The clinical management and outcomes of extremely preterm infants in Japan: past, present, and future
Extremely preterm infants: a brief overview of global statistics
Preterm birth complications are one of the major global health problems and the leading cause of global under-five child mortality (1). In addition, it features among the top five causes of global disability-adjusted life years and other major diseases (e.g., ischemic heart disease, cerebrovascular diseases) among adults (2). Although mortality due to preterm birth complications are typically high in developing countries, preterm births remain the major cause of child deaths even in developed countries (3). In particular, extremely preterm infants (i.e., infants born before 26 weeks of gestation) have very high mortality rates; further, even among survivors from developed countries, there is a high risk of long-term complications (4,5).
There is a wide variation in neonatal mortality rates across regions and countries (6). Japan has one of the lowest neonatal mortality rates in the world; further, the mortality rate of extremely preterm infants is much lower in Japan than in other developed countries (7). A recent study, which was conducted by the International Network for Evaluating Outcomes of Neonates (iNeo) across 11 developed regions and countries, reported that between-country differences in mortality rates widened as the gestational age of infants decreased; specifically, the mortality rate range was 16–65% at a gestational age of 24 weeks and 3–6% at 29 weeks (8).
This review article provides a comparative overview of the outcomes of extremely preterm infants in Japan and other countries; further, it introduces the unique features of clinical management of extremely preterm infants in Japan. Many clinical management systems that are described in this review do not have a strong evidence-base that supports their effectiveness or advantages. Accordingly, this review does not intend to recommend the application of such Japanese management systems in clinical practice in other countries. Instead, this review introduces the clinical management of preterm infants in Japan in order to generate ideas and opportunities for future research.
Development of neonatology in Japan
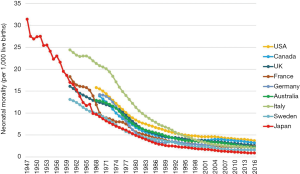
The neonatal mortality rate in Japan dramatically improved from the 1940s to 1960s (Figure 1). Several factors, which include the following, are considered to have contributed to this improvement: the introduction of the Maternal and Child Health Handbook (which was then referred to as the Maternal Handbook) in 1942, enforcement of several legislations to protect the health of mothers and children (Child Welfare Act in 1947, Maternal Health Act in 1948, and Maternal Child Health Law in 1965), and the establishment of the National Health Insurance System for all citizens in 1961 (10). In particular, the Maternal and Child Health Handbook was a unique Japanese system that enabled the public registration of pregnant women, systematic education and support of pregnant women and mothers of young children, and the development and promotion of regular health checkups and vaccination of newborns, infants, and children (10). In 1958, the Medical Care Benefits for Premature Babies was established to cover the medical expenses that the care of premature infants or low birth weight infants (<2,000 g) entails. These changes promoted the development of healthcare systems that supported pregnant women, mothers, newborns, infants, and children. During this period, the proportion of home births to all deliveries rapidly decreased from 95% in 1950 to 4% in 1960 (to 0.1% in 2015). This drastic shift in the birth place (i.e., from the home to clinics or hospitals) might account for the large reduction in neonatal mortality rates that have been evidenced across time (10). Since the 1960s, the neonatal mortality rate in Japan has remained one of the lowest in the world and is still improving (Figure 1). In the 1970s, the development of neonatal intensive care units (NICU), and the introduction of mechanical ventilation and the vital signs monitor systems improved the survival of sick newborns, particularly very low birth weight (VLBW) or very preterm infants. After 1980, the introduction of surfactant therapy, pulse oximetry, and high-frequency oscillation ventilation contributed to further decline in the mortality and morbidity rates of preterm infants. Japanese clinicians and researchers have played important roles in the development of these new drugs and devices (11-13).
Importantly, the preterm birth rate is lower in Japan (5.7%) than other countries such as the United States of America (USA; 9.6%), Canada (8.2%), and the United Kingdom (UK; 7.0%), and are comparable to those of Nordic countries such as Sweden (5.8%), Finland (5.8%), and Norway (5.8%) (14). In addition, the proportion of extremely preterm births is low in Japan. Live births at 22–25 weeks of gestation were found to be 1.3 per 1,000 births in Japan; this rate is lower than those of the USA (4.7), Canada (3.3), and the UK (3.2) and comparable to those of Nordic countries [e.g., Norway (1.3), Finland (1.2), Sweden (1.6)] (7). Since 1965, the Maternal and Child Health Act in Japan has recommended that local governments provide pregnant women with regular prenatal visits (i.e., once every 4 weeks until 23 weeks of gestation, once every 2 weeks between 24 and 35 weeks of gestation, and once a week after 36 weeks of gestation) by offering financial support. These factors as well as other public health interventions (e.g., maternal handbook) may have contributed to low preterm birth rates in Japan.
