
Regenerative therapies in young hearts with structural or congenital heart disease
Methods
This review summarizes the published achievements of cell-based cardiac therapies in children with severe heart failure (HF). The databases MEDLINE, Google Scholar and EMBASE were searched for literature addressing pediatric cardiac regeneration. For ongoing or upcoming trials, the www.clinicaltrials.gov homepage was included.
What is known
HF
Etiology of HF in childhood is manifold and can be familial, inflammatory, infectious, metabolic or occurs within congenital heart disease (CHD).
At the very most it is dilative cardiomyopathy (DCM) rather than hypertrophic or restrictive pathologies. The moment when DCM is diagnosed by the clinician usually marks a phase of the disease with already pronounced dysfunction and severe dilatation of the left ventricle. Life expectancy for these children is low with about 30% mortality within the first year after diagnosis is made (1).
Structural changes in pediatric DCM differs significantly from the findings seen in adult DCM which is most frequently ischemic with co-morbidities i.e., arterial hypertension concomitant cardiomyocyte hypertrophy and myocardial fibrosis, while children with DCM lack hypertrophy, have less incidence of cardiac fibrosis and show distinct differences in gene expression profiles and β-receptor regulation (2,3).
Pharmacologic support for the failing heart is rarely investigated by randomized clinical trials and there are uncertainties whether drugs used in the adult population have the same target in the heart of a young child, especially in the context of a single ventricle physiology (4,5).
Absence of adverse remodeling in DCM in childhood, downregulation of β2-receptors among others may explain why medications applied in adult HF are less effective in children.
Profound knowledge of structure and resources of the pediatric heart in its unique ability to develop a gain in function by optimized ventricular-ventricular interaction either electrical (6,7) or mechanical resynchronization by increased ventricular afterload using pulmonary artery banding (8) have been shown to improve symptoms in pediatric DCM patients and amelioration of echocardiographic parameters and biomarkers.
At the final end mechanical circulatory support can be offered bridging to heart transplantation (HTX), the latter one being palliative by nature and the first one at this point not selected for destination therapy in the pediatric age group. Long term mechanical circulatory support by pulsatile or continuous flow pumps have been used until a donor heart got available (9-11). Compared to the number of patients with HF including an increasing number of single ventricle patients presenting with failing Fontan circulation, only a minority has access to be either on a left ventricular assist device (LVAD) or to be transplanted. Despite progress in handling post-transplant immunology, it remains demanding to maintain function of a donor heart over decades.
Due to shortage of organs and limited effects of pharmacological treatment there has been an intense interest of physicians and researchers for alternative strategies to repair the malfunctioning myocardium by stimulating its regenerative forces. Experiences from animal studies have shown the enormous potential of renewal capacity when newborn mice were able to regenerate the whole cardiac apex after resection (12). In future another mainstay in treatment of pediatric HF may focus on regulation of stem cell activity by delivery of progenitor cells in the diseased heart.
Regenerative forces of the young heart
The shift in paradigm that proliferation of cardiomyocytes contributes to heart growth is meanwhile accepted and recently Mollova et al. described cardiomyocyte mitosis and cytokinesis until the age of 20 years, with the highest percentage in infants (13). A 3.4-fold increase of cardiomyocytes in the left ventricle between the age of 1 year until 20 years suggests that myocardium can regenerate and in the diseased heart, proliferation might be able to be stimulated.
It was demonstrated that human neonatal myocardium is enriched with cell populations representing human cardiac progenitor cells (hCPCs). Not only the number was increased but also marker genes of proliferation and differentiation as c-kit+, NKX2.5 and Ki67 among others were highly expressed (14). Significant information about progenitor cells comes from patients with CHD (15) that contributes to understanding of regeneration in the growing heart and its potential for cellular repair.
Several growth factors and cytokines released by human cardiosphere-derived cells (CDCs) have been shown to stimulate angiogenesis and suppress myocardial apoptosis. Again, neonatal hearts had the paramount but transient potential of regeneration with a quick drop of hCPCs during infancy. Nevertheless, CDCs could be isolated also from hearts of elder children and teenagers with CHD. However, CDCs known for the highest expression of c-kit+ and the greatest regenerative forces after transplantation in infarcted myocardium were again predominantly found in neonates.
