Two separated thrombi in deep veins associated with pulmonary embolism after Mycoplasma pneumoniae infection: a case in adolescent female
Introduction
Mycoplasma pneumoniae (M. pneumoniae) is commonly thought to be responsible for respiratory infection. However more and more reports have mentioned the extrapulmonary complications, in which vascular occlusion is extremely rare. As far as we know, there have been 16 cases of thromboembolic events in association with Mycoplasma pneumoniae concerning 25 patients. The age varies between four and forty years with the median age of eight. There are more male than female. The most involved vessel is arteria cerebri media, followed by artery in lower extremity. The other vessels, such as superior mesenteric artery (1), could be impacted but with single report. The mechanism of thrombosis after M. pneumoniae infection is still unknown but probably relating to immune modulation. Here we report one case of two separated thrombi in deep veins associated with pulmonary embolism after Mycoplasma pneumoniae infection.
Case report
A previously healthy twelve-year-old Chinese female complained of fever, dry cough, and a respiration aggravated chest pain for one day. There were no special family history or tuberculosis contacts. On admission, her body temperature was 40 °C and the oxygen saturation was 96% with no signs of tachypnea or dyspnea. Physical examination showed moderate congestion of her throat, and the breath sounds were coarse with moist rales over the left lung base. There were no other major abnormal findings from systemic physical examination. Laboratory test revealed elevated white blood cell count of 16.31 G/L, 84.3% neutrophils, and an elevated C-reactive protein of 115 mg/L. The platelet and myocardial enzymes were within normal ranges. No abnormality was identified in electrocardiogram or echocardiogram. Serum titer of M. pneumoniae antibody (Particle Agglutination assay, SERODIA-MYCOII, Fujirebio) was 1:160. Bacterial cultures in blood and sputum did not grow. Polymerase chain reaction tests for common virus were negative. The chest X-ray demonstrated blurry lung markings in the left lower lobe. She was diagnosed pneumonia presumed due to Mycoplasma pneumoniae and suspected pleurisy. Upon that intravenous azithromycin was given for five days, followed by a three-day course orally after a three-day break.
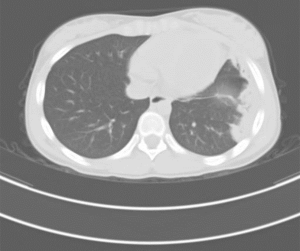
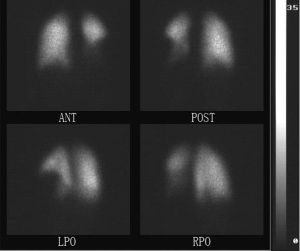
By the eleventh day, her fever and cough had gone with mitigated chest pain. Nevertheless, she began to suffer from swelling and a gradually deteriorating pain in the right lower limb. Meanwhile paradoxical tachypnea developed and the oxygen saturation attenuated to 85-90%. An ultrasonic examination was ordered with the result of thrombogenesis involving two separated parts, one of which extending from fibular vein to posterior tibial vein, and the other locating at vena femoralis. Lung CT scan revealed consolidation in the left lung (Figure 1). Clotting test and platelet count were normal except for D-dimer of 44,900 ng/mL. Anticardiolipin antibody was positive while ANA, ENA and DsDNA were negative. And the radionuclide pulmonary perfusion imaging showed segmental perfusion defect in the left lung (Figure 2). By that time, we were convinced with her multi-site thromboses.
Based on the guidelines of American College of Chest Physicians (2), we initialed the treatment with hypodermic low molecular weight heparin for 10 days, replacing by oral warfarin for six months, maintaining a target INR within 2.0-3.0. Methylprednisolone and intravenous immunoglobulin (IVIG, 2.0 g/Kg) were also used for immunosuppressive therapy. The necessity for thrombolysis was ruled out by her stable hemodynamic status. Thirteen days later, the swelling and pain in the right lower limb disappeared. Repeated D-dimer test reduced to 3,590 ng/mL. Azithromycin was taken orally for two more courses. The serum titer of M. pneumoniae indicating corresponding infection increased to 1:640 one month after the onset. Then she was discharged with the only requirement for warfarin. The anticardiolipin antibody was normal six months after the acute illness. Ultrasonography showed partial vessel recanalization. The chest X-ray was almost normal in follow-up.
