
Acute therapy of newborns with critical congenital heart disease
Introduction
Congenital heart defects (CHD) are the most common causes for acute cardiac failure in neonates. Critical CHD (cCHD) is defined by an associated systemic low cardiac output (LCO) requiring surgery or catheter-based intervention in the first year of life and has an incidence of approximately 15–25% of newborns with CHD (1,2). Despite considerable progress in the cardio-surgical, interventional and intensive care treatment of newborns, it remains a leading cause of infant morbidity and mortality with a mortality rate up to 25% in the first year of life (3-5). Therefore, early diagnosis and initiation of adequate treatment are of utmost importance.
The prevalence of CHDs is about 0.8–1% of all live births and is therefore one of the most common congenital malformations. Shunt lesions, representing the largest proportion of CHD, remain usually stable during the neonatal period, symptoms of congestive heart failure do not manifest themselves until the pulmonary vascular resistance (Rp) decreases within the first 3 months.
cCHD on the other hand, lead already to serious but unspecific symptoms in the newborn period. The lesion determines the time of the onset of symptoms, which can occur immediately after birth or within the first week of life.
The distribution of cCHD differs from the distribution of CHDs in general. Left heart obstructions have the largest share with 30–40%, followed by complete transposition of the great arteries (approx. 30%) and right heart obstructions (20–30%) (6,7). Sometimes, but much more rarely, congenital cardiomyopathy or arrhythmia are the underlying reason for acute cardiac failure in the first week of life.
The detection rate of critical heart defects has improved over the last decades, but is still inadequate despite improved prenatal diagnostics and postnatal clinical examinations. The sensitivity of prenatal diagnostics for critical heart defects is reported to be up to a maximum of 51% (8,9).
The sensitivity of the clinical examination in the first days of life for detection of cCHD is less than 50%. This may be due to a possible symptom-free interval (“diagnostic gap”) due to the delayed change from fetal to neonatal circulatory physiology (10). Pulse oximetry screening is a proposed method by which this postnatal “diagnostic gap” should be reduced (10-12). However, pulse oximetry screening does not replace a careful examination. Even a hardly noticeable cyanosis is accompanied by tachypnea (leading symptom) of the newborn, which is often recognized and reported by the parents.
Fetal cardiovascular physiology with a right-to-left shunting foramen ovale (FO) and ductus arteriosus (DA) with a high Rp and low systemic vascular resistance (Rs) allows to a large extent a normal fetal development (13) for the majority of cardiovascular malformations as long as gas exchange is guaranteed by the placenta. Umbilical clamp placement terminates the placental circulation, including the maternal extracorporeal oxygenation. The transition from the fetal parallel to the adult serial circulation is forced by the quality of postnatal pulmonary gas-mixture. Umbilical cut increases Rs and related to lung expansion decreases Rp over days to weeks, achieving low resistance of almost adult level after 6 weeks to 3 months (13). This phase of postnatal circulatory transition is very variable and influenced by functional and anatomical considerations. Open DA and FO can lead to inconspicuous clinical findings in newborns with critical CHD. This period is therefore also referred to as “diagnostic gap” (10). Transition to a serial circulatory physiology might lead to a life-threatening condition in the presence of critical heart defects (13). Several factors are responsible for postnatal duct closure: termination of the placental circulation and with it the main source of prostaglandin production; switchover from the intrauterine to extra-maternal environment with increase of pH and paO2; rise of the pulmonary prostaglandin dehydrogenase production.
The anatomy of FO enables a right-to-left, but not a left-to-right shunt, FO closure is related to a rise of the left ventricular afterload and increase of the left atrial pressure (LAP) usually to a level of 3–5 mmHg higher than the right atrial pressure (RAP). Contraction of the DA is finally followed by obliteration; in duct-dependent CHD, DA closure leads to a life-threatening situation due to compromised systemic blood flow (Qs). Restoring the parallel circulation with an arterial and/or atrial communication is therefore mandatory for most cCHD.
Medical reopening of a closed or narrowed DA by infusion of prostaglandin E1 (PGE1) is possible in any pediatric institution; in general, the lowest effective dosage which induces as few side effects as possible should be sought; an almost closed duct responds in most cases to continuous infused PGE1 in a dosage of 20 (rarely 50) ng/kg/min; high dosages of PGE1 have to be rapidly reduced after effective re-opening, in particular in CHD with duct-dependent systemic blood flow; a “prophylactic dosage” of 5–10 ng/kg/min remains usually effective with a low incidence of side-effects. Specialized pediatric heart centers are able to perform duct-stenting as an alternative to less effective PGE1 infusion or in non-responders (e.g., shock patients with metabolic acidosis).
A further measure, requiring also a pediatric cardiologist, is the manipulation of the atrial septum; whether enlargement of an ineffective (restrictive) FO or by creation of a new atrial communication. The technical arsenal ranges from a Rashkind maneuver (14) to static ballooning or stent placement; Rashkind procedures can be performed by ultrasound guidance on the pediatric intensive care unit. Extreme restrictive or a complete closed atrial septum require an advanced transcatheter intervention in specialized centers with a high level of expertise.
The parallel pulmonary and systemic circulation is a sensitive construct; massively influenced by the diameter of arterial communication and the ratio of Rp to Rs.