What the studies add
Cell types applied for cardiac regeneration in children
In the actual published clinical experience, exclusively autologous cells have been used for treatment of HF in childhood. Mononuclear bone marrow cells (BMCs) were harvested and processed from bone marrow, either as mesenchymal stem cells (MSCs) or as a subpopulation, the bone marrow mononuclear cells (BM-MNCs) representing hematopoietic stem cells (16-23). Different classes of cardiac derived CDCs were identified according their expression of stem cell markers. CDCs from atrial tissue with a proven mesenchymal phenotype, that is positive for signal-regulatory protein α (identifying cardiac lineage-committed cells) and markers for vascular endothelial precursors as well as cardiac transcription factors and even cardiac ion channel genes have been applied in patients with hypoplastic left heart syndrome (HLHS) and other forms of univentricular hearts (24-26). Peripheral blood stem cells (PBSCs) after stimulation with granulocyte-colony stimulating factor (G-CSF) after leukocyte apheresis (27,28) and finally umbilical cord mononuclear cells UC-MNCs (29) complete the portfolio of the recently transplanted cells in clinical trials in children.
Whether there is one specific cell type superior over another in pediatric HF cannot be determined yet. From animal studies for ischemic heart disease comparative efficacy has been shown between MSCs and CDCs (30) while paracrine effects were proven more efficacious in CDCs (31). CDCs taken from right atria showed the highest increase in LV-ejection fraction (LV-EF) (32). Human CDCs isolated from neonatal hearts are enriched with cardiac progenitor cells expressing c-kit+, flk-1 and Islet-1; the regenerative effect for ischemic myocardium to recover was significantly enhanced compared to adult CDCs in an animal model (33). BMCs have been studied extensively and are known to be safe in several scenarios of bone marrow reconstitution in ischemic HF of the adult. The unique possibility to yield high numbers of CDC in patients operated for CHD in the neonatal period and myocardial repopulation during a planned surgery at an early phase of myocardial dysfunction in infancy might be preventive for the known deterioration process in univentricular hearts (34,35).
The use of autologous progenitor cells is advantageous, as immunosuppression is not necessary and it might be offered repeatedly. Other cell types as myoblasts from skeletal muscle, amniotic fluid or placenta derived cells are conceivable for human use as well (36,37). Transplantation of embryonic stem cells is despite the probably highest potential of differentiation, limited by ethical issues and the risk of tumorgenicity.
Mechanisms of action
The exact mechanism of cellular cardiomyoplasty to improve ventricular function in the ischemic or miscellaneous diseased heart is still not completely understood. Angiogenesis and neovascularization play an important role (38). There is good evidence that release of paracrine factors and cell-cell contact or cell-to-cell fusion counteract apoptosis for myocardial protection and influences remodeling (39). This is supported by the observation that G-CSF itself as a hematopoietic factor is able to mobilize stem cells from bone marrow (40). Not all cells will be reprogrammed to cardiomyocytes. The degree of differentiation and mechanical integration determines the contractile force. The potential of cardiac precursor cells to differentiate to cardioblasts with contractile properties is probably higher than that of bone marrow derived cells (31,41). Finally stimulation of tissue resident progenitor cells has been identified to contribute to heart renewal in an animal model (42).
Based on the enhanced potential of a young heart to regenerate there is evidence that mobilization and liberation of endothelial progenitor cells is possible without cell based therapies (CBT) and can be induced by Sildenafil, shown for patients with CHD suffering from Eisenmenger syndrome (43). Additionally erythropoietin has an effect with rising numbers of circulating progenitor cells that either may work by improved vascularization or mobilization of progenitor cells (44). Hemodynamic changes themselves can initiate proliferation of progenitor cells. In infants pressure overload of the right ventricle by pulmonary artery banding has been able to increase the number of cardiac resident cells (c-kit+/mast cell tryptase-/CD45-) by three-fold (45). To counteract fibrosis, induced by pulmonary artery banding, CDCs from the right atrium were administered to the coronary circulation in a preclinical study of right heart failure. A remarkably reduced area of fibrosis in animals with CDC-application compared to controls and engraftment of 4.4% especially in the fibrotic spots indicated relevant myocyte regeneration as part of the repair mechanisms (46).