The Japanese perinatal healthcare system: general and regional perinatal medical centers (PMCs)
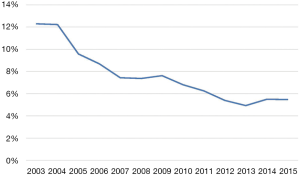
The Ministry of Health, Labour and Welfare in Japan formulated guidelines that pertain to the maintenance of perinatal medical systems; specifically, prefectural government bodies are required to designate general PMCs and regional PMCs based on the care level that is required by patients. The difference between general PMCs and regional PMCs is similar to that between level 3 NICUs (or tertiary NICUs) and level 2 NICUs (or step-down NICUs) in the USA (15). However, the important difference is that general PMCs are expected to provide not only high-level (or tertiary) intensive neonatal care but also high-level intensive obstetric maternal-fetal care. Therefore, standalone children’s hospitals without obstetric units cannot be designated as general PMCs that offer the highest level of care even if they have large tertiary NICUs. The requirement for the designation of general PMCs facilitated the development and expansion of high-level perinatal centers that have both NICUs and obstetric units with maternal fetal intensive care units, and discouraged hospitals from having high-level NICUs that do not have obstetric units. These factors helped reduce the neonatal transfer of sick newborns to hospitals with high-level NICUs and increase antenatal maternal transfer to high-level PMCs. In fact, the proportion of neonatal transfer among VLBW infants decreased from 12% in 2003 to 6% in 2015 (Figure 2). Such changes are important because outborn infants who are transferred to high-level NICUs after birth have poorer prognosis than inborn infants who are born in tertiary NICUs (17,18). Thus, lower neonatal transfer rates may explain the relatively good prognosis of preterm infants in Japan.
The perinatal center system in Japan is not without a weakness. One of them is a relatively low proportion of infants who receive antenatal corticosteroids that is well known to reduce the mortality and various morbidities of preterm infants (19). Although the proportion of antenatal steroids exposure has been improving recently, it was still around 60% of VLBW infants in Japan (16). In many other western countries, 80–90% of VLBW infants were exposed to antenatal corticosteroids (20). The reasons for the low proportion of antenatal corticosteroid exposure in Japan was not clear and it may be due to the fact that the use of maternal antenatal steroids (betamethasone) was off-label in Japan until 2009. By the clinical practice guidelines of the Japan Society of Obstetrics and Gynecology, antenatal corticosteroids administration is recommended to pregnant women with threatened preterm birth between 24- and 33-week gestations and considered for those at 22 to 23 weeks gestations (21). The repeated course of antenatal steroids can be an option for women who do not deliver within 1 week after antenatal corticosteroids, although it is not common in Japan yet (21).
The Neonatal Research Network of Japan (NRNJ)
The NRNJ was originally founded in 1998 with the aim of promoting clinical research, especially randomized controlled trials, in Japan (22). The NRNJ established a network database of VLBW infants across 38 NICUs in 2003. Since then, the number of participating NICUs has increased; further, as of January 2018, 202 NICUs participated in the NRNJ (22). The population coverage of VLBW infants was approximately 70% in 2012 (23). The NRNJ has been collecting the maternal and infant clinical data of VLBW (i.e., birth weight ≤1,500 g) and very preterm infants (i.e., <32 weeks of gestation) during their stay at the NICU and follow-up visits (i.e., at a corrected age of 18 months and chronological age of 3 years after NICU discharge). The goal of the NRNJ is to improve the quality of NICU care and the outcomes of sick newborn infants in Japan by using the NRNJ databases to benchmark NICUs, support follow-up care of sick newborns, and promote clinical research. Analysis of data that are housed by the NRNJ database showed wide variations in mortality and morbidity rates across NICUs in Japan (24). The NRNJ enables each NICU to benchmark the outcomes of VLBW infants in their NICUs against those of other NICUs.
A comparison of the outcomes of VLBW infants in Japan and other countries
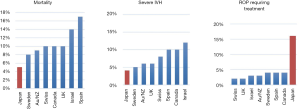
The NRNJ database has been used in international collaborative studies that have compared the outcomes of very preterm infants across countries (20,25). Initially, a cohort study using the databases of the NRNJ and the Canadian Neonatal Network (CNN) found differences in the outcomes of VLBW infants between the two networks (25). This study has evolved into a large multi-country collaborative project called the iNeo, which includes 10 neonatal networks that represent 11 regions or countries (19). Analysis of the iNeo databases showed that Japan had the lowest rates of mortality and severe neurological injury (e.g., severe intraventricular hemorrhage, periventricular leukomalacia) among the iNeo countries or regions (Figure 3). This study did not quantify the incidence of necrotizing enterocolitis (NEC) and late-onset sepsis due to heterogeneity in the diagnosis and definitions of the disease across participating networks. However, the study that compared the NRNJ and the CNN reported that VLBW infants in Japan had much lower incidences of NEC and late-onset sepsis than those in the CNN (NEC: 1.6% vs. 5.9%; late-onset sepsis: 5.0% vs. 16.6%) (25). In addition to these positive outcomes that were evidenced by the NRNJ data, the iNeo study found that very preterm infants in Japan had very high incidences of severe retinopathy of prematurity as well as chronic lung disease (Figure 3). Race or ethnicity may be one of the reasons that account for this difference in the incidence of retinopathy of prematurity. A population-based cohort study that was conducted in New York City reported that those of Asian race or ethnicity were at a higher risk of retinopathy of prematurity than those of a Caucasian race or ethnicity; however, no such group differences emerged with respect to the mortality and severity of intraventricular hemorrhage (26).