Clinical impairment might be the result of LCO based on pulmonary hyper-circulation. This is especially true for cCHD with left heart obstruction when the systemic flow depends on a high Rp and thus a postnatal right-left shunt on the ductal level. Diminished organ perfusion is clinically observed by tachypnea, high arterial oxygen saturation (SaO2) combined with a pale skin and a narrow systemic blood pressure amplitude. Considering these pathophysiological sequences, underlines the danger of using mean arterial pressure instead systolic and diastolic values for therapy monitoring and guiding in these particular high-risk patients.
An imbalance of the parallel circulation is often the result of inadequate administration of oxygen, application of excessive prostaglandin dosages, unnecessary mechanical ventilation, hyperventilation and inadequate bicarbonate administration.
Indiscriminate use of catecholamines with its systemic vasopressor effects and associated tachycardia resulting in increased oxygen consumption (VO2) and decreased diastolic filling time can also lead to an imbalance of the parallel circulation.
Considering a normal lung function, measurement of systemic arterial and venous oxygen saturation allows estimating the ratio of pulmonary and systemic blood flow (Qp/Qs) (15,16).
This ratio is calculated according to Fick’s formula:
Qp/Qs = (SaO2 − SvO2)/(SpvO2 − SpaO2)
Sa = arterial saturation (post-ductal), Sv = mixed venous saturation, Spv = pulmonary venous saturation (in healthy lungs 100% is assumed), Spa = pulmonary arterial saturation (corresponds to arterial saturation in a parallel circulation with complete mixing).
In most clinical settings the primary access for saturation measurements is restricted to arterial or transcutaneous oxygen saturation values.
Considering the above-mentioned formula, a post-ductal target saturation between 75% and 85% (= mild clinical cyanosis!) can in most cases be assumed to be equivalent to a balanced Qp/Qs.
CHD with a high-risk for critical hemodynamics are divided into following groups:
- CHD with duct-dependent systemic blood flow (SBF)
- Critical aortic stenosis;
- Critical aortic coarctation;
- Interrupted aortic arch;
- Hypoplastic left heart syndrome (HLHS) and hypoplastic left heart complex (HLHC).
- CHD with duct-dependent pulmonary blood flow (PBF)
- Critical pulmonary stenosis;
- Pulmonary atresia with intact septum;
- Pronounced types of Fallot’s tetralogy and pulmonary atresia with VSD;
- Atresia of the tricuspid valve with pulmonary stenosis/atresia;
- Ebstein’s anomaly or tricuspid valve dysplasia with pseudo- or anatomical atresia of the pulmonary valve.
- Complete transposition of the great arteries (d-TGA)
- Classified as non-mixture or inadequate shunting at atrial, ventricular or duct level.
- Others
- Total anomalous pulmonary venous return with obstructed pulmonary vein flow;
- Common arterial trunk with significant truncal valve regurgitation;
- Univentricular heart with imbalance of systemic or pulmonary circulation.
Complex CHD with a high-risk for congestive heart failure and cardiogenic shock need to be assumed on grounds of the patients’ history, which includes fetal echocardiography, clinical examination (tachypnea, chest palpation pulse status of all extremities as well as blood pressure and pulse oximetry), followed by immediate echocardiography determining cardiac function and morphology.
Further diagnostic procedures should be performed by indication.
CHD with duct-dependent SBF (left-heart obstruction)
Critical obstructions of the left heart, including the ascending aorta and aortic arch result in a duct-dependent systemic perfusion. As mentioned above, “critical” means impaired hemodynamic conditions as consequence of the cardiac obstruction leading to systemic LCO; an open arterial duct is the most important component avoiding LCO. All left ventricular structures might be affected as single or multiple lesions; ranging from critical inflow or outflow obstructions (mitral valve stenosis, aortic valve stenosis, aortic coarctation, interruption of the aortic arch, HLHS or HLH as Shone-complex or borderline left ventricle). However, in spite of left heart functional failure an acceptable SBF can, duct-dependent, be achieved.
Depending on the morphological or functional severity, the right ventricle has to take over the workload, supplying the pulmonary and systemic circulation completely or partially via an open duct.
In the absence of antegrade perfusion (highly impaired LV function, aortic atresia, HLHS) coronary and cerebral perfusion depends on retrograde perfusion into the ascending aorta via the arterial duct.
In general, hemodynamics of such a pathophysiology are very fragile; already a slight obstruction of the DA, all conditions decreasing Rp or the persistence of a relevant pre-ductal aortic coarctation are immediately associated with life-threatening brain or heart ischemia.
As obstructions of the left heart usually lead to a forward failure of the left ventricle, at least at the beginning, the pressure in the left atrium is increased and accordingly a left-right shunt is present via atrial communication. If a significant atrial left-right shunt is present, oxygen saturation is relatively high in the pulmonary artery and consecutive in the descending aorta. Cyanosis is not necessarily clinically detectable.
Postnatal screening by pulse oximetry has to be interpreted always in context of the clinical picture. Single cases are known, less published, in whom high SaO2 values obtained at the lower extremities were misinterpreted as beneficial and tachypnea as leading symptom of a severe impaired patient was not acknowledged resulting in the death of the patient.
