Heart transplantation in survivors of childhood cancer
Introduction
The American Cancer Society estimates that in 2019, there will be over 1.7 million new cases of cancer in the United States, of which nearly 16,000 are among pediatric patients with leukemia being most common (1). Since the 1970s, the incidence of childhood cancer has increased by 0.6% per year, though the mortality rate has decreased from 6.3 per 100,000 in 1970 to 2.2 per 100,000 in 2016 (1). The overall 5-year survival rate for childhood cancer is greater than 80%, yielding approximately 450,000 survivors (2,3). Therapies for childhood cancer include chemotherapy, radiation, targeted therapy, immunotherapy, and surgery. Complications, such as anthracycline-induced cardiomyopathy and resulting heart failure have been well described, and cardiovascular disease is the leading cause of non-cancer morbidity in cancer survivors (3). Cardiac-related mortality in cancer survivors has decreased in association with the utilization of more contemporary heart failure therapies, but despite this improvement the incidence of severe heart failure in this population has increased (4). As such, there is a population of patients, pediatric and adult, who will develop end-stage heart failure refractory to therapy and who warrant consideration for heart transplantation as a result of cancer-therapy related cardiotoxicity (CTRC). Herein, we briefly provide background on CTRC, and review current data regarding transplantation in survivors of childhood cancer.
Overview of cardiotoxic therapies
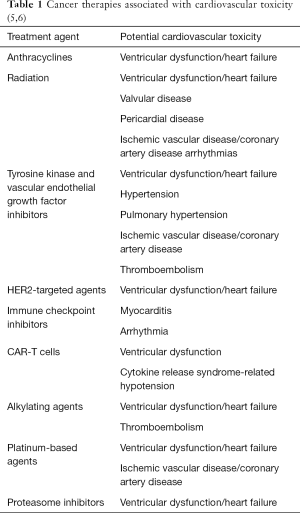
Many of the therapies used to treat pediatric cancers have known potential for CTRC (Table 1). This can include: symptomatic heart failure, asymptomatic ventricular dysfunction, acute myocarditis, hypertension, coronary artery disease, pericardial disease, valvular disease, arrhythmias, and thromboembolism (5,6).
Anthracyclines
Anthracyclines, such as doxorubicin, have been reported as the leading cause of cardiac complications in patients after cancer treatment. There is an incrementally increased risk of heart failure with higher cumulative anthracycline dose, and this risk persists beyond 45 years after treatment (3). In addition to high cumulative dose, a high single dose, underlying cardiac disease, mediastinal radiation, and younger age at treatment have been described as risk factors for development of cardiotoxicity (7). Reports vary regarding the exact cumulative anthracycline dose associated with cardiotoxicity, and has been reported as 250–500 mg/m2 in the literature (3,7). Over 50% of patients with anthracycline exposure have been reported to have some cardiac abnormalities on echocardiogram or gated nuclear angiography by 20 years after diagnosis (8). Even in those who do not develop symptoms of heart failure, abnormalities such as decreased left ventricular contractility, decreased left ventricular mass, and decreased wall thickness have been described (3). Among those who are affected, acute toxicity is defined as that occurring anytime within one week following administration of anthracycline based therapy, early-onset toxicity occurs after the first week but within 1 year post therapy, and late-onset toxicity is defined by presentation later than 1 year after therapy (3).
Targeted cancer therapies
Targeted cancer therapies, including tyrosine kinase inhibitors, vascular endothelial growth factor inhibitors, platelet derived growth factor inhibitors, and human epidermal growth factor-2 targeted therapies are demonstrated to have potential for CTRC (6). Tyrosine kinase inhibitors, e.g., sorafenib and imatinib, are used to target specific pathways within neoplastic tissue, particularly those affected by upregulated kinase enzymes (9). However, these medications also affect other kinase pathways, such as those in the myocardium, which can lead to left ventricular dysfunction. Prior studies looking at electron micrographs in adults who received therapy with imatinib have demonstrated abnormal myocytes, pleomorphic mitochondria with effaced cristae, vacuoles, and glycogen accumulation, among other changes (9). Clinically, this can lead to ventricular dysfunction, hypertension, pulmonary hypertension, and thromboembolism (6).