Mode of application
The most frequent delivery of precursor cells in children is intracoronary by using the stop-flow-technique to allow adhesion and engraftment. The injection of processed progenitor cells has been performed during cardiac catheterization in most cases with DCM, while the group in Riga/Latvia decided for percutaneous intramyocardial application into the interventricular septum or LV wall (16,23). In HLHS, Burkhart et al. (29) decided for a subepicardial injection of umbilical cord derived cells to the free wall of the RV with six single injections arranged in a radial pattern at the time of stage II of the palliative track of single ventricle repair. In an upcoming study (NCT03406884) it is planned to administer the cells also at the time of bidirectional cavopulmonary anastomosis (BDCPA) via the cardioplegia needle after completion of surgery. Figure 1 gives an overview of the possible sites of cell application.
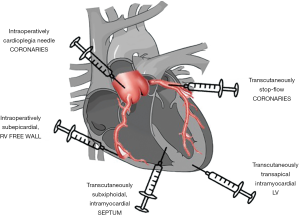
The number of cells that are injected varies considerably between the published series. It has to be expected that only a low number will be engrafted in the recipient’s heart. In an animal model only about 12.7% of transplanted cells stayed in the heart for the first 24 hrs with a rapid decline thereafter. This does not correlate with poor effectivity, pointing again on the crucial importance of paracrine mechanisms (47). The group in Okayama/Japan (24-26) based the dose regimen for their clinical trials of 3×105 CDCs/kg bodyweight on the convincing animal experiments mentioned above (46). Table 1 shows the cell types and numbers used for injection in the published pediatric series. For administration to the coronary circulation usually the stop-flow-technique is used. After intubation of the target coronary artery, a balloon catheter is advanced and after removal of the guide wire, low pressures of 1–2 atm are applied to stop flow from the aorta and allow cells that are injected slowly over 2–3 minutes to adhere and eventually migrate through the endothelium. The calculated number of cells is injected in two to three single shots to allow recovery of the circulation in between. Preventive administration of Amiodarone to avoid ventricular tachycardia or fibrillation, is part of the protocols equally a post CBT angiography to prove patency of the coronary vessel.

Full table
Target of regenerative cell therapies in children with HF
Left ventricular failure
While in adults CBT addresses mostly ischemic cardiomyopathy, the published literature in pediatric left ventricular failure includes DCM, either idiopathic, anthracycline-induced and post-myocarditis (16,23,27,28,48-50). CBT in ischemic HF is described in one case report in a patient with anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) (20) and finally in a patient suffering from Takayasu arteritis (21) who developed myocardial infarction.
Measures to demonstrate the effect of CBT in pediatric HF have been mostly improvement of the clinical status quoting the level of impairment from the NYHA class or Ross score or judgement of the treating physician (51). For primary LV-dysfunction as in DCM, LV-EF is the most frequently applied surrogate parameter. For the population with primarily LV-failure CBT increased LV-EF of 20.5% (3–37%) in mean. Figure 2 shows the increase of LV-EF in the published cases with DCM. The increase in LV-EF did not necessarily parallel the dimension of the left ventricle (23) pointing out the importance of the ventricular-ventricular interplay of the right and left ventricle in pediatric HF (52).
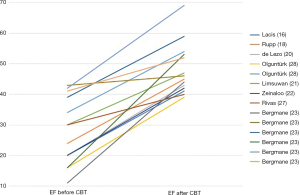
Brain natriuretic peptides (BNP) as biomarkers of HF have been investigated after CBT in LV-failure due to DCM (28). Admittedly not all patients had a decline in BNP despite of climbing up several steps in NYHA class from IV to I-II and improved function in Echo (48).
All patients with LV-failure that were selected for CBT had end stage HF. Follow-up times ranged from 2–34 months, being 14.6 months in mean for those 20 patients who survived with their own heart or underwent HTX. Among those, nine were less than 1 year, representing the patient group with the worst natural course if left untreated. One infant with anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) underwent coronary artery transfer and postoperative stent implantation to the left coronary artery preceding stem cell injection (20). Five patients were between 10 and 18 years and the reason for DCM varied from cardiotoxic chemotherapy, myocarditis to idiopathic DCM. From the data published in the case reports no clear conclusion can be drawn whether the younger DCM patients had a higher response of functional improvement.