If a shunt is missing or if the atrial communication is restrictive, blood will dam back into the lungs and can lead to passive pulmonary edema. Restriction at the atrial level can therefore lead to hypoxemia with cardiovascular shock immediately after birth. FO restriction or closure during fetal life might be associated with untreatable lymphangiectasia; even an early fetal intervention does often not solve this life-threatening condition.
Somewhat similar, is a restricted flow of the pulmonary veins in the setting of a total anomalous pulmonary venous return (TAPVR) (see also below).
Symptoms
Symptoms are related to the degree of lesion of the specific CHD. The main symptoms range from signs of acute cardiac failure up to the complete picture of a cardiogenic shock. Tachypnea is the early and therefore leading symptom; cyanotic patients with duct-dependent SBF are usually stable because of a sufficient systemic perfusion. Patients are referred due to acute cardiogenic shock or any other degree of cardio-vascular failure. Classical symptoms of chronic heart failure with feeding and weight gain problems are rare and only associated with some acquired cardio-vascular conditions of the newborn as cardiomyopathy, myocarditis, ischemic heart failure or initially less relevant CHD.
In this context, it has to be mentioned, that there are age-associated reasons for heart failure. Newborns with critical aortic valve stenosis are mostly symptomatic within the first week of life, a critical aortic coarctation mostly within the first 4 weeks with a typical history of a “3-week-old baby referred Friday afternoon, after failure of a sepsis therapy.” Considering the unspecific symptoms of congestive heart failure, sepsis or even fulminant sepsis is the differential diagnosis of choice; but therefore, also the initially most erroneous diagnosis.
In a newborn whose condition worsens clinically in the first days of life, the differential diagnosis of a critical heart defect must always be considered in addition to the suspected diagnosis of sepsis. This means that in case of doubt a therapy with PGE1 in a low dosage (see below) can always be initiated!
Treatment
Continuous infusion of prostaglandin-E1 in low dosage of 5–10 ng/kg/min is never wrong. Treatment should immediately be started in any newborn, whose condition worsens clinically in the first days of life; in particular, if the cardiovascular system cannot be immediately analyzed by echocardiography. This recommendation is also true, when sepsis is suspected or even confirmed by laboratory data.
Basic therapeutic goals are the stabilization of the patient with restoration of the fetal, parallel circulatory physiology as well as the optimization of the pulmonary and systemic resistance.
Key points in the treatment of suspected or confirmed CHD with duct-dependent SBF:
- Reopening of the arterial duct (initially high PGE1 dosage, rapid reduction);
- Keeping DA patent [“prophylactic” dosage of 5 to 10 ng/kg/min];
- Reduction of diastolic left to right shunt across a non-obstructive right-left-shunting DA;
- Avoidance of inadequate measures resulting in the reduction of the Rp;
- Reduction of Rs without jeopardizing adequate perfusion pressures;
- Rapid transfer of the patient to a pediatric cardiac center.
Considering the pivotal role of PGE1-treatment in neonatal critical CHD, the lowest effective dosage is mandatory, when used in any patient with DA-dependent SBF. High dosages of PGE-1 can easily compromise the hemodynamics by its side-effects: decrease in Rp, apnea with the need of oxygen or intubation and mechanical ventilation, fever and tachycardia with associated high oxygen consumption of the myocard and body as well as low diastolic filling time.
A non-obstructed, right-to-left shunting DA can be kept open with a prostaglandin dosage of 5–10 ng/kg/min, in premature babies even with a dosage of 1–2 ng/kg/min.
Stenting of the duct has to be considered, whenever, higher prostaglandin dosages are initially necessary or catecholamines beyond the use of inodilators as milrinone are required.
In general, administration of oxygen should be avoided or only be administrated with the objective of an arterial oxygen saturation of around 80%; targeting a Qp/Qs ~1 (SaO2 80% if SvO2 is about 60%). Treatment of a metabolic acidosis should be sought by improving systemic blood flow because the acidosis is usually systemic flow dependent; related to an obstructed duct or pulmonary run-off (meaning pulmonary hyper-perfusion at the expense of systemic perfusion). A vicious circle with decrease of pulmonary resistance and increase of systemic resistance must be prevented.
The systemic blood flow depends on a low systemic resistance, which is not synonymous with blood pressure, but adequate systolic and diastolic blood pressures. A critical organ perfusion pressure (diastolic pressure—central venous pressure >20 mmHg) should not be undercut (17).
In any critical care patient, volume depletion must always be ruled out, also in conditions with impaired ventricular function and pulmonary edema; in this case, however, volume, if missing (small, or collapsing inferior caval vein, low central venous pressure) must be administered cautiously. In cardio-vascular compromised univentricular hearts, hemoglobin level should be between 12–14 g/dL, in case of Hb <11 g/dL, blood transfusion should preferably be considered as volume replacement.
Pulmonary edema in HLHS, excluding a pulmonary hyperperfusion, is often caused by a pulmonary venous or atrial congestion. In contrast, systemic venous congestion is usually related to ischemic myocardial failure or valvular (tricuspid) dysfunction.
