Changes in interleukin-17 and transforming growth factor beta 1 levels in serum and bronchoalveolar lavage fluid and their clinical significance among children with asthma
Bronchial asthma (or asthma) is a chronic inflammatory airway disease caused by a variety of cells and cellular components, with airway inflammation and remodeling being the main pathological features. As one of proinflammatory cytokines, interleukin-17 (IL-17) is an early trigger of the T lymphocyte-induced inflammatory response and can induce and activate the neutrophil recruitment to the respiratory tract. Transforming growth factor beta 1 (TGF-β1) is a multifunctional cytokine that plays a key role in immune regulation, cell growth and differentiation, as well as synthesis and storage of extracellular matrix. In our current study, we detected the levels of IL-17 and TGF-β1 in the serum and bronchoalveolar lavage fluid (BALF) of asthma infants, with an attempt to explore the role of IL-17 and TGF-β1 in the pathogenesis of asthma and its clinical significance.
Subjects and methods
Subjects
A total of 56 asthma infants who were confirmed in the department of respiratory medicine of our hospital from January 2011 to June 2012 were enrolled as the asthma group. All the pediatric patients (18 females and 38 males, aged 1.4-11.0 years, weighing 14-32 kg) were diagnosed in strict accordance with the diagnostic criteria of asthma in children (1). Based on the disease severity, these patients were further divided into moderate or severe asthma (n=37) and mild asthma groups (n=19). In addition, 18 children (7 females and 11 males, aged 1.5-8.0 years, weighing 15-28 kg) without asthma were selected as the control group; these patients were admitted during the same period due to foreign body in bronchus (n=14, for 1-3 days) or tracheal malformation (n=4, for 3 months to 2 years). In addition, all the children in the control group had no history of infection or chronic disease, and any allergic or immune-related disorder was also ruled out. These three groups had no significant differences in terms of gender, age, and body weight (all P>0.05). Informed consents were obtained from the parents before the fiberoptic bronchoscopy and bronchoalveolar lavage performed.
Methods
Sample collection
Venous blood samples (2 mL) were obtained from all patients. After centrifugation, the serum was harvested and stored in a refrigerator at –70 °C refrigerator until required for analysis. When the fiberoptic bronchoscopy was performed, all the patients in the asthma group were in the remission stage, and the there was more than one week between each attack; bronchoalveolar lavage was then performed after the examination of the bronchial tree, and the right middle lobe of the lung was lavaged. In the control group, the right middle lobe of the lung (for patients with tracheal malformations) or the lung without inhaled foreign object was lavaged. After the fiberoptic bronchoscope was introduced into these sites, 10-20 mL sterile saline at 37 °C was injected via the bronchoscope for lavage for 2-4 times. After the bronchoscopic lavage (1 mL/kg each time, 3 times/day for patients weighing <20 kg; and 20 mL each time, with a total lavage volume reaching 3 mL/kg for patients weighing >20 kg), the fluid was suctioned with a maximum vacuum force of 100-150 mmHg. The procedure lasted 20-40 min. BALF from the first lavage was sent for etiological cultures. The remaining lavages were mixed. Each sample was centrifuged at 4 °C at 1,500 r/min (centrifugal radius: 13.5 cm) for 10 min. The sediment was used for cytological analysis, and the supernatant was stored at –70 °C for the analysis of cytokines.
Cytology
After the centrifugation of BALF, the sediment was used for cell counting using the counting chamber method, and the results are expressed as ×106/mL. The blood smear for differential count was used. The sediment smears were stained with Wright’s stain method. Count 600 cells in each smear, and then calculate the means of two smears.
Detection of cytokines
The serum and BALF levels of IL-17 and TGF-β1 were detected using enzyme-linked immunosorbent assay (ELISA) every week in strict accordance with the manufacturer’s instructions (Boster, Wuhan, China).
Statistical analysis
All data were analyzed using the SPSS 17.0 software. The normally distributed measurement data are described using means and standard deviations (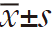 ). Comparisons between the means of two groups are performed using the t test. Non-normally distributed measurement data are expressed with medians and quartiles. Rank-sum test is applied for inter-group comparisons. Correlations are analyzed using Pearson’s or Spearman’s correlations. P<0.05 was considered significantly different.
). Comparisons between the means of two groups are performed using the t test. Non-normally distributed measurement data are expressed with medians and quartiles. Rank-sum test is applied for inter-group comparisons. Correlations are analyzed using Pearson’s or Spearman’s correlations. P<0.05 was considered significantly different.
Results
Total and differential cell counts in the BALF in three groups
There were no significant differences in total cell count and percentage of macrophages between the two asthma groups and the control group (P>0.05). The percentages of neutrophils, eosinophils and epithelial cells in BALF were significantly higher in the two asthma groups than in the control group (both P<0.05). Compared with the mild asthma group, the percentages of neutrophils, eosinophils and epithelial cells in BALF significantly increased in the moderate or severe asthma group (Z=5.731, 5.660, and 5.227, respectively; all P<0.01) (Table 1).