While there has been a positive effect in almost all patients in short and intermediate follow up, gradual deterioration is reported Zeinaloo et al. (50) indicating a transient effectivity. Obviously endogenous repair mechanisms in childhood DCM and CBT alone cannot balance the myocardial damage of DCM at least when the patients present at a later stage of LV-failure.
Right ventricular failure
The architecture and functional capacity of the right ventricle in infancy are unique and allow adaptation to increased pressure load for years. Children with hypoplastic heart syndrome, missing the left ventricle are able to withstand failure of the pressure overloaded right ventricle for a while. Working in subaortic position and providing both the pulmonary and systemic circulation, finally RV function deteriorates and clinical symptoms occur. Not surprisingly attempts of myocardial renewal by CBT where undertaken in these patients. Two case reports describe improvement of RV ejection fraction from 22% to 44% and 30–35% to 50% respectively (17,29).
There is one prospective controlled trial of intracoronary application of CDC, the trans-coronary Infusion of Cardiac Progenitor Cells in Patients with Single-Ventricle Physiology (TICAP) (24). Seven patients with HLHS received cardiac progenitor cells that where harvested from the patient’s atrial tissue during stage one of the palliative surgical track, propagated and injected within 1 month after surgery. This group was compared to seven patients with HLHS who got the same type of surgery but no cell transfusion. Already in the early follow-up after 3 months, patients with autologous CDC-transfusion had a significant increase in RV ejection fraction from 46.9% to 52.8%. A sustained effect and superior clinical parameters of HF could be measured in midterm follow-up after 18 months, the RV-EF being 31.5%±6.8% in the untreated versus 40.4%±7.6% in the CDC treatment group. Meanwhile 3 years follow-up data are available with continuing improved heart function in the active group measured as an increase in RV-EF of +8.0% vs. +2.2% paralleled by decreased biomarker levels, better somatic growth and superior clinical HF scoring (26). Based on this experience in the TICAP-Trial, the group in Japan expanded the application of CDC to other variants of functional single ventricle. In a phase II trial (PERSEUS) (25) a total number of 34 patients had CDC treatment according the protocol of the TICAP-trial with application in all main coronary vessels. The portion of non HLHS single ventricle patients with either morphological right or left subaortic ventricle was 33–47% representing a broad spectrum of congenital heart defects. Therefore, results can be interpreted to be representative for the entity of functional single ventricle patients and it is by now the largest published number of patients treated with cardiac cell transfusion. Half of the patients were treated primarily, another 17 patients that were originally in the control group with standard surgical care received CDC after 3 months as a late infusion if ejection fraction was <60%. Surrogate parameters of ventricular function like ejection fraction, ventricular volumes, global strains, and strain rate uniformly improved not only in the patients with early CDC injection 4–5 weeks after primary surgery but also in the group with late CDC injection 3 months after standard care. The effects lasted over a longer period of twelve months. Moreover, the study revealed parameters indicative of reverse remodeling measured as cardiac elastance and stiffness. Cardiac MRI could demonstrate a lower end-diastolic volume index which has been described indicative for transplant free survival in patients with Fontan circulation. A significant reduction of myocardial fibrosis was evident by analysis of scar volume, size and mass in late gadolinium enhancement studies (LGE). P values were highly significant (P=0.001) in favor for improved clinical signs of HF, proportionate weight gain, quality of life and a decrease of natriuretic peptides.
The most recent analysis of the group in Okayama compared the 41 pooled patients of the TICAP and PERSEUS trials with 60 controls of single ventricle patients with standard surgery without CDC treatment for improvement of cardiac function and mortality as primary endpoint 2 years after either BDCPA or total cavopulmonary anastomosis (TCPA) (53). While there was no difference for deaths between the cohorts, that where matched also for the stage of surgical palliation, significant increase of EF was noted in the active group of cell therapy: stage 2: +8.4% vs. +1.6%, (P=0.03); stage 3: +7.9% vs. −1.1%, (P<0.001). Analysis of late failure, characterized by late death, intensified pharmacologic or surgical management, higher HF scores, inability to reach TCPA, take down of stage 2 or 3 procedure or the development of protein-losing enteropathy was favorable for patients after CDC treatment with a hazard ratio of 0.387 (P=0.028) together with less adverse events. Deeper insights were gained in discriminating between effects on HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF). Mortality was significantly reduced by CDC administration in HFrEF patients (P=0.038) while diastolic dysfunction was less amenable by cell transfusion. From animal experiments remodeling to reduce fibrosis could be demonstrated after CDC injection. Either by autocrine or paracrine effects or eventually cell differentiation, Interleukin-6 and Collagen type 3 expression is suppressed after CDC administration, irrespective of the phenotype of myocardial failure, while collagen type 1, matrix metalloproteinase type 2 and atrial natriuretic peptide where significantly suppressed in HFpEF (53).