Underlying causes should be immediately investigated. Myocardial dysfunction should be treated preferentially by inodilators; Phosphodiesterase inhibitors (e.g., Milrinon®) are particularly suitable for this purpose. They have a very favorable mode of action by supporting myocardial function and simultaneously reducing afterload and should therefore be considered at an early stage (18).
Catecholamines should be used with caution; the side-effects—elevated heart rate, reduced diastolic filling time, increased myocardial oxygen consumption and negative influence on the Rp/Rs ratio—should always weight against the desired effects.
Nowadays, transcatheter and/or cardiosurgical procedures are not only used for definitive or palliative repair, but also for effective high-urgency measures. Therefore, the newborn should be immediately transferred to a full-equipped pediatric heart center (see interventional therapy).
CHD with duct-dependent PBF (right-heart obstruction) & transposition of the large vessels (TGA)
Cyanosis is the main and common symptom of transposition of the great arteries (d-TGA), and heart defects with duct-dependent PBF.
d-TGA is defined by atrial-ventricular concordance and ventricular-arterial discordance and therefore consists of two parallel but separate circuits. An efficient atrial defect is the most important communication avoiding cyanosis with consecutive life threating hypoxic cardiovascular dysfunction. This is especially true in simple d-TGA, simple means in this context without further malformations (75% of the cases) (19).
An open DA alone is not able to route both sufficiently deoxygenated blood to the pulmonary tract and at the same time prevent life-threatening hypoxemia in the systemic circulation. The systemic circulation is enriched with oxygen via a left-right shunt at the atrial level. An open duct increases the pulmonary blood flow, keeps the sub-pulmonary left ventricle longer under high pressure level and contributes to the left-right shunt by contributing higher volumes via the pulmonary veins if there is a sufficient atrial communication. Accordingly, a persistent FO or a very restrictive atrial septal defect leads to a pronounced cyanosis as well as to tachy- and dyspnea up to pulmonary edema (19). Consecutive tachypnea and stress-hormone related tachycardia aggravates the mismatch of oxygen delivery (DO2) and oxygen consumption (VO2) leading to a vicious circle.
CHD with duct-dependent PBF
Morphological obstructions of the right ventricular inflow or sub-pulmonary outflow tract have obligatory intracardiac communications and in most cases an arterial-pulmonary vascular communication in form of an open duct or major aorto-pulmonary collaterals (MAPCA’s). Some rare anatomical constellations do not need any surgical or catheter-based interventions during the neonatal period, such as a tricuspid atresia (Tricuspid atresia Ib, IIB) or pulmonary atresia with VSD with MAPCA associated balanced pulmonary to systemic blood flow.
In critical pulmonary stenosis or pulmonary atresia with an intact septum the entire cardiac output has to be directed into the sub-aortic ventricle via an intra-atrial communication to the left atrium. In these cases, a restrictive atrial communication is rare. If it is present, the patient presents signs of a systemic venous congestion including hepatosplenomegaly.
If the arterial duct is the only source for the pulmonary perfusion, the central cyanosis increases with increasing occlusion of the duct, leading to death due to hypoxemia without intervention.
Symptoms
Cyanosis is the leading symptom of d-TGA and right heart obstructions with duct-dependent pulmonary flow. Usually, the cyanotic newborn has compensated hemodynamics with a sufficient DO2. Therefore, the cyanotic newborn is vivid and has almost no clinical impairment. Despite an apparent vivid newborn, but because of the apparent cyanosis, breathing support, oxygen administration and unnecessary intubation and mechanical ventilation are often performed as first measures. The diagnosis of a cyanotic cardiac heart disease is frequently made after and because these measures do not result in a significant improvement of the saturation. Cyanosis becomes critical when arterial oxygen saturation decreases continuously on less than <60% with a paO2 <25 mmHg or at the latest when metabolic acidosis becomes obvious (17). Even today, the following guiding principle is still valid: “The cause of a cyanotic but vital newborn is a heart defect until the opposite is proven”.
Initial therapy
Therapy has to be based on an assumed better on an established diagnosis; first therapeutic steps should be accompanied by echocardiography as soon as possible.
The basic therapeutic goal is to keep the cyanosis at a level which does not threaten the child and ensures normal somatic and neurological development. Usually, the systemic cardiac output increments with the increase of cyanosis (until the anaerobic threshold is reached). Therefore, there is rarely a hypoxemia-related cerebral damage (DO2 = CO × CaO2. DO2, oxygen delivery; CO, cardiac output; CaO2, arterial oxygen content).
In case of a d-TGA, a balloon atrial-septostomy (Rashkind procedure) is still the most effective emergency measure, apart from the start of a PGE1 infusion. However, based on the experience of especially Asian countries, in which a Rashkind procedure is not available or not performed, a hypoxic newborn with d-TGA can be sufficiently treated by utilizing immediately cardio-pulmonary bypass (CPB) followed by an arterial switch operation (ASO). A little delayed reperfusion time can be useful for further recovering.
A newborn with d-TGA should be delivered to a pediatric heart center (PHC), if possible. In case of a postnatal diagnosis, transfer to a PHC should be immediately prepared. If a Rashkind-procedure is feasible, it can be performed on any pediatric/neonatal intensive care unit, even by umbilical venous access and also by using a diagnostic balloon catheter (wedge-catheter) instead of an atrioseptostomy catheter as a transient measure.