Radiation therapy
Radiation therapy can lead to tissue damage and fibrosis, as well as vascular injury. Fibrosis is caused by an increase in the total collagen deposition and increased proportion of type I collagen compared to type III, which alters myocardial compliance and leads to diastolic dysfunction(9). Chest radiotherapy with doses over 35 Gy, or over 15 Gy when in combination with anthracycline use, has been described as increasing the risk for development of cardiomyopathy (7). Patients who have received mediastinal radiation therapy during cancer treatment are at increased risk to develop cardiomyopathy, valvar dysfunction, constrictive pericarditis, and coronary artery disease. Transplantation becomes an option for these patients if they develop symptomatic heart failure or when pericardial and mediastinal adhesions are extensive, limiting the ability to perform necessary valvar or coronary artery bypass graft surgery (10).
Chimeric antigen receptor T-cell (CAR-T cells)
CAR-T cells target CD 19 cells, stimulating T cell proliferation, and leading to cytolysis and release of inflammatory cytokines. This can result in a systemic inflammatory response, also called cytokine release syndrome (11,12). A recent single center study looking at the cardiac profile of CAR-T cells showed approximately 25% of patients developed hypotension requiring inotropic support, of which 40% had systolic dysfunction by echocardiogram (10% of total patients in the study). By hospital discharge, a total of 7% of patients had either persistent cardiac dysfunction, or new cardiac dysfunction by echocardiography (12). Long-term effects are not yet known.
Prevention strategies
Medications to both prevent and reverse cardiotoxicity are used in the care of patients with childhood and adult cancers. Dexrazoxane has been used off label for decades in children and adolescents with leukemia undergoing therapy with anthracyclines to prevent development of cardiomyopathy (13,14). However, given some concern over its effect on therapies or development of secondary malignancy, it is not uniformly used. Medications to reverse cardiotoxic effects will be discussed below.
Cancer therapy-induced cardiomyopathy
For survivors of childhood cancers, the Childhood Cancer Survivor Study has been a wealth of information regarding risk to develop CTRC. For development of heart failure specifically, important risk factors included female gender, age less than 10 years at diagnosis, treatment era, and higher doses of anthracycline and/or radiation (15). Using these data, an online risk calculator (https://ccss.stjude.org/tools-and-documents/calculators-and-other-tools/ccss-cardiovascular-risk-calculator.html), focused on anthracycline and radiation therapy, is available to predict risk of heart failure, ischemic heart disease, and stroke by age 50 in survivors of childhood cancer (16).
There is no standard definition for cancer therapy-induced ventricular dysfunction, or cardiomyopathy. Suggested definitions range from a scale based on symptomatic heart failure to one that uses a percentage decrease in left ventricular ejection fraction (LVEF) (17). In adult literature, a common definition is a decrease in LVEF of ≥5% in symptomatic patients, and ≥10% in asymptomatic patients from the reported baseline to an EF of <55% (18).
Monitoring for cardiomyopathy
Guidelines are available for the imaging assessment of adult patients throughout cancer therapy (19). Both 3D LVEF and parameters of tissue deformation (myocardial strain and tissue Doppler) have demonstrated the ability to detect subclinical dysfunction in adult and pediatric studies (18,20,21). Despite this, the majority of current screening efforts in pediatric patients focus on shortening fraction and LVEF. In considering the guidance from various organizing bodies, the International Late Effects of Childhood Cancer Guideline Harmonization Group recommends echocardiography as the primary method of surveillance for assessment of left ventricular systolic function in patients previously treated with anthracyclines and/or chest radiation, to be initiated no later than 2 years after completion of therapy, and repeated a maximum of every 5 years. More frequent and lifelong surveillance can be considered in high risk survivors. It is also considered reasonable to use cardiac MRI for cardiomyopathy surveillance in at-risk survivors when echocardiography is technically suboptimal. In addition to imaging surveillance, this document also recommends that “assessment of cardiac blood biomarkers… in conjunction with imaging studies may be reasonable in instances where symptomatic cardiomyopathy is strongly suspected or in individuals who have borderline cardiac function during primary surveillance” (22).