Keynotes of clinical experience in pediatric CBT
- In total there are 66 pediatric patients published worldwide that received autologous CBT for myocardial repair;
- Cell application was feasible in all age groups;
- There was no tumorigenicity;
- The majority, forty-five patients, had CHD;
- Cardiac engraftment of transplanted cells has been demonstrated;
- A clear benefit of one cell type for CBT cannot be assumed due to heterogeneity of cell preparations and applications;
- Cardiac derived cells from newborn hearts might be advantageous for including not only a high cell number but also the most fortunate milieu for myocardial regeneration, neoangiogenesis, and secretion of trophic factors;
- The effect of CBT was at least transient in the majority of patients but with lasting improvement in the randomized trials;
- Surrogate parameters for improved cardiac function after CBT were positive for failure of the LV and RV;
- Univentricular hearts responded equally with reduced ventricular volumes and less fibrosis after CDC transfusion;
- The highest increase of cardiac function was pronounced in the sickest and youngest patients;
- HFrEF phenotypes seem to benefit more than those with HFpEF.
Future perspectives
The needs of the increasing number of patients with univentricular circulation that will reach adulthood and will present with HF are not met yet. It seems therefore reasonable to focus on further research of regenerative therapies. Table 2 gives an overview of the actual ongoing studies in the field of stem cell application for myocardial failure in children with univentricular hearts. Besides safety trials, more randomized investigations, one of them the APPOLLON-trial as a multicenter phase III successor of the aforementioned TICAP and PERSEUS CDC trials for single ventricle, are recruiting or will start soon (NCT02781922).
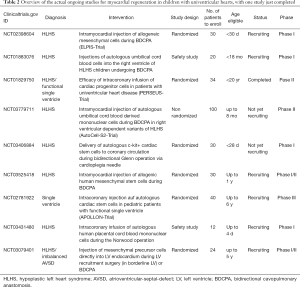
Full table
Except two trials using allogenic stem cells, the majority sticks to autologous mesenchymal, umbilical/placental cord or cardiac precursors. Patients within the concept of staged palliation offer the possibility of direct myocardial injection of different cell types at stage II eventually at stage III avoiding intracoronary application. Whether the myocardial architecture with small intercellular space is suitable to accept and integrate the high number of cells delivered, remains to be elucidated. There is one upcoming study focusing on stimulating growth of a border line left ventricle rather than healing of volume- or pressure loaded single ventricles (NCT03079401).
Recently neonatal CPC-derived exosomes have been classified to promote regeneration (54). It is suggested it is these cell free vesicles that carry the mixture of factors that can stimulate cardiac repair.
Tissue and circulating noncoding RNA’s have been identified to be involved in the process of myocardial regeneration and post-infarction remodeling, and again there seems to be an age specific regulation of these noncoding RNA’s (55,56). Whole-genome microRNA screening may provide insights also for patients with CHD.
Whenever new therapeutic strategies for treatment of HF are developed, the differences between adult and pediatric patients have to be considered. Cardiac fibrosis is present only in 28% in Fontan patients and 16% in pediatric DCM (57,58) pointing on the distinct possibility to reverse ventricular dysfunction. Stunning or hibernating myocardium expressing a viable architecture rather than an irreversibly damaged fibrotic ventricle has to be assumed for pediatric HF (59) and together with absence of atherosclerosis, can explain the recovery of severely depressed heart function in CHD after surgical correction (60). Especially cyanotic CHD provide a unique possibility to analyze cardiac reprogramming in a hypoxic environment that might have an impact on cell mobilization like in the fetal circulation (61). Additional insights might be gained from the hypoxia inducing factor (HIF)-pathways, that have been characterized to be involved in cardiac protection.