As mentioned above, without echocardiographic availability as an immediate diagnostic tool, continuous PGE1-infusion in a low dosage of 5–10 (up to 20) ng/kg/min can be applicated in any cyanotic newborn without additional risk, with the chance for immediate improvement in case of duct-dependent cardiovascular function.
Therefore, the following therapeutic measures have to be considered in case of d-TGA:
- Reopening/keeping the arteriosus duct open by PGE1-infusion;
- Balloon atrial-septostomy (d-TGA with restrictive atrial communication);
- Rarely, catecholamines, i.e., nor-epinephrine can become necessary in combination with high dosed prostaglandin to increase systemic afterload and to decrease Rp, and therefore improving pulmonary perfusion. It should be considered that the use of catecholamines is accompanied by an increased oxygen consumption (heart rate) and can negate the benefit of the saturation improvement and even lead to metabolic acidosis.
However, newborns with duct-dependent PBF tolerate the application of higher dosages of prostaglandin, oxygen and mechanical ventilation quite better than patients with duct-dependent SBF. - Prophylactic measures as mechanical ventilation, inadequate oxygen administration, high dosages of prostaglandin should be avoided, even for helicopter transport;
- A lack of volume must always be ruled out, since intravascular volume depletion increases cyanosis (rare exception: presence of pulmonary edema). Diuretics are therefore rarely indicated;
Depending on the severity of cyanosis, volume should be generously administered in a dose of 10–30 mL/kg as a challenge dose. - However, a well-prepared rapid transfer of the patient to a pediatric cardiac center is the most important measure;
- If a prostaglandin-refractory duct stenosis is present, a stent implantation in the arterial duct or aorto-pulmonary shunt surgery are therapeutic emergency options.
Complex heart defects and rare diseases
There are further complex CHD, which might be associated with an acute decompensation in the postnatal period.
In principle, these complex heart defects exhibit usually the same clinical picture as the here described cCHD either with duct-dependent systemic or pulmonary perfusion.
On basis of the leading symptoms the clinician should be able to initiate the first adequate measures until a definitive diagnosis exists and a specific therapy is possible.
Nevertheless, one heart defect should still be mentioned briefly: total anomalous pulmonary venous return (TAPVR) with obstruction. These patients are seriously ill with pronounced cyanosis and tachy- and dyspnea, which can develop within the first hours of life. In addition, pulmonary edema develops rapidly, in particular when prostaglandin is applicated. The clinical picture can resemble a respiratory distress syndrome and thus delay the diagnosis. X-ray images show signs of pulmonary congestion with pulmonary edema or lymphangiectasis (white lung). These patients cannot really be stabilized by conservative therapy measures. It is a rare heart defect, so it is important to not to forget it when in doubt. The therapy consists in the fastest possible transfer to cardiac surgery or cardiac catheter intervention, each time delay worsens the prognosis dramatically (20).
Opposite to newborns with white lung syndrome, a transparent (black lung) X-ray, needs also immediately to be diagnosed, but differently treated; in case of a postnatally restricted pulmonary perfusion, as seen in persistent pulmonary hypertension of the newborn (PPHN). The ratio of Rs to Rp should be improved by decreasing Rp, by increasing Rs or by both measures simultaneously, with the goal to achieve a sufficient systemic oxygenation. Contrary to idiopathic pulmonary arterial hypertension in older children and adults, patients with PPHN benefit from an open atrial communication and/or patent arterial duct. Should the degree of cyanosis be too severe because of right-left shunts on two level, one communication, preferentially the atrial communication, should be closed, if possible; depending on the atrial septum morphology closure can be performed by a transcatheter approach. A closed DA might be associated with supra-systemic pulmonary arterial pressures and failing right ventricle; reopening of the DA by continuous infusion of PGE1 is therefore an efficient measure also in newborns with PPHN without any structural heart defect (21).
A special case scenario might be associated with a pre- or postnatal symptomatic Epstein-anomaly considering a truly or pseudo-atresia of the pulmonary valve. Some therapeutic rules are summarized: the duct dependent newborn needs to be treated by continuous infusion of PGE1; the pulmonary vasodilative effect is desirable. Application of inhaled NO might help to differentiate between a true or pseudo atresia of the pulmonary valve. Transcatheter trials to open the pulmonary valve are forbidden as long as the right ventricular pressure is lower than the pulmonary artery pressure, because this would lead to a pulmonary run-off up to the atrial level due to the associated incompetent Epstein tricuspid valve. Surgical procedures as neonatal Epstein repair or perforated closure of the Epstein valve with or without right heart tissue resection are associated with high mortality rates and in most cases only an ultima ratio, if the patient cannot be stabilized and transferred.
Finally, neonates born with a severely impaired left ventricular function with limited systemic perfusion might benefit from a left-to-right shunting via an atrial communication and right-to-left shunt at the arterial level. Prenatal or neonatal myocarditis, sequelae of a neonatal myocardial infarction caused by thrombo-embolization or insufficient coronary perfusion need to be treated by appropriate intensive medical measures; however, a patent or re-opened DA can be a life-saving option treating bi-ventricular dysfunction and might even be able to avoid extra-corporal membrane oxygenation.
