cardiovasc Imaging 2009;2:1126-40. [Crossref] [PubMed]
- Khan UA, Williams SG, Fildes JE, et al. The pathophysiology of chronic graft failure in the cardiac transplant patient. Am J Transplant 2009;9:2211-6. [Crossref] [PubMed]
- Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon 2011;9:160-7. [Crossref] [PubMed]
- Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2013;32:1147-62. [Crossref] [PubMed]
- Chih S, Chong AY, Mielniczuk LM, et al. Allograft Vasculopathy: The Achilles' Heel of Heart Transplantation. J Am Coll Cardiol 2016;68:80-91. [Crossref] [PubMed]
- Schumacher KR, Gajarski RJ, Urschel S. Pediatric coronary allograft vasculopathy--a review of pathogenesis and risk factors. Congenit Heart Dis 2012;7:312-23. [Crossref] [PubMed]
- Bhalodolia R, Cortese C, Graham M, et al. Fulminant acute cellular rejection with negative findings on endomyocardial biopsy. J Heart Lung Transplant 2006;25:989-92. [Crossref] [PubMed]
- Veiga Barreiro A, Crespo Leiro M, Domenech Garcia N, et al. Severe cardiac allograft dysfunction without endomyocardial biopsy signs of cellular rejection: incidence and management. Transplant Proc 2004;36:778-9. [Crossref] [PubMed]
- Angelini A, Andersen CB, Bartoloni G, et al. A web-based pilot study of inter-pathologist reproducibility using the ISHLT 2004 working formulation for biopsy diagnosis of cardiac allograft rejection: the European experience. J Heart Lung Transplant 2011;30:1214-20. [Crossref] [PubMed]
- Wong RC, Abrahams Z, Hanna M, et al. Tricuspid regurgitation after cardiac transplantation: an old problem revisited. J Heart Lung Transplant 2008;27:247-52. [Crossref] [PubMed]
- Crumbley AJ 3rd, Van Bakel AB. Tricuspid valve repair for biopsy-induced regurgitation after cardiac transplantation. Ann Thorac Surg 1994;58:1156-60. [Crossref] [PubMed]
- Nguyen V, Cantarovich M, Cecere R, et al. Tricuspid regurgitation after cardiac transplantation: how many biopsies are too many? J Heart Lung Transplant 2005;24:S227-31. [Crossref] [PubMed]
- Zhorne D, Petit CJ, Ing FF, et al. A 25-year experience of endomyocardial biopsy safety in infants. Catheter Cardiovasc Interv 2013;82:797-801. [Crossref] [PubMed]
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35. [Crossref] [PubMed]
- Pollack A, Nazif T, Mancini D, et al. Detection and imaging of cardiac allograft vasculopathy. JACC Cardiovasc Imaging 2013;6:613-23. [Crossref] [PubMed]
- Zinn MD, Wallendorf MJ, Simpson KE, et al. Impact of routine surveillance biopsy intensity on the diagnosis of moderate to severe cellular rejection and survival after pediatric heart transplantation. Pediatr Transplant 2018;22:e13131. [Crossref] [PubMed]
- Ingvarsson A, Werther Evaldsson A, Waktare J, et al. Normal Reference Ranges for Transthoracic Echocardiography Following Heart Transplantation. J Am Soc Echocardiogr 2018;31:349-60. [Crossref] [PubMed]
- Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465-95. [Crossref] [PubMed]
- Margossian R, Chen S, Sleeper LA, et al. The reproducibility and absolute values of echocardiographic measurements of left ventricular size and function in children are algorithm dependent. J Am Soc Echocardiogr 2015;28:549-58.e1. [Crossref] [PubMed]
- Soslow JH, Xu M, Slaughter JC, et al. Evaluation of Echocardiographic Measures of Left Ventricular Function in Patients with Duchenne Muscular Dystrophy: Assessment of Reproducibility and Comparison to Cardiac Magnetic Resonance Imaging. J Am Soc Echocardiogr 2016;29:983-91. [Crossref] [PubMed]
- Samyn MM, Saudek DE, Dasgupta M, et al. Are echocardiographic and cardiac magnetic resonance assessments reliable and reproducible in patients with a systemic right ventricle? Abstract presented at the American Society of Echocardiography Scientific Sessions 2009.