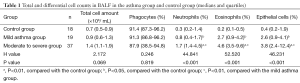
Full table
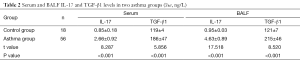
Comparison of serum and BALF IL-17 and TGF-β1 levels in three groups
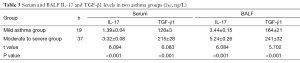
The two asthma groups had significantly higher levels of IL-17 and TGF-β1 in serum and BALF than in the control group (both P<0.01) (Table 2). In addition, the serum and BALF levels of IL-17 and TGF-β1 were also significantly higher in the moderate or severe asthma group than in the mild asthma group (P<0.01) (Table 3).
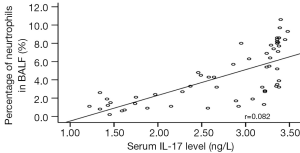
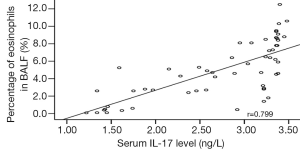
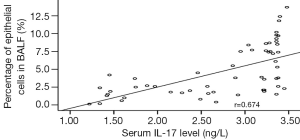
Correlations of the serum IL-17 level with the percentages of neutrophils, eosinophils, and epithelial cells in BALF in the asthma group
Rank correlation analysis showed that the serum IL-17 level was positively correlated with the percentages of neutrophils, eosinophils, and epithelial cells in BALF in the asthma group (r=0.802, 0.799, and 0. 674, all P<0.01) (Figures 1-3).
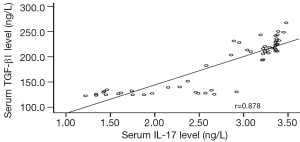
Correlations of serum and BALF IL-17 and TGF-β1 levels in asthma group
As shown by the linear correlation analysis, in the asthma group, the serum IL-17 and TGF-β1 levels were positively correlated with those in BALF (r=0.935, r=0.943, both P<0.05). Also in the asthma group, the serum IL-17 level was positively correlated with serum TGF-β1 level (r=0.878, P<0.01) (Figure 4).
Discussion
Airway inflammation and airway remodeling are two main pathological features of asthma. IL-17 is an early trigger of the T lymphocyte-induced inflammatory response and has potent chemotactic activity for inflammatory cells. It can remarkably increase recruitment of inflammatory cells in the airways. IL-17 can also activate the epithelial cells, fibroblasts, and macrophages to release granulocyte-macrophage colony stimulating factor (GM-CSF), IL-1, IL-6, and IL-8; by doing so, it not only exaggerates airway inflammation but also participates in airway remodeling. IL-17 can be involved in airway inflammation through a variety of ways: (I) it can induce bronchial epithelial cells, bronchial fibroblasts, and venous endothelial cells to release proinflammatory cytokines such as IL-6, IL-8, GM-CSF, and TNF-γ, which can stimulate the inflammatory tissues, and thus indirectly causes tissue invasion and tissue damage (2); (II) it can promote dendritic cell maturation and stimulate the relevant cells to produce a series of inflammatory mediators and cytokines, causing inflammatory response (3); and (III) the Th17 cells can produce IL-17 and thus mediate the endothelial cells, fibroblasts, and macrophages to secret a series of inflammatory chemokines, which promotes the aggression of inflammatory cells and the chronic inflammation, resulting in immune-inflammatory damage (4). In our current study, compared with the control group, the moderate to severe asthma group and mild asthma group had significantly elevated percentages of neutrophils, eosinophils, and epithelial cells. The asthma group also had significantly higher levels of IL-7 in serum and BALF than in the control group, whereas the moderate to severe asthma group has significantly higher levels of IL-7 in serum and BALF in the mild asthma group. Furthermore, the serum IL-17 level was positively correlated with the percentages of neutrophils, eosinophils, and epithelial cells in BALF, indicating that IL-17 plays a key role in the acute attack and exacerbations of asthma. Chen et al. established mouse asthma models using allergic egg protein and found that, compared with the control group, the asthma group had significantly higher total cell amount and higher percentages of lymphocytes, eosinophilss, and neutrophils in BALF (5). In the study conducted by Bajoriuniene et al. (6), subjects underwent bronchial challenge with D. pteronyssinus. Twenty-four hours after challenge, the IL-17 level was significantly higher in patients with allergic asthma than those in the control group, suggesting that IL-17 may have an important role in the development of asthma. Wang et al. (7) also found that the serum IL-17 levels were significantly higher in moderate to severe asthma group and mild attack group than in the healthy control group, and the percentage of neutrophils in peripheral blood was also significantly higher in the asthma group than in the control group.