Transcatheter therapy
Transcatheter interventions are nowadays decisive in the treatment of neonates with critical CHD (22). Surgical-interventional procedures are the most effective therapeutic measure apart from immediate prostaglandin application, in particular if the latter is ineffective. Live-threating hypoxemia can be effectively treated by atrial septum manipulation; compromised systemic blood flow due to duct obstruction by transcatheter stenting, pulmonary run off by bilateral surgical pulmonary banding and critical aortic coarctation by balloon angioplasty as bridging procedure to surgical repair.
The most well-known and oldest emergency procedure is the balloon atrial septostomy (BAS), also known as Rashkind procedure (14,23). In neonates with d-TGA and restrictive FO/ASD, BAS improves the left to right shunt and consecutively oxygen saturation. Besides d-TGA, BAS can be necessary in newborns with HLHS and restrictive ASD or rarely in patients with tricuspid atresia.
BAS is nowadays more often performed under echo guidance than as fluoroscopic procedure (23). In particular in d-TGA with a normal sized left atrium the procedure is routinely carried out in the intensive care unit. Vascular access is gained by a 5 of 6 Fr sheath in the femoral or umbilical veins. The atria are displayed by echocardiography and the balloon septostomy catheter tip is visualized as it enters the left atrium. After satisfactory localization of the catheter tip the balloon is filled with saline (1–2 mL according to the balloon specifications) and abruptly drawn back into the right atrium (“maximum force, defined distance”). The procedure may be repeated two or more times until a satisfactory result is achieved. Usually the saturation will improve rapidly and echocardiography will confirm unrestricted left-to-right shunt.
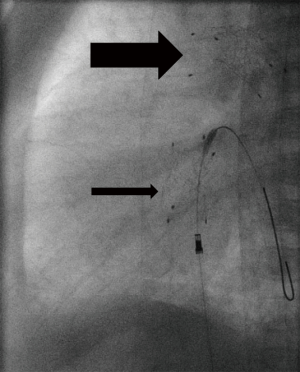
In case of a restrictive atrial septum in HLHS, atrial decompression tends to be more complicated due to an associated small left atrial dimension, atypically positioned PFO or even complete closed atrial septum; requiring fluoroscopic guidance by an experienced interventionalist (24). The challenging cross of the PFO or small restrictive ASD needs oftentimes a special technique, as by utilizing a 2.5 curved right Judkins (4 Fr) together with a floppy 0.014 inch coronary wire; once the wire is crossed and curved in the LA or directed to one of the pulmonary veins, the Judkins-catheter is than advanced over the floppy-wire exchanging the floppy to a stiff coronary guide wire to perform a Rashkind procedure or gradual static balloon dilatation of the atrial septum. Sometimes the balloon technique including the use of cutting balloons remains insufficient; in these cases stent implantation is required. In our institution, we use nowadays self-expandable stents (Sinus SuperFlex DS; 8×15 or 9×15 or 18 mm) instead of balloon expandable stents. The open-cell design of self-expandable stents reduces the risk of stent embolization, and if it should occur, the stent design facilitates percutaneous retrieval (Figure 1). In case of a closed atrial septum, perforation of the septum is performed by Brockenbrough trans-septal needle technique or by radio-frequency technique.
Interestingly, nature adapts the morphology of an arterial duct to postnatal function. In CHD with duct-dependent SBF, the arterial duct remains wide open; depending on the additional amount of the passing cardiac output the duct is often larger than normal; in extreme, the total amount of the body’s blood flow has to pass through the DA. Body weight dependent, the DA diameter ranges between 6 to 9 mm and is usually located at a typical position between pulmonary arterial bifurcation and descending aorta. The arterial duct is angiographically delineated by repetitive hand-injection of contrast medium through a 2.5 right Judkins (4 Fr) catheter before and additionally after a floppy 0.014 inch coronary wire is passed through the duct.
In Europe, self-expandable stents with CE-mark for duct stenting of newborns with duct-dependent circulations are available, which can be advanced through a 4 Fr vascular access, preferably the femoral artery. For DA-stenting of patients with duct-dependent SBF, the stent diameter should be between 7 and 9 mm, mostly 8 mm; stents of less than 7 mm should be avoided. Early duct obstructions have to be considered despite or because of DA stenting; close in-hospital and inter-stage follow-up observations are mandatory. From a technical point of view, DA-stents should not have any, even slight kinking or insignificant obstruction. In case of obstructions the stent should be redilated by a Tyshak-balloon catheter, which can also be advanced through a 4 Fr sheath. Despite availability of variable stent lengths between 12 to 24 mm, it is not unusual, that two or three stents need to be placed in telescope technique to avoid that parts of the duct remain uncovered. Additionally, it has to be considered, that any manipulation including the stent-material irritates the duct tissue and therefore favors its constriction.
A duct stenosis might be a necessary requirement for balloon-expandable stents to avoid embolization. If self-expandable stents are used in a stenotic duct, prostaglandin should be stopped shortly before and during the procedure, but continued for a minimum of 24 hours after the procedure. Additionally, heparin 300 U/kg/day should be continuously administered. An antiaggregating drug is not used routinely except if more than one stent is implanted either within the duct, the atrial septum or aortic isthmus. We prefer clopidogrel as an oral antiaggregating drug. High attention is necessary in patients with an obligatory retrograde aortic blood flow. If possible the isthmus region of the descending aorta should not be crossed by the duct stent; if this is not possible, it should be avoided that struts of two stents cross this vascular area. A morphological even slightly obstructed isthmus should be balloon dilated before the duct is stented. If significant aortic coarctation is present after duct stenting, the coarctation should be stented additionally utilizing a short 5 or 6 mm balloon- or self-expandable stent.