- Simsek E, Nalbantgil S, Ceylan N, et al. Assessment of right ventricular systolic function in heart transplant patients: Correlation between echocardiography and cardiac magnetic resonance imaging. Investigation of the accuracy and reliability of echocardiography. Echocardiography 2017;34:1432-8. [Crossref] [PubMed]
- Jiang L, Wiegers S, Weyman AE. Right Ventricle. In: Weyman AE, editor. Principles and practice of echocardiography. 2nd ed. Philadelphia: Lea & Febiger, 1994:901-21.
- Dedieu N, Silva Vieira M, Fenton M, et al. The importance of qualitative and quantitative regional wall motion abnormality assessment at rest in pediatric coronary allograft vasculopathy. Pediatr Transplant 2018;22:e13208. [Crossref] [PubMed]
- Scheurer M, Bandisode V, Ruff P, et al. Early experience with real-time three-dimensional echocardiographic guidance of right ventricular biopsy in children. Echocardiography 2006;23:45-9. [Crossref] [PubMed]
- Parthiban A, Li L, Kindel SJ, et al. Mechanical Dyssynchrony and Abnormal Regional Strain Promote Erroneous Measurement of Systolic Function in Pediatric Heart Transplantation. J Am Soc Echocardiogr 2015;28:1161-70, e2.
- Pauliks LB, Pietra BA, Kirby S, et al. Altered ventricular mechanics in cardiac allografts: a tissue Doppler study in 30 children without prior rejection events. J Heart Lung Transplant 2005;24:1804-13. [Crossref] [PubMed]
- Pauliks LB, Pietra BA, DeGroff CG, et al. Non-invasive detection of acute allograft rejection in children by tissue Doppler imaging: myocardial velocities and myocardial acceleration during isovolumic contraction. J Heart Lung Transplant 2005;24:S239-48. [Crossref] [PubMed]
- Behera SK, Trang J, Feeley BT, et al. The use of Doppler tissue imaging to predict cellular and antibody-mediated rejection in pediatric heart transplant recipients. Pediatr Transplant 2008;12:207-14. [Crossref] [PubMed]
- Aggarwal S, Blake J, Sehgal S. Right Ventricular Dysfunction as an Echocardiographic Measure of Acute Rejection Following Heart Transplantation in Children. Pediatr Cardiol 2017;38:442-7. [Crossref] [PubMed]
- Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol 1995;26:357-66. [PubMed]
- Harrington JK, Richmond ME, Woldu KL, et al. Serial Changes in Right Ventricular Systolic Function Among Rejection-Free Children and Young Adults After Heart Transplantation. J Am Soc Echocardiogr 2019;32:1027-1035.e2. [Crossref] [PubMed]
- Leonard GT Jr, Fricker FJ, Pruett D, et al. Increased myocardial performance index correlates with biopsy-proven rejection in pediatric heart transplant recipients. J Heart Lung Transplant 2006;25:61-6. [Crossref] [PubMed]
- Mooradian SJ, Goldberg CS, Crowley DC, et al. Evaluation of a noninvasive index of global ventricular function to predict rejection after pediatric cardiac transplantation. Am J Cardiol 2000;86:358-60. [Crossref] [PubMed]
- Flanagan R, Cain N, Tatum GH, et al. Left ventricular myocardial performance index change for detection of acute cellular rejection in pediatric heart transplantation. Pediatr Transplant 2013;17:782-6. [Crossref] [PubMed]
- Moran AM, Lipshultz SE, Rifai N, et al. Non-invasive assessment of rejection in pediatric transplant patients: serologic and echocardiographic prediction of biopsy-proven myocardial rejection. J Heart Lung Transplant 2000;19:756-64. [Crossref] [PubMed]