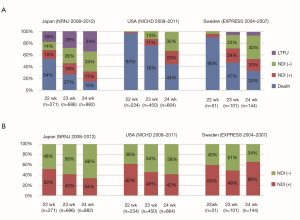
The iNeo study has not examined long term neonatal outcomes (e.g., neurodevelopmental impairments). Figure 4 summarizes the incidences of death and neurodevelopment impairments in extremely preterm infants born at 22–24 weeks of gestation across three countries, namely, Japan, Sweden, and the USA (4,5,27). These results must be interpreted with caution because the NRNJ database evidenced a high proportion of missing information due to a lack of follow up. However, it is noteworthy that the neurodevelopmental outcomes of extremely preterm infants in Japan were better than or comparable to those in Sweden and the USA. This result suggests that the high survival rate of extremely preterm infants in Japan did not result in a high incidence of neurodevelopment impairments in later years.
Decision making pertaining to the active treatment of periviable infants in Japan
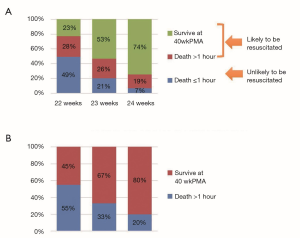
In Japan, the limit of viability changed from 28 to 24 completed weeks of gestation in 1977; subsequently, in 1991, it was changed from 24 to 22 completed weeks of gestation by amendments that were made to the Maternal Protection Act (which was then referred to as the Eugenic Protection Act). As a result of such changes, the outcomes of extremely preterm infants have improved across the years. A national survey that was conducted in Japan in 2012 (response rate =75%, i.e., 208 out of 277 NICUs) reported that active resuscitation of extremely preterm infants that were born at 22 and 23 weeks of gestation was performed in 81% and 85% of NICUs, respectively; of these, 42% and 75% of NICUs resuscitated these infants, regardless of parents’ wishes (28). A population-based cohort study that analyzed vital Japanese statistics yielded information that was used to estimate the number of infants born at 22–24 weeks of gestation that had received resuscitation and active postnatal treatment (29). The study reported that the proportions of infants who had survived an hour since birth to those who had survived the onset of labor were 51%, 79%, and 93% at 22, 23, and 24 weeks of gestation, respectively (Figure 5) (29). Because most extremely preterm infants born at 22–24 weeks of gestation are likely to die shortly after birth, unless they receive resuscitation or active treatment, these numbers can be considered as estimates of the proportions of infants who receive resuscitation and active treatment after birth. Given that death within an hour of birth included intrapartum fetal death (i.e., stillbirth) as well as death after resuscitation in this study, these figures are likely to be underestimates. In the NICHD centers in the USA, the proportions of infants that received active treatment at 22, 23, and 24 weeks of gestation were 21%, 70%, and 96%, respectively (4). A previous study reported that NICUs, where active treatment is more likely to be initiated, promoted better prognosis of extremely preterm infants than other NICUs (30). Therefore, the Japanese practice of active resuscitation of infants that are born during the 22nd week of gestation may contribute to the good prognosis of extremely preterm infants in Japan (30).