Conclusions
Cell-based therapies in pediatric HF have made the step into clinical practice. The initial experience in single cases or small cohorts proved feasibility and safety with at least temporary improvement of heart function. From controlled studies in CHD patients with univentricular hearts medium-term follow-up demonstrates a lasting effect after CDC injection compared to controls. Further trials should distinguish the risks and benefits in a larger population. Actually, CBT is an adjunct to standard treatment of severe HF; the potential of cardiac healing may be higher than in adults. Insights to molecular and paracrine activity have been gathered from the clinical experience and will contribute to further optimized patient selection and refinements to increase the lifespan of transplanted cells or their paracrine effects.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Alvarez JA, Wilkinson JD, Lipshultz SE. Outcome predictors for pediatric dilated cardiomyopathy: A systematic review. Prog Pediatr Cardiol 2007;23:25-32. [Crossref] [PubMed]
- Patel MD, Mohan J, Schneider C, et al. Pediatric and adult dilated cardiomyopathy represent distinct pathological entities. JCI Insight 2017;2. [Crossref] [PubMed]
- Miyamoto SD, Stauffer BL, Nakano S, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J 2014;35:33-41. [Crossref] [PubMed]
- Nakano SJ, Nelson P, Sucharov CC, et al. Myocardial response to milrinone in single right ventricle heart disease. J Pediatr 2016;174:199-203.e5. [Crossref] [PubMed]
- Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation 2010;122:333-40. [Crossref] [PubMed]
- Motonaga KS, Dubin AM. Cardiac resynchronization therapy for pediatric patients with heart failure and congenital heart disease: a reappraisal of results. Circulation 2014;129:1879-91. [Crossref] [PubMed]
- Schiller O, Dham N, Greene EA, et al. Pediatric dilated cardiomyopathy patients do not meet traditional cardiac resynchronization criteria. J Cardiovasc Electrophysiol 2015;26:885-9. [Crossref] [PubMed]
- Schranz D, Akintuerk H, Bailey L. Pulmonary artery banding for functional regeneration of end-stage dilated cardiomyopathy in young children: world network report. Circulation 2018;137:1410-2. [Crossref] [PubMed]
- Fan Y, Weng YG, Xiao YB, et al. Outcomes of ventricular assist device support in young patients with small body surface area. Eur J Cardiothorac Surg 2011;39:699-704. [Crossref] [PubMed]
- Fraser CD, Jaquiss RDB, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med 2012;367:532-41. [Crossref] [PubMed]
- Miera O, Kirk R, Buchholz H, et al. A multicenter study of the HeartWare ventricular assist device in small children. J Heart Lung Transplant 2016;35:679-81. [Crossref] [PubMed]
- Polizzotti BD, Ganapathy B, Walsh S, et al. Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window. Sci Transl Med 2015;7:281ra45. [Crossref] [PubMed]
- Mollova M, Bersell K, Walsh S, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A 2013;110:1446-51. [Crossref] [PubMed]
- Amir G, Ma X, Reddy VM, Hanley FL, et al. Dynamics of human myocardial progenitor cell populations in the neonatal period. Ann Thorac Surg 2008;86:1311-9. [Crossref] [PubMed]
- Ghazizadeh Z, Vahdat S, Fattahi F, et al. Isolation and characterization of cardiogenic, stem-like cardiac precursors from heart samples of patients with congenital heart disease. Life Sciences 2015;137:105-15. [Crossref] [PubMed]
- Lacis A, Erglis A. Intramyocardial administration of autologous bone marrow mononuclear cells in a critically ill child with dilated cardiomyopathy. Cardiol Young 2011;21:110-2. [Crossref] [PubMed]
- Rupp S, Zeiher AM, Dimmeler S, et al. A regenerative strategy for heart failure in hypoplastic left heart syndrome: intracoronary administration of autologous bone marrow-derived progenitor cells. J Heart Lung Transplant 2010;29:574-7. [Crossref] [PubMed]