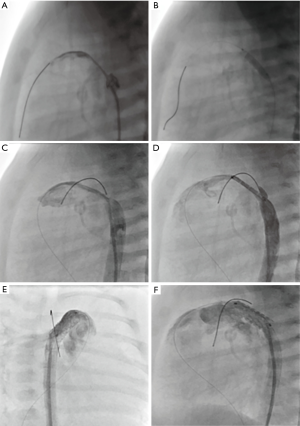
Cases of prostaglandin-refractory duct stenosis, as seen in newborns admitted with cardiogenic shock and metabolic acidosis, should be immediately transferred to the catheter laboratory for stent implantation; immediate duct stenting is the most effective and quickest therapy (24,25). Requiring however, a well-prepared cardiac team and a sufficiently equipped catheter stock (Figure 2A,B,C,D,E,F).
CHDs with right heart obstructions and duct-dependent PBF are related to quite different duct morphologies. The arterial duct has mostly not a uniform diameter, its widths range between 2 to 6 mm, the most narrow part is mostly at the area of the pulmonary insertion. With the exception of newborns with pulmonary atresia and intact ventricular septum, the duct mostly winds from the inner curvature of the aortic arch or rises from head-neck vessels. In rare cases one lung is supplied by an arterial duct, while the other lung is perfused from a major aorto-pulmonary collateral vessel.
Duct-stenting should only be performed if the procedure is in consideration of the duct morphology feasible and a therefore a minimal invasive alternative to a surgically performed aorto-pulmonary shunt.
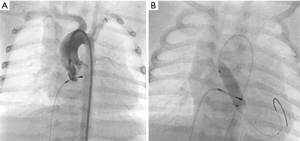
Critical aortic valve stenosis as well as aortic coarctation are defined by its associated LCO and failing heart function. The pressure gradient is usually not related to the severity of the lesion. Obstructions of the DA aggravate heart failure. Palliation strategies consist of re-opening the duct and/or of balloon valvular- or angioplasty. Balloon valvuloplasty needs guidewire crossing of the highly stenosed valve; several techniques and tips are described. A retrograde approach is the most common method (Figure 3A,B). In general, aortic valvuloplasty should be performed under rapid pacing to ensure balloon stability during inflation. The rapid pacing technique is usually not recommended in neonates with a critical aortic stenosis and low output heart failure, but this valve saving technique should be considered after a first valvuloplasty, if repetitive catheterization with balloon inflations have to be performed. Furthermore, stiff guide-wires, low pressure balloon catheters with a width of maximal 90% of the aortic anulus, but a triple length of the anulus size should be additionally considered, favoring satisfying results. Hemodynamic, angiographic and echocardiographic evaluations should be performed to assess the immediate result of the procedure. If necessary, the procedure has to be repeated with bigger balloons (26-29).
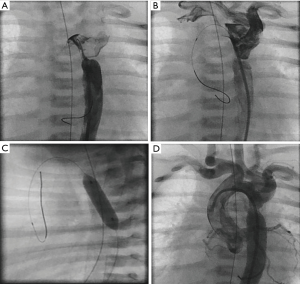
Newborns with critical aortic coarctation, associated myocardial failure and no response to prostaglandin can be effectively palliated by balloon angioplasty; it seems to be the method of choice as bridging procedure to corrective surgery (Figure 4A,B,C,D). Without describing the technique in detail, after angiographic delineation of the lesion, diameters of the transverse aortic arch, the descending aorta and minimum diameter of the coarctation are measured and a high-pressure balloon is chosen with a diameter not exceeding the diameter of the transverse arch or of the descending aorta at diaphragm level. Repetitive angioplasties with different balloons may be required. After angioplasty, angiography and hemodynamic measurements should be repeated. Considering the high re-stenosis rate in neonates, balloon angioplasty is only a palliation and serve as bridge-to-surgery procedure (30,31).