Circulatory management
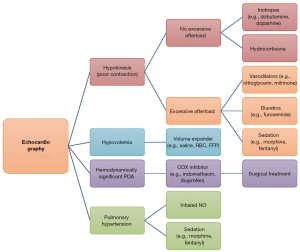
The reason for the lower mortality rate and lower rate of severe intraventricular hemorrhage in Japan than in other countries is not clear; the circulatory management of preterm infants in Japan may account for these international differences (31). A cross-national study on preterm infants’ outcomes found that the rates of mortality and severe intraventricular hemorrhage were significantly lower in Japan than in Canada; however, the differences in the rates of both the outcomes were found only in infants with patent ductus arteriosus (PDA) (32). This result indicated that the management of PDA as well as circulation may contribute to good outcomes among preterm infants in Japan. A national survey on the circulatory management of very preterm infants in Japan reported that most Japanese neonatologists perform functional echocardiography (33). In most NICUs, echocardiography is performed very frequently (i.e., two or more times per day) on extremely preterm infants within 3 days of birth (33). Using echocardiography, Japanese neonatologists assess (I) cardiac function and volume status [i.e., left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVDd)], left atrium to aortic root ratios (LA/Ao), and diameter of inferior vena cava (IVC)], (II) ductus arteriosus status (i.e., diameter and shunt flow of PDA, diastolic flow of left pulmonary artery), (III) peripheral organ circulation [i.e., blood flow pattern of the anterior cerebral artery (ACA), renal artery (RA), and superior mesenteric artery (SMA)], and (IV) pulmonary hypertension (i.e., shape and motion of the interventricular septum, tricuspid regurgitation). These echocardiographic findings, along with clinical signs, guide the circulatory management of extremely preterm infants (Figure 6). Furthermore, there was another aspect of circulatory management that was found to be unique to Japan; specifically, approximately two-thirds of NICUs monitored the end-systolic wall stress (ESWS) of the left ventricle to assess the afterload of the left ventricle and treat the poor left ventricular function that results from excessive afterload (i.e., high ESWS) using vasodilators (e.g., nitroglycerin, milrinone) as well as diuretics and sedation (Figure 6) (33). This use of ESWS in the prevention of intraventricular hemorrhage was originally proposed by Dr. Katsuaki Toyoshima, a Japanese neonatologist and cardiologist, and was reported to be effective in reducing intraventricular haemorrhage (31,34,35). High ESWS accompanied by poor cardiac function) is considered to be a risk factor for intraventricular hemorrhage due to afterload mismatch.
Respiratory management
The respiratory management of extremely preterm infants in Japanese NICUs is relatively conservative or invasive, whereby infants are frequently intubated and placed on mechanical ventilation during the acute phase (i.e., 72 hours after birth). The iNeo conducted an international questionnaire-based survey on the respiratory management of very preterm infants across 10 neonatal networks that represented 11 countries or regions (36). With regard to the initial respiratory management of respiratory distress in spontaneously breathing infants, approaches differed based on the gestational age of the infants. In all countries or regions including Japan, the invasive approach (i.e., intubating and placing infants on mechanical ventilation) emerged as the most common respiratory management strategy that was used with infants born at 23–24 weeks of gestation. However, with regard to infants born at 25–28 weeks of gestation, the non-invasive approach [i.e., placing infants on continuous positive airway pressure (CPAP) with or without surfactant administration] was found to be more common than invasive approaches in most countries or regions except Japan. In Japan, most NICUs used the invasive approach with all infants born at 23–28 weeks of gestation. Previous systematic reviews have reported that the non-invasive approach is more effective in reducing the likelihood of death or chronic lung disease than the invasive approach (37,38). In addition, the non-invasive approach with surfactant administration (e.g., INSURE: intubate-surfactant-extubate; LISA: less-invasive-surfactant-administration) was reported to be effective in reducing the likelihood of death and chronic lung disease (38). According to the results of the iNeo survey, the INSURE and LISA approaches were commonly used in many countries or regions with infants born at 25–28 weeks of gestation; however, they were not common in Japan (36). The invasive respiratory management in Japan may lead to a high incidence of chronic lung disease in Japanese NICUs (25). Greater practice of the invasive approach in Japan may be due to Japanese neonatologists’ apprehensions that the non-invasive approach may increase the risk of intraventricular hemorrhage in extremely preterm infants; it is noteworthy that the conclusions of previous systematic reviews do not offer support to these concerns (37,38). A national survey on the management of chronic lung disease in Japan reported that inhaled and systemic postnatal corticosteroids were often or sometimes used in 44% and 13% of NICUs as prevention and 47% and 67% of NICUs as treatment, respectively (39). Although the types of systemic corticosteroids were not assessed in the survey, the use of hydrocortisone has been increasing instead of dexamethasone due to the concern of neurodevelopmental adverse effect associated with the use of dexamethasone (40).