- Rupp S, Bauer J, Tonn T, et al. Intracoronary administration of autologous bone marrow-derived progenitor cells in a critically ill two-yr-old child with dilated cardiomyopathy. Pediatr Transplant 2009;13:620-3. [Crossref] [PubMed]
- Rupp S, Jux C, Bönig H, et al. Intracoronary bone marrow cell application for terminal heart failure in children. Cardiol Young 2012;22:558-63. [Crossref] [PubMed]
- de Lezo JS, Pan M, Herrera C. Combined percutaneous revascularization and cell therapy after failed repair of anomalous origin of left coronary artery from pulmonary artery. Catheter Cardiovasc Interv 2009;73:833-7. [Crossref] [PubMed]
- Limsuwan A, Pienvichit P, Limpijankit T, et al. Transcoronary bone marrow-derived progenitor cells in a child with myocardial infarction: first pediatric experience. Clin Cardiol 2010;33:E7-12. [Crossref] [PubMed]
- Zeinaloo A, Zanjani KS, Bagheri MM, et al. Intracoronary administration of autologous mesenchymal stem cells in a critically ill patient with dilated cardiomyopathy. Pediatr Transplant 2011;15:E183-6. [Crossref] [PubMed]
- Bergmane I, Lacis A, Lubaua I, et al. Follow-up of the patients after stem cell transplantation for pediatric dilated cardiomyopathy. Pediatr Transplant 2013;17:266-70. [Crossref] [PubMed]
- Ishigami S, Ohtsuki S, Tarui S, et al. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome. Circ Res 2015;116:653-64. [Crossref] [PubMed]
- Ishigami S, Ohtsuki S, Eitoku T, et al. Intracoronary cardiac progenitor cells in single ventricle physiology. Circ Res 2017;120:1162-73. [Crossref] [PubMed]
- Tarui S, Ishigami S, Ousaka D, et al. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: Three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients With Single-Ventricle Physiology (TICAP) trial. J Thorac Cardiovasc Surg 2015;150:1198-1207, 8.e1-2.
- Rivas J, Menendez JJ, Arrieta R, et al. Usefulness of intracoronary therapy with progenitor cells in patients with dilated cardiomyopathy: bridge or alternative to heart transplantation? An Pediatr (Barc) 2011;74:218-25. [Crossref] [PubMed]
- Olguntürk R, Kula S, Sucak GT, et al. Peripheric stem cell transplantation in children with dilated cardiomyopathy: Preliminary report of first two cases. Pediatr Transplant 2010;14:257-60. [Crossref] [PubMed]
- Burkhart HM, Qureshi MY, Peral SC, et al. Regenerative therapy for hypoplastic left heart syndrome: first report of intraoperative intramyocardial injection of autologous umbilical-cord blood-derived cells. J Thorac Cardiovasc Surg 2015;149:e35-7. [Crossref] [PubMed]
- Weil BR, Suzuki G, Leiker MM, et al. Comparative efficacy of intracoronary allogeneic mesenchymal stem cells and cardiosphere-derived cells in swine with hibernating myocardium. Circ Res 2015;117:634-44. [Crossref] [PubMed]
- Li TS, Cheng K, Malliaras K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 2012;59:942-53. [Crossref] [PubMed]
- Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378:1847-57. [Crossref] [PubMed]
- Simpson DL, Mishra R, Sharma S, et al. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation 2012;126:S46-53. [Crossref] [PubMed]
- Atz AM, Zak V, Mahony L, et al. Longitudinal Outcomes of Patients With Single Ventricle After the Fontan Procedure. J Am Coll Cardiol 2017;69:2735-44. [Crossref] [PubMed]
- Liu MY, Zielonka B, Snarr BS, et al. Longitudinal assessment of outcome from prenatal diagnosis through fontan operation for over 500 fetuses with single ventricle-type congenital heart disease: The Philadelphia Fetus-to-Fontan Cohort Study. J Am Heart Assoc 2018;7:e009145. [Crossref] [PubMed]
- Walther G, Gekas J, Bertrand OF. Amniotic stem cells for cellular cardiomyoplasty: promises and premises. Catheter Cardiovasc Interv 2009;73:917-24. [Crossref] [PubMed]
- Yamada S, Nelson TJ, Crespo-Diaz RJ, et al. Embryonic stem cell therapy of heart failure in genetic cardiomyopathy. Stem Cells 2008;26:2644-53. [Crossref] [PubMed]
- Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest 2005;115:572-83. [Crossref] [PubMed]