Acknowledgements
Parts of this publication are based on M Khalil’s article, “Principles of postnatal acute treatment in patients with congenital heart diseases”. Monatsschr Kinderheilkd 2017;165:952. https://doi.org/10.1007/s00112-017-0366-1.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation 1971;43:323-32. [Crossref] [PubMed]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900. [Crossref] [PubMed]
- Dolk H, Loane M, Garne E. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation 2011;123:841-9. [Crossref] [PubMed]
- Oster ME, Lee KA, Honein MA, et al. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 2013;131:e1502-8. [Crossref] [PubMed]
- Oster ME, Kim CH, Kusano AS, et al. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol 2014;113:1036-40. [Crossref] [PubMed]
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:e46-215. [PubMed]
- Samanek M. Children with congenital heart disease: probability of natural survival. Pediatr Cardiol 1992;13:152-8. [PubMed]
- Khoshnood B, De Vigan C, Vodovar V, et al. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983-2000: a population-based evaluation. Pediatrics 2005;115:95-101. [Crossref] [PubMed]
- Lindinger A, Schwedler G, Hense HW. Prevalence of congenital heart defects in newborns in Germany: Results of the first registration year of the PAN Study (July 2006 to June 2007). Klin Padiatr 2010;222:321-6. [Crossref] [PubMed]
- Riede FT, Worner C, Dahnert I, et al. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine--results from a prospective multicenter study. Eur J Pediatr 2010;169:975-81. [Crossref] [PubMed]
- Diller CL, Kelleman MS, Kupke KG, et al. A Modified Algorithm for Critical Congenital Heart Disease Screening Using Pulse Oximetry. Pediatrics 2018. [Crossref] [PubMed]
- Grosse SD, Peterson C, Abouk R, et al. Cost and Cost-Effectiveness Assessments of Newborn Screening for Critical Congenital Heart Disease Using Pulse Oximetry: A Review. Int J Neonatal Screen 2017;3:34. [Crossref] [PubMed]
- Rudolph AM. Congenital cardiovascular malformations and the fetal circulation. Arch Dis Child Fetal Neonatal Ed 2010;95:F132-6. [Crossref] [PubMed]
- Rashkind WJ, Miller WW. Creation of an atrial septal defect without thoracotomy. A palliative approach to complete transposition of the great arteries. JAMA 1966;196:991-2. [Crossref] [PubMed]
- Austin EH, Santamore WP, Barnea O. Balancing the circulation in hypoplastic left heart syndrome. J Cardiovasc Surg (Torino) 1994;35:137-9. [PubMed]
- Hoffman GM, Tweddell JS, Ghanayem NS, et al. Alteration of the critical arteriovenous oxygen saturation relationship by sustained afterload reduction after the Norwood procedure. J Thorac Cardiovasc Surg 2004;127:738-45. [Crossref] [PubMed]
- Schranz D. Pädiatrische Intensivtherapie. Elsevier, Munich: 1993.
- Burkhardt BE, Rucker G, Stiller B. Prophylactic milrinone for the prevention of low cardiac output syndrome and mortality in children undergoing surgery for congenital heart disease. Cochrane Database Syst Rev 2015.CD009515. [PubMed]
- Van Praagh R, Jung WK. The arterial switch operation in transposition of the great arteries: anatomic indications and contraindications. Thorac Cardiovasc Surg 1991;39 Suppl 2:138-50. [Crossref] [PubMed]
- Lacour-Gayet F. Surgery for pulmonary venous obstruction after repair of total anomalous pulmonary venous return. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2006.45-50. [Crossref] [PubMed]
- Latus H, Apitz C, Moysich A, et al. Creation of a functional Potts shunt by stenting the persistent arterial duct in newborns and infants with suprasystemic pulmonary hypertension of various etiologies. J Heart Lung Transplant 2014;33:542-6. [Crossref] [PubMed]
- Kutty S, Zahn EM. Interventional therapy for neonates with critical congenital heart disease. Catheter Cardiovasc Interv 2008;72:663-74. [Crossref] [PubMed]
- Doshi H, Venugopal P, MacArthur K. Does a balloon atrial septostomy performed before arterial switch surgery increase adverse neurological outcomes? Interact Cardiovasc Thorac Surg 2012;15:141-3. [Crossref] [PubMed]
- Schranz D, Michel-Behnke I. Advances in interventional and hybrid therapy in neonatal congenital heart disease. Semin Fetal Neonatal Med 2013;18:311-21. [Crossref] [PubMed]
- Schranz D, Michel-Behnke I, Akintuerk H. Letter by Schranz et al regarding article, "Comparison of the profiles of postoperative systemic hemodynamics and oxygen transport in neonates after the hybrid or the Norwood procedure: a pilot study". Circulation 2008;117:e296; author reply e297-8.
- Boe BA, Zampi JD, Kennedy KF, et al. Acute Success of Balloon Aortic Valvuloplasty in the Current Era: A National Cardiovascular Data Registry Study. JACC Cardiovasc Interv 2017;10:1717-26. [Crossref] [PubMed]
- Donald JS, Konstantinov IE. Surgical Aortic Valvuloplasty Versus Balloon Aortic Valve Dilatation in Children. World J Pediatr Congenit Heart Surg 2016;7:583-91. [Crossref] [PubMed]
- Mozumdar N, Burke E, Schweizer M, et al. A Comparison of Anterograde Versus Retrograde Approaches for Neonatal Balloon Aortic Valvuloplasty. Pediatr Cardiol 2018;39:450-8. [Crossref] [PubMed]
- Rao PS. Balloon aortic valvuloplasty. Indian Heart J 2016;68:592-5. [Crossref] [PubMed]
- Rao PS, Chopra PS. Role of balloon angioplasty in the treatment of aortic coarctation. Ann Thorac Surg 1991;52:621-31. [Crossref] [PubMed]
- Adjagba PM, Hanna B, Miro J, et al. Percutaneous angioplasty used to manage native and recurrent coarctation of the aorta in infants younger than 1 year: immediate and midterm results. Pediatr Cardiol 2014;35:1155-61. [Crossref] [PubMed]