Nutritional management
The incidence of NEC is lower in Japan (1.6% of VLBW infants) than in other countries (3–10%) (25,41). The low rate of NEC can be attributed to the nutrition management of extremely preterm infants in Japan, which is characterized by (I) the promotion of breastfeeding, (II) early minimal enteral feeding, (III) routine glycerin enema, and (IV) probiotics administration. It was reported that 99% of NICUs tried to solely use breast milk as much as possible and avoided formula milk, when enteral feeding was initiated in extremely preterm infants (42). Significant benefits were observed in breastfed infants, when compared to formula-fed infants; these benefits included the reduction of late-onset infections and NEC, improvement in neurodevelopmental outcomes, and protection against metabolic syndromes in later years (43). Minimal enteral feeding and administration of oral drops of the mother’s breast milk are actively practiced in many Japanese NICUs, typically within 24–48 hours of birth (42). Although a systematic review reported that minimal enteral feeding yielded no significant benefits when compared to enteral fasting, there were not enough data to assess its effectiveness with extremely preterm infants (44). Although donor milk was reported to be effective in preventing NEC, a recent national survey of Japanese NICUs found that only a minority of NICUs (35%) used donor milk (45). Therefore, donor-milk use is an unlikely explanation for the low incidence of NEC in Japan (46,47). The same survey also reported that 71% of NICUs used 3-hour feeding intervals rather than 2-hour feeding intervals. The incidence of NEC was slightly lower in NICUs with 3-hour feeding intervals (1.9%) than those with 2-hour intervals (2.7%); however, this difference was not statistically significant (45). A recent systematic review reported that the reviewed trials did not have sufficient sample sizes to assess variations in NEC incidences between different feeding intervals (48). Therefore, the relationship between feeding intervals and NEC incidence requires further exploration. Another unique aspect of gastrointestinal management in Japanese NICUs is the routine administration of glycerin enema (25% glycerin, 1–2 mL/kg/dose, 3–6 times per day) to accelerate the passage of meconium in extremely preterm infants. A systematic review reported that neither prophylactic glycerin enema nor suppositories hastened the transition to enteral feeding or reduced NEC (49). However, the total sample size of all studies included in the meta-analysis was small; therefore, further trials are needed to assess the effectiveness of glycerin enema. Probiotics have been found to be effective in reducing NEC and late-onset infection (50). Since one of the classic trials on the use of probiotics in improving gastrointestinal outcomes among VLBW infants was conducted in Japan, probiotics (mainly Bifidobacterium species) tend to be commonly administered across many NICUs in Japan (51).
Neurological management
In Japan, the clinical management of extremely preterm infants focuses on the prevention of intraventricular hemorrhage during the acute phase (72 hours after birth). Along with the aforementioned circulatory and respiratory managements, many NICUs in Japan promote neuroprotection by adopting the following: (I) minimal handling of infants, (II) sedation of ventilated infants, and (III) close monitoring of infants using brain ultrasounds.
Neonatologists and NICU nurses try to minimize the handling of infants who are in the acute phase by placing them in comfortable positions, placing arterial lines for blood sampling, and avoiding unnecessary oral or airway suctioning and physical examination. In addition, sedatives and analgesics that contain fentanyl, morphine, midazolam, or phenobarbital are used to stabilize the respiratory and circulatory statuses of ventilated infants in many NICUs (52). A systematic review including more than 1,000 infants reported that the opioids use for infants on mechanical ventilation did not reduce intraventricular hemorrhage (either any grades or grades 3/4) (53). However, the previous trials were likely conducted in NICUs where neonatologist-performed echocardiography was not commonly available unlike in Japan. This is important because the use of opioids was associated with hypotension and therefore circulatory management is important for infants on opioids (54). There is no sufficient data regarding the effectiveness of opioids for ventilated infants when the circulation is closely monitored and managed with neonatologist-performed echocardiography.
In Japanese NICUs, brain ultrasounds of extremely preterm infants with a high risk of intraventricular hemorrhage are frequently undertaken (mostly a few times per day) during the initial 72 hours after birth. The close monitoring of infants using brain ultrasounds enables the early detection of intraventricular haemorrhage and consequent changes in clinical management during the early stages of a disease (e.g., deepening sedations, closer monitoring of cardiopulmonary status); however, there are no clear guidelines about such management. Furthermore, early detection of intraventricular hemorrhage is important because it allows care providers to reflect on its causes (e.g., desaturation after suctioning, high blood pressure) and improve the management of future infants with the condition. Performing serial ultrasound of brain or heart is not in line with the policy of minimal handling. Therefore, Japanese neonatologists are trained to perform the ultrasound quickly and gently to minimize invasive stimuli on infants as much as possible.
A recent topic that has gained interest among Japanese neonatologists is the monitoring of fluctuation in the venous blood flow of the internal cerebral vein (ICV) (55). A single-center cohort study reported that high-grade fluctuation of blood flow in the ICV was associated with a high risk of intraventricular hemorrhage among extremely low birth weight infants (55). Based on this finding, many Japanese NICUs have started measuring the ICV flow pattern, along with routine brain ultrasounds, during the acute phase of extremely preterm infants. However, the relationship between the new index and intraventricular hemorrhage, as well as its usefulness in preventing the condition requires further exploration.