- Hernandez LE, Shepard CW, Menk J, et al. Global left ventricular relaxation: A novel tissue Doppler index of acute rejection in pediatric heart transplantation. J Heart Lung Transplant 2015;34:1190-7. [Crossref] [PubMed]
- Hernandez LE, Chrisant MK, Valdes-Cruz LM. Global Left Ventricular Relaxation: A Useful Echocardiographic Marker of Heart Transplant Rejection and Recovery in Children. J Am Soc Echocardiogr 2019;32:529-36. [Crossref] [PubMed]
- Dodd DA, Brady LD, Carden KA, et al. Pattern of echocardiographic abnormalities with acute cardiac allograft rejection in adults: correlation with endomyocardial biopsy. J Heart Lung Transplant 1993;12:1009-17. [PubMed]
- Tantengco MV, Dodd D, Frist WH, et al. Echocardiographic abnormalities with acute cardiac allograft rejection in children: correlation with endomyocardial biopsy. J Heart Lung Transplant 1993;12:S203-10. [PubMed]
- Putzer GJ, Cooper D, Keehn C, et al. An improved echocardiographic rejection-surveillance strategy following pediatric heart transplantation. J Heart Lung Transplant 2000;19:1166-74. [Crossref] [PubMed]
- Boucek MM, Mathis CM, Boucek RJ Jr, et al. Prospective evaluation of echocardiography for primary rejection surveillance after infant heart transplantation: comparison with endomyocardial biopsy. J Heart Lung Transplant 1994;13:66-73. [PubMed]
- Frommelt MA, Snider AR, Crowley DC, et al. Echocardiographic indexes of allograft rejection in pediatric cardiac transplant recipients. J Am Soc Echocardiogr 1992;5:41-7. [Crossref] [PubMed]
- Derumeaux G, Redonnet M, Mouton-Schleifer D, et al. Dobutamine stress echocardiography in orthotopic heart transplant recipients. VACOMED Research Group. J Am Coll Cardiol 1995;25:1665-72. [Crossref] [PubMed]
- Dipchand AI, Bharat W, Manlhiot C, et al. A prospective study of dobutamine stress echocardiography for the assessment of cardiac allograft vasculopathy in pediatric heart transplant recipients. Pediatr Transplant 2008;12:570-6. [Crossref] [PubMed]
- Pahl E, Crawford SE, Swenson JM, et al. Dobutamine stress echocardiography: experience in pediatric heart transplant recipients. J Heart Lung Transplant 1999;18:725-32. [Crossref] [PubMed]
- Wisotzkey BL, Jorgensen NW, Albers EL, et al. Feasibility and interpretation of global longitudinal strain imaging in pediatric heart transplant recipients. Pediatr Transplant 2017. [Crossref] [PubMed]
- Godown J, Dodd DA, Stanley M, et al. Changes in left ventricular strain parameters following pediatric heart transplantation. Pediatr Transplant 2018;22:e13166. [Crossref] [PubMed]
- Antonczyk K, Niklewski T, Antonczyk R, et al. Evaluation of the Graft Mechanical Function Using Speckle-Tracking Echocardiography During the First Year After Orthotropic Heart Transplantation. Ann Transplant 2018;23:554-60. [Crossref] [PubMed]
- Kailin JA, Miyamoto SD, Younoszai AK, et al. Longitudinal myocardial deformation is selectively decreased after pediatric cardiac transplantation: a comparison of children 1 year after transplantation with normal subjects using velocity vector imaging. Pediatr Cardiol 2012;33:749-56. [Crossref] [PubMed]
- Saleh HK, Villarraga HR, Kane GC, et al. Normal left ventricular mechanical function and synchrony values by speckle-tracking echocardiography in the transplanted heart with normal ejection fraction. J Heart Lung Transplant 2011;30:652-8. [Crossref] [PubMed]