Discussion
This case refers to multiple thromboses approximately twelve days after respiratory infection. The comparison of paired specimens collected four weeks apart indicated recent M. pneumoniae infection. And no more pathogens were identified. Moreover, the patient had no proof of coagulation disorder, cardiovascular disease or any autoimmune disorders, neither was there any recent history of trauma. Therefore, we considered the thromboses and M. pneumoniae to be relational.
The mechanism of thrombosis after M. pneumoniae infection is still unknown but probably relating to immune modulation. On rare occasions, lipoprotein and glycolipid of M. pneumoniae would be treated as common antigens with sorts of human tissues. Organs may be influenced when immune system overreacts (3). So as to proof that, anticardiolipin antibody has been detected according to some other documents (4-11). Anticardiolipin antibody is thought to be a kind of autoantibody, aiming for the structure of cardiolipin on the surface of platelet and vessel endothelial cells (ECs). Once injured, ECs would express procoagulant and antifibrinolytic components, such as vWF, TXA3, P-selectin and PAI-1. In addition, owing to their dependence on healthy state of ECs surface, some physiologic coagulation inhibitors including protein C system and tissue factor pathway inhibitor might be secondary impaired (3).
According to the review we made, thrombus has been identified in only one location while shedding happens sometimes. Graw-Panzer KD et al. has reported a thirteen-year-old child who developed popliteal vein thrombosis and pulmonary embolism after M.pneumonia infection. Protein S deficiency and transient antiphospholipid antibodies were considered to be responsible (6). However, whether pulmonary embolism was secondary to popliteal vein thrombosis was not answered. In our case, the chronological relationship between deep venous thrombosis and pulmonary embolism was acataleptic too. But two separated thrombi in deep veins of lower limb were found at the same time. This phenomenon gives a strong hint to systemic abnormity which anticardiolipin antibody contributed to very likely. Furthermore, it seems logical to surmise that the remarkably high C-reactive protein at the very beginning stood for excessive immune reaction.
As for follow-up, more attention is needed on the chronic but frequent complication so-called post-thrombosis syndrome, which was characterized by the destruction of venous valves and persistent outflow obstruction. In this regard, our patient was lucky.
Conclusions
Thrombosis should be considered for those who have significantly increased C-reactive protein and positive anticardiolipin antibody after Mycoplasma pneumoniae infection. Though its mechanism is unknown immune modulation might play a key role. More cases and correlative fundamental researches are worth expecting.
Acknowledgements
Written consent was obtained from the patient’s family. All authors have read and approved this manuscript. Yu Chen played a major role in writing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wilson ML, Menjivar E, Kalapatapu V, et al. Mycoplasma pneumoniae associated with hemolytic anemia, cold agglutinins, and recurrent arterial thrombosis. South Med J 2007;100:215-7. [PubMed]
- Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e737S-801S.
- Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother 2010;16:162-9. [PubMed]
- Bakshi M, Khemani C, Vishwanathan V, et al. Mycoplasma pneumonia with antiphospholipid antibodies and a cardiac thrombus. Lupus 2006;15:105-6. [PubMed]
- Brown SM, Padley S, Bush A, et al. Mycoplasma pneumonia and pulmonary embolism in a child due to acquired prothrombotic factors. Pediatr Pulmonol 2008;43:200-2. [PubMed]
- Graw-Panzer KD, Verma S, Rao S, et al. Venous thrombosis and pulmonary embolism in a child with pneumonia due to Mycoplasma pneumoniae. J Natl Med Assoc 2009;101:956-8. [PubMed]
- Nagashima M, Higaki T, Satoh H, et al. Cardiac thrombus associated with Mycoplasma pneumoniae infection. Interact Cardiovasc Thorac Surg 2010;11:849-51. [PubMed]
- Padovan CS, Pfister HW, Bense S, et al. Detection of Mycoplasma pneumoniae DNA in cerebrospinal fluid of a patient with M. pneumoniae infection-“associated” stroke. Clin Infect Dis 2001;33:E119-21. [PubMed]
- Snowden N, Wilson PB, Longson M, et al. Antiphospholipid antibodies and Mycoplasma pneumoniae infection. Postgrad Med J 1990;66:356-62. [PubMed]
- Witmer CM, Steenhoff AP, Shah SS, et al. Mycoplasma pneumoniae, splenic infarct, and transient antiphospholipid antibodies: a new association? Pediatrics 2007;119:e292-5. [PubMed]
- Tanir G, Aydemir C, Yilmaz D, et al. Internal carotid artery occlusion associated with Mycoplasma pneumoniae infection in a child. Turk J Pediatr 2006;48:166-71. [PubMed]