The ethical attitude and medical management of critically ill infants with severe neurological injuries is different among countries (56). The withdrawal of life-sustaining treatment (e.g., mechanical ventilation) is not common in Japan even for seriously ill newborns for whom health care providers consider sustaining treatment futile and instead the withholding of treatment can be chosen (56). It is different from many western countries (e.g., Canada, USA, Australia) where the deaths after withdrawal of life sustaining treatment are relatively common (56-58). Although the reluctance to withdraw life-sustaining treatments may contribute to the low mortality in Japan, it was reassuring that neurodevelopmental outcomes of extremely preterm infants in Japan were at least comparable to those in Sweden and the USA as in Figure 4.
Infection management
The incidence of late-onset sepsis (i.e., ≥7 days after birth) was found to be much lower in Japan (5% of VLBW infants) than in Canada (18% of VLBW infants) (25). Standard precautions (e.g., hand hygiene, isolation precaution) that have been recommended by the guidelines of the Centers for Disease Control and Prevention in the USA are followed in Japan (59-61). Although the guidelines recommend that healthcare providers wear gloves, masks, and gowns when they contact infectious materials (e.g., blood, mucous, stool) or transmissible infectious agents (e.g., drug-resistant organisms), these personal protective equipment are commonly worn by medical personnel even during the provision of routine care to infants (e.g., physical examination) in many Japanese NICUs.
A unique aspect of infection management in Japanese NICUs is the serial measurement of C-reactive protein (CRP) during the acute phase of extremely preterm infants. Several limitations in the use of the CRP have been reported; these include the risk of false-positive results due to a physiological increase in CRP after birth or a non-infectious response (e.g., post-surgery response) as well as the risk of false negative results due to a delayed response to an infection (approximately 10 to 12 hours) (62,63). However, by taking these limitations into account and monitoring temporal changes in CRP, serial CRP measurements can be used to detect a slow-onset infection before it becomes symptomatic, rule out possible infection, and monitor infant responses to the treatment for the infection (63).
Summary and future perspectives
This review has delineated the various unique features of clinical management of extremely preterm infants in Japanese NICUs, which are summarized in Table 1. Although some of these management strategies are supported by high-quality evidence, many are not supported by enough data or empirical findings. Therefore, future studies are needed to assess the effectiveness of these Japanese management strategies that are aimed at improving the outcomes of extremely preterm infants.

Full table
Acknowledgments
The author would like to thank Dr. Katsuaki Toyoshima (Kanagawa Children’s Medical Center, Yokohama, Japan) for reviewing Figure 6 (Circulatory management guided by neonatologist-performed echocardiography) and providing useful comments that helped refine it. The author would like to thank Editage (www.editage.jp) for English language editing.
Funding: The Grant of National Center for Child Health and Development supported the cost for editing this manuscript.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- GBD 2015 Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1725-74. [Crossref] [PubMed]
- GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1603-58. [Crossref] [PubMed]
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430-40. [Crossref] [PubMed]
- Younge N, Goldstein RF, Bann CM, et al. Survival and Neurodevelopmental Outcomes among Periviable Infants. N Engl J Med 2017;376:617-28. [Crossref] [PubMed]
- Serenius F, Kallen K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. Jama 2013;309:1810-20. [Crossref] [PubMed]
- United Nations Inter-agency Group for Child Mortality Estimation (UN IGME), ‘Levels & Trends in Child Mortality: Report 2017, Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation’, United Nations Children’s Fund, New York, 2017. Available online: https://childmortality.org/
- Smith LK, Morisaki N, Morken NH, et al. An International Comparison of Death Classification at 22 to 25 Weeks' Gestational Age. Pediatrics 2018. [Crossref] [PubMed]
- Helenius K, Sjors G, Shah PS, et al. Survival in Very Preterm Infants: An International Comparison of 10 National Neonatal Networks. Pediatrics 2017;140. [Crossref] [PubMed]