- Watanabe K, Schafer M, Cassidy C, et al. Right atrial function in pediatric heart transplant patients by echocardiographic strain measurements. Pediatr Transplant 2019;23:e13383. [Crossref] [PubMed]
- Sera F, Kato TS, Farr M, et al. Left ventricular longitudinal strain by speckle-tracking echocardiography is associated with treatment-requiring cardiac allograft rejection. J Card Fail 2014;20:359-64. [Crossref] [PubMed]
- Mingo-Santos S, Monivas-Palomero V, Garcia-Lunar I, et al. Usefulness of Two-Dimensional Strain Parameters to Diagnose Acute Rejection after Heart Transplantation. J Am Soc Echocardiogr 2015;28:1149-56. [Crossref] [PubMed]
- Kato TS, Oda N, Hashimura K, et al. Strain rate imaging would predict sub-clinical acute rejection in heart transplant recipients. Eur J Cardiothorac Surg 2010;37:1104-10. [Crossref] [PubMed]
- Podrouzkova H, Bedanova H, Tretina M, et al. Decrease in longitudinal strain in heart transplant recipients is associated with rejection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159:601-6. [Crossref] [PubMed]
- Gursu HA, Varan B, Sade E, et al. Evaluation of Acute Rejection by Measuring Strain and Strain Rate in Children With Heart Transplant: A Preliminary Report. Exp Clin Transplant 2017;15:561-6. [PubMed]
- Sehgal S, Blake JM, Sommerfield J, et al. Strain and strain rate imaging using speckle tracking in acute allograft rejection in children with heart transplantation. Pediatr Transplant 2015;19:188-95. [Crossref] [PubMed]
- Godown J, McEachern WA, Dodd DA, et al. Temporal changes in left ventricular strain with the development of rejection in paediatric heart transplant recipients. Cardiol Young 2019;29:954-9. [Crossref] [PubMed]
- Clemmensen TS, Logstrup BB, Eiskjaer H, et al. Evaluation of longitudinal myocardial deformation by 2-dimensional speckle-tracking echocardiography in heart transplant recipients: relation to coronary allograft vasculopathy. J Heart Lung Transplant 2015;34:195-203. [Crossref] [PubMed]
- Cote AT, Hosking M, Voss C, et al. Coronary artery intimal thickening and ventricular dynamics in pediatric heart transplant recipients. Congenit Heart Dis 2018;13:663-70. [Crossref] [PubMed]
- Zoeller BB, Miyamoto SD, Younoszai AK, et al. Longitudinal Strain and Strain Rate Abnormalities Precede Invasive Diagnosis of Transplant Coronary Artery Vasculopathy in Pediatric Cardiac Transplant Patients. Pediatr Cardiol 2016;37:656-62. [Crossref] [PubMed]
- Boruta RJ, Miyamoto SD, Younoszai AK, et al. Worsening in Longitudinal Strain and Strain Rate Anticipates Development of Pediatric Transplant Coronary Artery Vasculopathy as Soon as One Year Following Transplant. Pediatr Cardiol 2018;39:129-39. [Crossref] [PubMed]
- Clemmensen TS, Eiskjaer H, Logstrup BB, et al. Left ventricular global longitudinal strain predicts major adverse cardiac events and all-cause mortality in heart transplant patients. J Heart Lung Transplant 2017;36:567-76. [Crossref] [PubMed]
- Barakat AF, Sperry BW, Starling RC, et al. Prognostic Utility of Right Ventricular Free Wall Strain in Low Risk Patients After Orthotopic Heart Transplantation. Am J Cardiol 2017;119:1890-6. [Crossref] [PubMed]
- Colquitt JL, Jeewa A, Morris SA, et al. Diminished Global Longitudinal Strain Predicts Late Allograft Failure in Pediatric Heart Transplant Recipients. JACC Cardiovasc Imaging 2017;10:1529-31. [Crossref] [PubMed]