Medical management of heart failure in CTRC
Patients with CTRC manifested as heart failure are treated in accordance with available heart failure guidelines (23,24). The goals of therapy are to diurese if needed, provide afterload reduction, and promote myocardial remodeling. Diuretics (e.g., furosemide, bumetanide, chlorothiazide) are used to manage symptoms of fluid overload: pulmonary edema, hepatomegaly, extremity edema.
Oral medications such as angiotensin converting enzyme inhibitors (ACE-I) (e.g., lisinopril, enalapril) or angiotensin receptor blockers (ARBs) (e.g., losartan) provide afterload reduction through inhibitory effects on the renin-angiotensin-aldosterone system. Both ACE-I/ARBs as well as aldosterone receptor antagonists (e.g., spironolactone, eplerenone) have myocardial remodeling effects (23,25). Beta blockers (e.g., carvedilol) are used when patients have symptomatic heart failure or asymptomatic heart failure with systemic LV dysfunction. Multiple studies in adults have demonstrated improvement and even normalization of LV systolic function in patients with chemotherapy-related cardiomyopathy who were treated with an ACE-I +/− beta blocker, with better results on dual therapy (26,27). There are limited data available in pediatric patients, however a clinical trial is currently enrolling to investigate the efficacy of beta blockers in preventing development of CTRC in survivors or pediatric cancers (28). Intravenous medications such as milrinone, epinephrine, dopamine, dobutamine and calcium may be used when oral therapies are insufficient.
Mechanical circulatory support in CTRC
Mechanical circulatory support is utilized when medical therapies are no longer adequate to support metabolic demand, and options range from extracorporeal membrane oxygenator support to ventricular assist devices (VAD). The type of VAD chosen for a patient varies based on patient size and institutional preference. A VAD can be considered as a bridge to recovery, bridge to eligibility or candidacy, bridge to transplantation, or as destination therapy (29). This may be particularly important in a patient who is still temporally close to cancer diagnosis or chemotherapy course and may not yet be appropriate for transplant consideration (see below). Multiple case reports and 2 small cohort studies have described use of left VAD as a bridge to recovery in adult patients with chemotherapy-induced cardiotoxicity (30-33). In both of the cohort studies, survival was similar to other cardiomyopathies, however in one, there was a higher need for subsequent right VAD support in the chemotherapy group (32). Data in pediatric patients are limited to a case report (34). With currently available techniques, a VAD may be explanted for myocardial function recovery, with ability for reinsertion if function should decline (35). This may be an appropriate strategy for patients with acute toxicity from chemotherapy that has a higher chance for recovery.
Eligibility for heart transplant in CTRC
Heart transplantation is considered only when other treatment options have been exhausted, and the patient’s current life expectancy is less than the median expected lifetime of a transplanted heart. Some of the first reports of transplantation for anthracycline-induced cardiomyopathy in pediatric patients date back to the early 1990s (36,37). According to the ISHLT 2016 guidelines for listing criteria for heart transplantation, in patients with a cancer diagnosis, eligibility for listing is dependent on a variety of factors such as type of neoplasm, response to provided therapies, and absence of metastases (29). There is no defined time period for observation between a diagnosis of remission and listing for heart transplant—rather evaluation and discussion between cardiologists and oncologists is necessary to determine eligibility and, if appropriate, optimal timing of listing. The majority of relapses for childhood cancer occur within 2 years after therapy is completed (38), thus defining a timeframe some centers currently use. Contraindications to transplant include active neoplasm, presence of metastases, ongoing therapy with either chemotherapy or radiation, active infection, and multisystem organ dysfunction.
Listing status
Listing status is based on the Organ Procurement and Transplant Network and the United Network of Organ Sharing guidelines, with pediatric statuses of 1A, 1B, 2, and 7 (inactive). Factors such as primary diagnosis and disease severity determine the listing status. Patients on the heart transplant waitlist with a diagnosis of anthracycline-induced cardiomyopathy wait longer for a heart than patients listed for other indications (39). This is in part due to status listing and that patients with anthracycline induced cardiomyopathy, even on high dose inotropes, will be listed 1B, compared to patients with congenital heart disease requiring the same inotropic support that qualify for status 1A. Additionally, patients who have undergone cancer therapy typically have received multiple blood products and transfusions which can lead to allosensitization and longer time to find an acceptable match. Of note, this listing schema is applicable only for those patients covered by the Organ Procurement and Transplant Network and the United Network of Organ Sharing, and therefore may not be universally generalizable.