- Ministry of Health Labour and Welfare in Japan, e-Stat (Vital Statistics, Infant mortality, Table 6-2). Available online: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450011&tstat=000001028897&result_back=1&second2=1
- Japan International Cooperation Agency (JICA). Chapter 3: Maternal and Child Health. In: Japan’s Experiences in Public Health and Medical Systems. Available online: https://www.jica.go.jp/jica-ri/IFIC_and_JBICI-Studies/english/publications/reports/study/topical/health/index.html
- Fujiwara T, Maeta H, Chida S, et al. Artificial surfactant therapy in hyaline-membrane disease. Lancet 1980;1:55-9. [Crossref] [PubMed]
- Severinghaus JW. Takuo Aoyagi: discovery of pulse oximetry. Anesth Analg 2007;105:S1-4. [Crossref] [PubMed]
- Bohn DJ, Miyasaka K, Marchak BE, et al. Ventilation by high-frequency oscillation. J Appl Physiol Respir Environ Exerc Physiol 1980;48:710-6. [PubMed]
- Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37-e46. [Crossref] [PubMed]
- Stark AR. Levels of neonatal care. Pediatrics 2004;114:1341-7. [Crossref] [PubMed]
- NPO Neonatal Research Network, Japan. Neonatal Research Network Database Japan. Available online: http://plaza.umin.ac.jp/nrndata/indexe.htm
- Boland RA, Davis PG, Dawson JA, et al. Outcomes of infants born at 22-27 weeks' gestation in Victoria according to outborn/inborn birth status. Arch Dis Child Fetal Neonatal Ed 2017;102:F153-61. [Crossref] [PubMed]
- Amer R, Moddemann D, Seshia M, et al. Neurodevelopmental Outcomes of Infants Born at <29 Weeks of Gestation Admitted to Canadian Neonatal Intensive Care Units Based on Location of Birth. J Pediatr 2018;196:31-7.e1. [Crossref] [PubMed]
- Roberts D, Brown J, Medley N, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454. [PubMed]
- Shah PS, Lui K, Sjors G, et al. Neonatal Outcomes of Very Low Birth Weight and Very Preterm Neonates: An International Comparison. J Pediatr 2016;177:144-52.e6. [Crossref] [PubMed]
- "Sanfujinka Shinryo Gaidorain - Sankahen 2017"(Clinical Practice Guidelines of Obstetrics and Gynecology -Version of Obstetrics 2017-) P152-157 (In Japanese) Nihon Sanfujinka Gakkai (The Japan Society of Obstetrics and Gynecology). Available online: https://minds.jcqhc.or.jp/n/med/4/med0097/G0000984
- Neonatal Research Network of Japan (NRNJ). Special Report of the Neonatal Research Network of Japan (Summary of the first 10 years of the network database). Available online: http://plaza.umin.ac.jp/nrndata/indexe.htm
- Nakanishi H, Suenaga H, Uchiyama A, et al. Trends in the neurodevelopmental outcomes among preterm infants from 2003-2012: a retrospective cohort study in Japan. J Perinatol 2018;38:917-28. [Crossref] [PubMed]
- Kusuda S, Fujimura M, Sakuma I, et al. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics 2006;118:e1130-8. [Crossref] [PubMed]
- Isayama T, Lee SK, Mori R, et al. Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics 2012;130:e957-65. [Crossref] [PubMed]
- Janevic T, Zeitlin J, Auger N, et al. Association of Race/Ethnicity With Very Preterm Neonatal Morbidities. JAMA Pediatr 2018;172:1061-9. [Crossref] [PubMed]
- Kono Y, Yonemoto N, Nakanishi H, et al. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks' gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatr Open 2018;2:e000211. [Crossref] [PubMed]
- Kunikata T, Saito A, Kawasaki H, et al. Zaitai 24 shuu miman no chiryou sennryaku ni kansuru zenkoku ankeito chousa houkoku Shuusaki Igakugakkai Zasshi 2013;49:83-7. (in Japanese).
- Morisaki N, Isayama T, Samura O, et al. Socioeconomic inequity in survival for deliveries at 22-24 weeks of gestation. Arch Dis Child Fetal Neonatal Ed 2018;103:F202-7. [Crossref] [PubMed]
- Rysavy MA, Li L, Bell EF, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med 2015;372:1801-11. [Crossref] [PubMed]
- Toyoshima K, Kawataki M, Ohyama M, et al. Tailor-made circulatory management based on the stress-velocity relationship in preterm infants. J Formos Med Assoc 2013;112:510-7. [Crossref] [PubMed]
- Isayama T, Mirea L, Mori R, et al. Patent Ductus Arteriosus Management and Outcomes in Japan and Canada: Comparison of Proactive and Selective Approaches. Am J Perinatol 2015;32:1087-94. [Crossref] [PubMed]
- Miyata M, Toyoshima K, Yoda H, et al. Extensive use of vasodilator agents and functional echocardiography to monitor extremely-low-birth-weight infants in Japan. J Neonatal Perinatal Med 2016;9:261-9. [Crossref] [PubMed]
- Toyoshima K, Kawataki M, Sato Y, et al. Serial echocardiographic assessment of mVcfc-ESWS relation in very low birth infant with perinatal complication Japan Soc Premature Newborn Med 2002;14:45-52. (in Japanese).
- Toyoshima K, Watanabe T, Kawataki M, et al. Usefulness of evaluating stress-velocity relation in extremely premature infants Japan Soc Perinatal Neonatal Med 2005;41:535-42. (in Japanese).