- Mirotsou M, Jayawardena TM, Schmeckpeper J, et al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 2011;50:280-9. [Crossref] [PubMed]
- Srinivas G, Anversa P, Frishman WH. Cytokines and myocardial regeneration: a novel treatment option for acute myocardial infarction. Cardiol Rev 2009;17:1-9. [Crossref] [PubMed]
- Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005;433:647-53. [Crossref] [PubMed]
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763-76. [Crossref] [PubMed]
- Diller GP, van Eijl S, Okonko DO, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 2008;117:3020-30. [Crossref] [PubMed]
- Bahlmann FH, de Groot K, Spandau JM, et al. Erythropoietin regulates endothelial progenitor cells. Blood 2004;103:921-6. [Crossref] [PubMed]
- Rupp S, Bauer J, von Gerlach S, et al. Pressure overload leads to an increase of cardiac resident stem cells. Basic Res Cardiol 2012;107:252. [Crossref] [PubMed]
- Oh H. Cell therapy trials in congenital heart disease. Circ Res 2017;120:1353-66. [Crossref] [PubMed]
- Hong KU, Guo Y, Li QH, et al. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One 2014;9:e96725. [Crossref] [PubMed]
- Rupp S, Jux C, Bönig H, et al. Intracoronary bone marrow cell application for terminal heart failure in children. Cardiol Young 2012;22:558-63. [Crossref] [PubMed]
- Rupp S, Bauer J, Tonn T, et al. Intracoronary administration of autologous bone marrow‐derived progenitor cells in a critically ill two‐yr‐old child with dilated cardiomyopathy. Pediatr Transplant 2009;13:620-3. [Crossref] [PubMed]
- Zeinaloo Aa, Zanjani KS, Khosroshahi AG. Further follow up of the cardiomyopathic patient treated by intracoronary administration of autologous mesenchymal stem cells. Pediatr Transplant 2011;15:442. [Crossref] [PubMed]
- Pavo IJ, Michel-Behnke I. Clinical cardiac regenerative studies in children. World J Cardiol 2017;9:147-53. [Crossref] [PubMed]
- Schranz D. Can the Right Ventricle Support the Failing Left Ventricle? In: Friedberg M, Redington A. editors. Right Ventricular Physiology, Adaptation and Failure in Congenital and Acquired Heart Disease. Cham: Springer, 2018:93-7.
- Sano T, Ousaka D, Goto T, et al. Impact of cardiac progenitor cells on heart failure and survival in single ventricle congenital heart disease. Circ Res 2018;122:994-1005. [Crossref] [PubMed]
- Agarwal U, George A, Bhutani S, et al. Experimental, systems, and computational approaches to understanding the microRNA-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ Res 2017;120:701-12. [Crossref] [PubMed]
- Eulalio A, Mano M, Dal Ferro M, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012;492:376-81. [Crossref] [PubMed]
- Agarwal U, Smith AW, French KM, et al. Age-dependent effect of pediatric cardiac progenitor cells after juvenile heart failure. Stem Cells Transl Med 2016;5:883-92. [Crossref] [PubMed]
- Rathod RH, Prakash A, Kim YY, et al. Cardiac magnetic resonance parameters predict transplantation-free survival in patients with fontan circulation. Circ Cardiovasc Imaging 2014;7:502-9. [Crossref] [PubMed]
- Latus H, Gummel K, Klingel K, et al. Focal myocardial fibrosis assessed by late gadolinium enhancement cardiovascular magnetic resonance in children and adolescents with dilated cardiomyopathy. J Cardiovasc Magn Reson 2015;17:34. [Crossref] [PubMed]
- Bayeva M, Sawicki KT, Butler J, et al. Molecular and cellular basis of viable dysfunctional myocardium. Circ Heart Fail 2014;7:680-91. [Crossref] [PubMed]
- Azakie A, Russell JL, McCrindle BW, et al. Anatomic repair of anomalous left coronary artery from the pulmonary artery by aortic reimplantation: early survival, patterns of ventricular recovery and late outcome. Ann Thorac Surg 2003;75:1535-41. [Crossref] [PubMed]
- Gaber N, Gagliardi M, Patel P, et al. Fetal reprogramming and senescence in hypoplastic left heart syndrome and in human pluripotent stem cells during cardiac differentiation. Am J Pathol 2013;183:720-34. [Crossref] [PubMed]