- Pattynama PM, Lamb HJ, van der Velde EA, et al. Left ventricular measurements with cine and spin-echo MR imaging: a study of reproducibility with variance component analysis. Radiology 1993;187:261-8. [Crossref] [PubMed]
- Fratz S, Chung T, Greil GF, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 2013;15:51. [Crossref] [PubMed]
- Latus H, Hachmann P, Voges I, et al. Reduced biventricular volumes and myocardial dysfunction long-term after pediatric heart transplantation assessed by CMR. Transplantation 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475-87. [Crossref] [PubMed]
- Butler CR, Kumar A, Toma M, et al. Late gadolinium enhancement in cardiac transplant patients is associated with adverse ventricular functional parameters and clinical outcomes. Can J Cardiol 2013;29:1076-83. [Crossref] [PubMed]
- Butler CR, Kim DH, Chow K, et al. Cardiovascular MRI predicts 5-year adverse clinical outcome in heart transplant recipients. Am J Transplant 2014;14:2055-61. [Crossref] [PubMed]
- Pedrotti P, Vittori C, Facchetti R, et al. Prognostic impact of late gadolinium enhancement in the risk stratification of heart transplant patients. Eur Heart J Cardiovasc Imaging 2017;18:130-7. [Crossref] [PubMed]
- Krieghoff C, Barten MJ, Hildebrand L, et al. Assessment of sub-clinical acute cellular rejection after heart transplantation: comparison of cardiac magnetic resonance imaging and endomyocardial biopsy. Eur Radiol 2014;24:2360-71. [Crossref] [PubMed]
- Taylor AJ, Vaddadi G, Pfluger H, et al. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur J Heart Fail 2010;12:45-51. [Crossref] [PubMed]
- Butler CR, Savu A, Bakal JA, et al. Correlation of cardiovascular magnetic resonance imaging findings and endomyocardial biopsy results in patients undergoing screening for heart transplant rejection. J Heart Lung Transplant 2015;34:643-50. [Crossref] [PubMed]
- Simsek E, Nalbantgil S, Ceylan N, et al. Diagnostic performance of late gadolinium enhancement in the assessment of acute cellular rejection after heart transplantation. Anatol J Cardiol 2016;16:113-8. [PubMed]
- Greenway SC, Dallaire F, Kantor PF, et al. Magnetic resonance imaging of the transplanted pediatric heart as a potential predictor of rejection. World J Transplant 2016;6:751-8. [Crossref] [PubMed]
- Braggion-Santos MF, Lossnitzer D, Buss S, et al. Late gadolinium enhancement assessed by cardiac magnetic resonance imaging in heart transplant recipients with different stages of cardiac allograft vasculopathy. Eur Heart J Cardiovasc Imaging 2014;15:1125-32. [Crossref] [PubMed]
- Madani MH, Canter CE, Balzer DT, et al. Noninvasive detection of transplant coronary artery disease with contrast-enhanced cardiac MRI in pediatric cardiac transplants. J Heart Lung Transplant 2012;31:1234-5. [Crossref] [PubMed]
- Hussain T, Fenton M, Peel SA, et al. Detection and grading of coronary allograft vasculopathy in children with contrast-enhanced magnetic resonance imaging of the coronary vessel wall. Circ Cardiovasc Imaging 2013;6:91-8. [Crossref] [PubMed]
- Schelbert EB, Testa SM, Meier CG, et al. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson 2011;13:16. [Crossref] [PubMed]
- Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75. [Crossref] [PubMed]
- Haaf P, Garg P, Messroghli DR, et al. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 2016;18:89. [Crossref] [PubMed]
- Ferreira VM, Piechnik SK, Dall'Armellina E, et al. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:42. [Crossref] [PubMed]