- Beltempo M, Isayama T, Vento M, et al. Respiratory Management of Extremely Preterm Infants: An International Survey. Neonatology 2018;114:28-36. [Crossref] [PubMed]
- Schmölzer GM, Kumar M, Pichler G, et al. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ 2013;347:f5980. [Crossref] [PubMed]
- Isayama T, Iwami H, McDonald S, et al. Association of Noninvasive Ventilation Strategies With Mortality and Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review and Meta-analysis. JAMA 2016;316:611-24. [Crossref] [PubMed]
- Miyake F, Ito M, Minami H, et al. The management of neonatal chronic lung disease in perinatal center in 2015. Japan Soc Neonatal Health Dev 2015;27:494.
- Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev 2014;5:CD001146. [PubMed]
- Hein-Nielsen AL, Petersen SM, Greisen G. Unchanged incidence of necrotising enterocolitis in a tertiary neonatal department. Dan Med J 2015.62. [PubMed]
- Kanai M, Sakazaki S, Yamada S, et al. Zaitai 22, 23 shuu shussei no chou souzanji ni okeru kyuuseiki kanri ni tsuiteno zenkoku chousa Shuusaki Igakugakkai Zasshi 2013;49:88-9. (in Japanese).
- Schanler RJ. Outcomes of human milk-fed premature infants. Semin Perinatol 2011;35:29-33. [Crossref] [PubMed]
- Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst Rev 2013.CD000504. [PubMed]
- Ashina M, Fujioka K, Totsu S, et al. Feeding interval and use of donor breast milk for very low birthweight infants: A nationwide survey in Japan. Pediatr Neonatol 2019;60:245-51. [Crossref] [PubMed]
- Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2014.CD002971. [PubMed]
- O'Connor DL, Gibbins S, Kiss A, et al. Effect of Supplemental Donor Human Milk Compared With Preterm Formula on Neurodevelopment of Very Low-Birth-Weight Infants at 18 Months: A Randomized Clinical Trial. JAMA 2016;316:1897-905. [Crossref] [PubMed]
- Binchy Á, Moore Z, Patton D. Feeding Intervals in Premature Infants ≤1750 g: An Integrative Review. Adv Neonatal Care 2018;18:168-78. [Crossref] [PubMed]
- Livingston MH, Shawyer AC, Rosenbaum PL, et al. Glycerin enemas and suppositories in premature infants: a meta-analysis. Pediatrics 2015;135:1093-106. [Crossref] [PubMed]
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014.CD005496. [PubMed]
- Kitajima H, Sumida Y, Tanaka R, et al. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 1997;76:F101-7. [Crossref] [PubMed]
- Toyoshima K. The management of circulation in acute phase of high-risk infants and quality improvement project in perinatal medicine [in Japanese]. Records of the 9th Annual Meeting of the Japanese Perinatal Circulation Management Study Group, Sendai, Japan 2011 (slides). Available online: https://blogs.yahoo.co.jp/nicu_sp25/11670434.html
- Bellù R, de WKA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews, 2008. Available online: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004212.pub3/abstract
- Hall RW, Kronsberg SS, Barton BA, et al. Morphine, hypotension, and adverse outcomes among preterm neonates: who's to blame? Secondary results from the NEOPAIN trial. Pediatrics 2005;115:1351-9. [Crossref] [PubMed]
- Ikeda T, Amizuka T, Ito Y, et al. Changes in the perfusion waveform of the internal cerebral vein and intraventricular hemorrhage in the acute management of extremely low-birth-weight infants. Eur J Pediatr 2015;174:331-8. [Crossref] [PubMed]
- Morisaki N, Helenius K, Kusuda S, et al. Variation in management of critically ill infants in preterm neonates: An international survey. May 9, 2017. Pediatric Academil Societies Meeting, San Francisco, CA (Abstract). Available online: https://2019.pas-meeting.org/past-programs/
- Hellmann J, Knighton R, Lee SK, et al. Neonatal deaths: prospective exploration of the causes and process of end-of-life decisions. Arch Dis Child Fetal Neonatal Ed 2016;101:F102-7. [Crossref] [PubMed]
- James J, Munson D, DeMauro SB, et al. Outcomes of Preterm Infants following Discussions about Withdrawal or Withholding of Life Support. J Pediatr 2017;190:118-23.e4. [Crossref] [PubMed]
- Boyce JM, Pittet D; Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep 2002;51:1-45, quiz CE1-4.
- Siegel JD, Rhinehart E, Jackson M, et al. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (2007). Available online: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html
- Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev 2012;88 Suppl 2:S69-74. [Crossref] [PubMed]
- Kawamura M, Nishida H. The usefulness of serial C-reactive protein measurement in managing neonatal infection. Acta Paediatr 1995;84:10-3. [Crossref] [PubMed]
- Sharma D, Farahbakhsh N, Shastri S, et al. Biomarkers for diagnosis of neonatal sepsis: a literature review. J Matern Fetal Neonatal Med 2018;31:1646-59. [Crossref] [PubMed]