As a multifunctional cytokine, TGF-β1 can induce epithelial cell damage, airway inflammation, airway smooth muscle cell proliferation, and Goblet cell hyperplasia and promote vascular remodeling and many other cellular responses. Also, it can cause vascular remodeling by promoting the hyperplasia of Goblet cells and inducing the release of vascular endothelial growth factor and be involved in airway remodeling by promoting the hyperplasia, hypertrophy, and migration of airway smooth muscles and damaging the epithelial cell layer (8). Asthma patients can also experience airway remodeling, which is featured by the thickening of airway walls, airway smooth muscles, and basement membranes, the angiogenesis, and the mucosal metaplasia. As shown in our current study, the serum and BALF TGF-β1 levels were significantly higher in asthma group than in control group, whereas the moderate to severe asthma group had significantly higher levels of TGF-β1 in serum and BALF than in the mild asthma group, indicating that the TGF-β1 level is closely related with the severity of asthma, and the pediatric asthma patients may have varying degrees of airway remodeling. Using the fluorescent in situ hybridization, Minshall et al. (9) found that the TGF-β1 levels in bronchial mucosa and BALF in asthma patients were positively correlated with the disease severity. Our study also found that the serum and BALF IL-17 levels were positively correlated with the TGF-β1 levels, indicating that the increased TGF-β1 levels in serum and BALF may be resulted from the increase of IL-17. IL-17 can cause the remodeling of airway and lung tissue, which may because IL-17 can activate the neutrophils to produce neutrophil elastase, which can degrade the elastin, promote the secretion of gland cells, and thus affect the structures of airway and lung tissues (10). The activated neutrophils release matrix metalloproteinase-8 and -9, resulting in the massive degradation of the components of the extracellular matrix and the changes in airway structures (11). IL-17 stimulates the bronchial fibroblasts to produce and secret IL-11, causing the aggression of collagen and matrix metalloproteinase inhibitor-1, which can inhibit the degradation of extracellular matrix and thus indirectly promote fibrosis or directly induce the migration of airway smooth muscle cells, facilitating the airway remodeling in asthma patients (12).
Our study also found that, in the pediatric asthma patients, the levels of IL-17 and TGF-β1 were significantly higher in BALF than in serum, and there was a positive correlation between IL-17 and TGF-β1. It is therefore speculated that the increased IL-17 and TGF-β1 levels in serum are secondary to those in BALF via the bloodstream pathway. Therefore, detection of serum IL-17 and TGF-β1 levels can indirectly reflects the levels of IL-17 and TGF-β1 in bronchi and alveoli.
In summary, IL-17 and TGF-β1 are involved in the airway inflammation and airway remodeling in pediatric asthma patients, and therefore key roles in the pathogenesis of asthma. However, their exact mechanisms are complex and require further investigations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Subspecialty Group of Respiratory Diseases Society of Pediatrics, Chinese Medical Association, Chinese Journal of Pediatrics Editorial Board. Guideline for the diagnosis and optimal management of asthma in children. Zhonghua Er Ke Za Zhi 2008;46:745-53. [PubMed]
- Tanaka S, Yoshimoto T, Naka T, et al. Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol 2009;183:7523-30. [PubMed]
- Besnard AG, Togbe D, Guillou N, et al. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol 2011;41:1675-86. [PubMed]
- Bettelli E, Korn T, Oukka M, et al. Induction and effector functions of T(H)17 cells. Nature 2008;453:1051-7. [PubMed]
- Chen WC, Liu EM, Deng Y, et al. Impact of neonatal bacillus Calmette-Guerin vaccination on lung Th17 cells and IL-17 in murine asthma model. Zhongguo Dang Dai Er Ke Za Zhi 2010;12:650-3. [PubMed]
- Bajoriuniene I, Malakauskas K, Lavinskiene S, et al. Response of peripheral blood Th17 cells to inhaled Dermatophagoides pteronyssinus in patients with allergic rhinitis and asthma. Lung 2012;190:487-95. [PubMed]
- Wang X, Ma CY, Zhang YJ, et al. Expressions of Serum Interleukin -17 and Leukotriene B4 in Childhood Asthma and Their Clinical Significance. Journal of Applied Clinical Pediatrics 2012;27:257-9.
- Li T, Dong M. Role of transforming growth factor-β in bronchial asthma airway smooth muscle remodeling. International Journal of Respiration 2010;30:169-72.
- Minshall EM, Leung DY, Martin RJ, et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1997;17:326-33. [PubMed]
- Wilson RH, Whitehead GS, Nakano H, et al. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 2009;180:720-30. [PubMed]
- Lindén A. Interleukin-17 and airway remodelling. Pulm Pharmacol Ther 2006;19:47-50. [PubMed]
- Al-Alwan LA, Chang Y, Baglole CJ, et al. Autocrine-regulated airway smooth muscle cell migration is dependent on IL-17-induced growth-related oncogenes. J Allergy Clin Immunol 2012;130:977-85.e6.