- Ferreira VM, Piechnik SK, Dall'Armellina E, et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013;6:1048-58. [Crossref] [PubMed]
- Imran M, Wang L, McCrohon J, et al. Native T1 Mapping in the Diagnosis of Cardiac Allograft Rejection: A Prospective Histologically Validated Study. JACC Cardiovasc Imaging 2019;12:1618-28. [Crossref] [PubMed]
- Vermes E, Pantaleon C, Auvet A, et al. Cardiovascular magnetic resonance in heart transplant patients: diagnostic value of quantitative tissue markers: T2 mapping and extracellular volume fraction, for acute rejection diagnosis. J Cardiovasc Magn Reson 2018;20:59. [Crossref] [PubMed]
- Feingold B, Salgado CM, Reyes-Mugica M, et al. Diffuse myocardial fibrosis among healthy pediatric heart transplant recipients: Correlation of histology, cardiovascular magnetic resonance, and clinical phenotype. Pediatr Transplant 2017;21. [Crossref] [PubMed]
- Ide S, Riesenkampff E, Chiasson DA, et al. Histological validation of cardiovascular magnetic resonance T1 mapping markers of myocardial fibrosis in paediatric heart transplant recipients. J Cardiovasc Magn Reson 2017;19:10. [Crossref] [PubMed]
- Kramer CM, Barkhausen J, Flamm SD, et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 2008;10:35. [Crossref] [PubMed]
- Freed BH, Narang A, Bhave NM, et al. Prognostic value of normal regadenoson stress perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2013;15:108. [Crossref] [PubMed]
- Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453-60. [Crossref] [PubMed]
- Vijarnsorn C, Noga M, Schantz D, et al. Stress perfusion magnetic resonance imaging to detect coronary artery lesions in children. Int J Cardiovasc Imaging 2017;33:699-709. [Crossref] [PubMed]
- Al-Mallah MH, Arida M, Garcia-Sayan E, et al. Safety of adenosine pharmacologic stress myocardial perfusion imaging in orthotopic cardiac transplant recipients: a single center experience of 102 transplant patients. Int J Cardiovasc Imaging 2011;27:1105-11. [Crossref] [PubMed]
- Korosoglou G, Osman NF, Dengler TJ, et al. Strain-encoded cardiac magnetic resonance for the evaluation of chronic allograft vasculopathy in transplant recipients. Am J Transplant 2009;9:2587-96. [Crossref] [PubMed]
- Hofmann NP, Voss A, Dickhaus H, et al. Long-term outcome after heart transplantation predicted by quantitative myocardial blush grade in coronary angiography. Am J Transplant 2013;13:1491-502. [Crossref] [PubMed]
- Hofmann NP, Steuer C, Voss A, et al. Comprehensive bio-imaging using myocardial perfusion reserve index during cardiac magnetic resonance imaging and high-sensitive troponin T for the prediction of outcomes in heart transplant recipients. Am J Transplant 2014;14:2607-16. [Crossref] [PubMed]
- Korosoglou G, Riedle N, Erbacher M, et al. Quantitative myocardial blush grade for the detection of cardiac allograft vasculopathy. Am Heart J 2010;159:643-51.e2. [Crossref] [PubMed]
- Erbel C, Mukhammadaminova N, Gleissner CA, et al. Myocardial Perfusion Reserve and Strain-Encoded CMR for Evaluation of Cardiac Allograft Microvasculopathy. JACC Cardiovasc Imaging 2016;9:255-66. [Crossref] [PubMed]
- Flyer JN, Zuckerman WA., Richmond ME, et al. Prospective Trial of Adenosine on Atrioventricular Nodal Conduction in the Pediatric Transplanted Heart. American Heart Association Scientific Sessions 2016; November 11, 2016; New Orleans, LA: Circulation; 2016.