Post-transplant outcomes in CTRC
Early period
After a heart transplant, the most critical period occurs in the first week, and requires intensive monitoring for bleeding, hemodynamic support, acute kidney injury in the setting of being on bypass, and with initiation of immunosuppressive medications. In patients who previously underwent radiotherapy, given the amount of mediastinal fibrosis and technical difficulties with the procedure, bleeding may be a significant concern.
Long-term outcomes
Adult data report similar 1-, 2-, and 5-year survival for patients transplanted for anthracycline-induced cardiomyopathy compared to patients with other nonischemic cardiomyopathies (40). Consistent with adult reports, the Pediatric Heart Transplant Study (PHTS) group reported no difference in graft survival among pediatric patients (<18 years of age) transplanted for anthracycline-induced cardiomyopathy compared to those with dilated cardiomyopathy. Among patients transplanted for anthracycline-induced cardiomyopathy, the most common cause of death was due to infection (39).
For patients who underwent mediastinal radiotherapy during cancer treatment, a unique process that can contribute to morbidity and mortality at any point is respiratory failure secondary to radiation induced lung disease (10).
Post-transplant morbidity in CTRC
Rejection
The frequency of treated rejection in the first year post-transplant has decreased over time. There is less reported rejection with the use of tacrolimus when compared to cyclosporine, regardless of whether the patient underwent induction therapy or no induction therapy (41). With respect to patients transplanted for anthracycline-induced cardiomyopathy, according to PHTS, the timing of the first episode of treated rejection and was similar in this group when compared to those transplanted for dilated cardiomyopathy (39).
Risk of cancer
In pediatric heart transplant recipients, survival free from any malignancy is 98% at 1 year and 91% at 10 years (41). Neither the use of induction therapy, nor the type of calcineurin inhibitor used in maintenance therapy is associated with development of post-transplant malignancy (42). Post-transplant lymphoproliferative disease (PTLD) is the most common malignancy to develop in all patients post-transplant, and it occurs in similar numbers in patients who undergo heart transplant for anthracycline-induced cardiomyopathy compared to dilated cardiomyopathy (39). The PHTS study from 2017 included 1,985 patients with dilated cardiomyopathy (79 with anthracycline-induced cardiomyopathy who were matched 2:1 with dilated cardiomyopathy for propensity-matched cohort analysis), and reported no recurrence of pre-transplant malignancy (39). The reported 5-year freedom from malignancy was 93% in patients with anthracycline-induced cardiomyopathy compared to 96% in patients with dilated cardiomyopathy (P=N.S.) (39). Despite a handful of case reports and case series documenting cancer recurrence or new malignancy after heart transplantation in pediatric patients with a history of cancer therapy (43-45), all malignancies in the anthracycline group from the PHTS report were due to PTLD.
Coronary artery vasculopathy
There has been an overall decrease in coronary artery vasculopathy or cardiac allograft vasculopathy over time. However, cardiac allograft vasculopathy is seen with increased frequency in those patients who have episodes of rejection in the first year post-transplant (41). There is no difference in the timing of development of coronary artery vasculopathy among patients transplanted for anthracycline-induced cardiomyopathy compared to dilated cardiomyopathy (39).
Summary
The number of survivors of childhood cancer is increasing, and with this comes an increase in the number of patients developing cardiotoxicity as a result of cancer therapies. Attention should be placed on close surveillance of cardiac function, with early intervention with medical and advanced therapies when cardiac dysfunction or symptoms of heart failure develop. A portion of patients will have continued decline in function requiring heart transplantation. Complications and overall survival are similar for patients transplanted for anthracycline-induced cardiomyopathy compared to those with dilated cardiomyopathy, demonstrating that this is a viable treatment option for this population. As other cancer therapies become more common, new cardiovascular toxicities are recognized. Whether heart transplantation will be appropriate for all patients with CTRC will require demonstration of similarly good outcomes to ensure proper allocation of organs.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- American Cancer Society. Cancer Facts & Figures. American Cancer Society, 2018.
- Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64:83-103. [Crossref] [PubMed]
- Trachtenberg BH, Landy DC, Franco VI, et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol 2011;32:342-53. [Crossref] [PubMed]
- Feijen EAML, Font-Gonzalez A, Van der Pal HJH, et al. Risk and Temporal Changes of Heart Failure Among 5-Year Childhood Cancer Survivors: a DCOG-LATER Study. J Am Heart Assoc 2019;8:e009122. [Crossref] [PubMed]
- Babiker HM, McBride A, Newton M, et al. Cardiotoxic effects of chemotherapy: A review of both cytotoxic and molecular targeted oncology therapies and their effect on the cardiovascular system. Crit Rev Oncol Hematol 2018;126:186-200. [Crossref] [PubMed]
- Ryan TD, Nagarajan R, Godown J. Pediatric Cardio-Oncology: Development of Cancer Treatment-Related Cardiotoxicity and the Therapeutic Approach to Affected Patients. Curr Treat Options Oncol 2019;20:56. [Crossref] [PubMed]
- Neudorf U, Schönecker A, Reinhardt D. Cardio-toxicity in childhood cancer survivors “Cure is not enough.” J Thorac Dis 2018;10:S4344-50. [Crossref] [PubMed]
- Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol 2004;22:820-8. [Crossref] [PubMed]
- Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013;128:1927-95. [Crossref] [PubMed]
- Saxena P, Joyce LD, Daly RC, et al. Cardiac transplantation for radiation-induced cardiomyopathy: The Mayo Clinic experience. Ann Thorac Surg 2014;98:2115-21. [Crossref] [PubMed]
- Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol 2019;16:45-63. [Crossref] [PubMed]
- Burstein DS, Maude S, Grupp S, et al. Cardiac Profile of Chimeric Antigen Receptor T Cell Therapy in Children: A Single-Institution Experience. Biol Blood Marrow Transplant 2018;24:1590-5. [Crossref] [PubMed]
- Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 2010;11:950-61. [Crossref] [PubMed]
- Lipshultz SE, Rifai N, Dalton VM, et al. The Effect of Dexrazoxane on Myocardial Injury in Doxorubicin-Treated Children with Acute Lymphoblastic Leukemia. N Engl J Med 2004;351:145-53. [Crossref] [PubMed]
- Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the childhood cancer survivor study cohort. BMJ 2009;339:b4606. [Crossref] [PubMed]
- Chow EJ, Chen Y, Kremer LC, et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol 2015;33:394-402. [Crossref] [PubMed]
- Bloom MW, Hamo CE, Cardinale D, et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure Part 1: Definitions, Pathophysiology, Risk Factors, and Imagin. Circ Hear Fail 2017;37:784-90.
- Thavendiranathan P, Poulin F, Lim KD, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J Am Coll Cardiol 2014;63:2751-68. [Crossref] [PubMed]
- Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911-39. [Crossref] [PubMed]
- Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: Comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol 2012;30:2876-84. [Crossref] [PubMed]
- Yu AF, Raikhelkar J, Zabor EC, et al. Two-Dimensional Speckle Tracking Echocardiography Detects Subclinical Left Ventricular Systolic Dysfunction among Adult Survivors of Childhood, Adolescent, and Young Adult Cancer. Biomed Res Int 2016;2016:9363951.
- Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015;16:e123-36. [Crossref] [PubMed]
- Kirk R, Dipchand AI, Rosenthal DN, et al. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary. J Heart Lung Transplant 2014;33:888-909. [Crossref] [PubMed]
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J 2016;37:2768-801. [Crossref] [PubMed]
- O’Connor MJ, Rosenthal DN, Shaddy RE. Outpatient Management of Pediatric Heart Failure. Heart Fail Clin 2010;6:515-29. [Crossref] [PubMed]
- Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981-8. [Crossref] [PubMed]
- Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-Induced Cardiomyopathy. Clinical Relevance and Response to Pharmacologic Therapy. J Am Coll Cardiol 2010;55:213-20. [Crossref] [PubMed]
- Armenian SH, Hudson MM, Chen MH, et al. Rationale and design of the Children’s Oncology Group (COG) study ALTE1621: A randomized, placebo-controlled trial to determine if low-dose carvedilol can prevent anthracycline-related left ventricular remodeling in childhood cancer survivors at high risk. BMC Cardiovasc Disord 2016;16:187. [Crossref] [PubMed]
- Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant 2016;35:1-23. [Crossref] [PubMed]
- Sayin OA, Ozpeker C, Schoenbrodt M, et al. Ventricular assist devices in patients with chemotherapy-induced cardiomyopathy: new modalities. Acta Cardiol 2015;70:430-4. [Crossref] [PubMed]
- Appel JM, Sander K, Hansen PB, et al. Left ventricular assist device as bridge to recovery for anthracycline-induced terminal heart failure. Congest Heart Fail 2012;18:291-4. [Crossref] [PubMed]
- Oliveira GH, Dupont M, Naftel D, et al. Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support: Outcomes from the intermacs registry (interagency registry for mechanically assisted circulatory support). J Am Coll Cardiol 2014;63:240-8. [Crossref] [PubMed]
- Thomas GR, McDonald MA, Day J, et al. A Matched Cohort Study of Patients With End-Stage Heart Failure from Anthracycline-Induced Cardiomyopathy Requiring Advanced Cardiac Support. Am J Cardiol 2016;118:1539-44. [Crossref] [PubMed]
- Krasnopero D, Asante-Korang A, Jacobs J, et al. Case report and review of the literature: the utilisation of a ventricular assist device as bridge to recovery for anthracycline-induced ventricular dysfunction. Cardiol Young 2018;28:471-5. [Crossref] [PubMed]
- Shugh SB, Tunuguntla HP, Jeewa A, et al. Reinitiation of centrifugal ventricular assist device support after failed attempt at cardiac recovery. J Heart Lung Transplant 2018;37:1041-2. [Crossref] [PubMed]
- Aricò M, Pedroni E, Nespoli L, et al. Long term survival after heart transplantation for doxorubicin induced cardiomyopathy. Arch Dis Child 1991;66:985-6. [Crossref] [PubMed]
- McManus RP, O'Hair DP. Pediatric heart transplantation for doxorubicin-induced cardiomyopathy. J Heart Lung Transplant 1992;11:375-6. [PubMed]
- Meister R, Katzenstein HM. Heart transplantation for anthracycline cardiomyopathy: Pump up the volume. Pediatr Transplant 2017;21:e12946. [Crossref] [PubMed]
- Bock MJ, Pahl E, Rusconi PG, et al. Cancer recurrence and mortality after pediatric heart transplantation for anthracycline cardiomyopathy: A report from the Pediatric Heart Transplant Study (PHTS) group. Pediatr Transplant 2017;21:1-8. [Crossref] [PubMed]
- Oliveira GH, Qattan MY, Al-Kindi S, et al. Advanced Heart Failure Therapies for Patients With Chemotherapy-Induced Cardiomyopathy. Circ Heart Fail 2014;7:1050-8. [Crossref] [PubMed]
- Rossano JW, Dipchand AI, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Nineteenth Pediatric Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1185-95. [Crossref] [PubMed]
- Rossano JW, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-first pediatric heart transplantation report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1184-95. [Crossref] [PubMed]
- Ward KM, Binns H, Chin C, et al. Pediatric heart transplantation for anthracycline cardiomyopathy: Cancer recurrence is rare. J Heart Lung Transplant 2004;23:1040-5. [Crossref] [PubMed]
- Mangat JS, Rao K, Kingston J, et al. Early Pediatric Anthracycline Cardiotoxicity: Managed by Serial Heart and Bone Marrow Transplantation. J Heart Lung Transplant 2007;26:658-60. [Crossref] [PubMed]
- Menon NM, Katsanis E, Khalpey Z, et al. Pediatric secondary chronic myeloid leukemia following cardiac transplantation for anthracycline-induced cardiomyopathy. Pediatr Blood Cancer 2015;62:166-8. [Crossref] [PubMed]