- Noel CV, Krishnamurthy R, Masand P, et al. Myocardial Stress Perfusion MRI: Experience in Pediatric and Young-Adult Patients Following Arterial Switch Operation Utilizing Regadenoson. Pediatr Cardiol 2018;39:1249-57. [Crossref] [PubMed]
- Noel CV, Krishnamurthy R, Moffett B, et al. Myocardial stress perfusion magnetic resonance: initial experience in a pediatric and young adult population using regadenoson. Pediatr Radiol 2017;47:280-9. [Crossref] [PubMed]
- Kazmirczak F, Nijjar PS, Zhang L, et al. Safety and prognostic value of regadenoson stress cardiovascular magnetic resonance imaging in heart transplant recipients. J Cardiovasc Magn Reson 2019;21:9. [Crossref] [PubMed]
- Narang A, Blair JE, Patel MB, et al. Myocardial perfusion reserve and global longitudinal strain as potential markers of coronary allograft vasculopathy in late-stage orthotopic heart transplantation. Int J Cardiovasc Imaging 2018;34:1607-17. [Crossref] [PubMed]
- Waller AH, Blankstein R, Kwong RY, et al. Myocardial blood flow quantification for evaluation of coronary artery disease by positron emission tomography, cardiac magnetic resonance imaging, and computed tomography. Curr Cardiol Rep 2014;16:483. [Crossref] [PubMed]
- Lubbers DD, Janssen CH, Kuijpers D, et al. The additional value of first pass myocardial perfusion imaging during peak dose of dobutamine stress cardiac MRI for the detection of myocardial ischemia. Int J Cardiovasc Imaging 2008;24:69-76. [Crossref] [PubMed]
- Gebker R, Jahnke C, Manka R, et al. Additional value of myocardial perfusion imaging during dobutamine stress magnetic resonance for the assessment of coronary artery disease. Circ Cardiovasc Imaging 2008;1:122-30. [Crossref] [PubMed]
- Mirelis JG, Garcia-Pavia P, Cavero MA, et al. Magnetic Resonance for Noninvasive Detection of Microcirculatory Disease Associated With Allograft Vasculopathy: Intracoronary Measurement Validation. Rev Esp Cardiol (Engl Ed) 2015;68:571-8. [Crossref] [PubMed]
- Voigt JU, Flachskampf FA. Strain and strain rate. New and clinically relevant echo parameters of regional myocardial function. Z Kardiol 2004;93:249-58. [Crossref] [PubMed]
- Donofrio MT, Clark BJ, Ramaciotti C, et al. Regional wall motion and strain of transplanted hearts in pediatric patients using magnetic resonance tagging. Am J Physiol 1999;277:R1481-7. [PubMed]
- Miller CA, Naish JH, Shaw SM, et al. Multiparametric cardiovascular magnetic resonance surveillance of acute cardiac allograft rejection and characterisation of transplantation-associated myocardial injury: a pilot study. J Cardiovasc Magn Reson 2014;16:52. [Crossref] [PubMed]
- Shah AD, Morris MA, Hirsh DS, et al. Magnetic resonance imaging safety in nonconditional pacemaker and defibrillator recipients: A meta-analysis and systematic review. Heart Rhythm 2018;15:1001-8. [Crossref] [PubMed]
- Cheng Z, Wang X, Duan Y, et al. Low-dose prospective ECG-triggering dual-source CT angiography in infants and children with complex congenital heart disease: first experience. Eur Radiol 2010;20:2503-11. [Crossref] [PubMed]
- Arraiza M, Azcarate PM, De Cecco CN, et al. Assessment of left ventricular parameters in orthotopic heart transplant recipients using dual-source CT and contrast-enhanced echocardiography: comparison with MRI. Eur J Radiol 2012;81:3282-8. [Crossref] [PubMed]
- Mittal TK, Panicker MG, Mitchell AG, et al. Cardiac allograft vasculopathy after heart transplantation: electrocardiographically gated cardiac CT angiography for assessment. Radiology 2013;268:374-81. [Crossref] [PubMed]
- Gunther A, Andersen R, Gude E, et al. The predictive value of coronary artery calcium detected by computed tomography in a prospective study on cardiac allograft vasculopathy in heart transplant patients. Transpl Int 2018;31:82-91. [Crossref] [PubMed]
- Gunther A, Aaberge L, Abildgaard A, et al. Coronary computed tomography in heart transplant patients: detection of significant stenosis and cardiac allograft vasculopathy, image quality, and radiation dose. Acta Radiol 2018;59:1066-73. [Crossref] [PubMed]
- Oebel S, Hamada S, Higashigaito K, et al. Comprehensive morphologic and functional imaging of heart transplant patients: first experience with dynamic perfusion CT. Eur Radiol 2018;28:4111-21. [Crossref] [PubMed]


