
Percutaneous pulmonary valve implantation (PPVI) in non-obstructive right ventricular outflow tract: limitations and mid-term outcomes
Introduction
Anomalies of the right ventricular outflow tract (RVOT) are approximately found in 20% of children born with a congenital heart defect (1). In addition to congenital anomalies as tetralogy of Fallot (TOF), double outlet right ventricle (DORV), truncus arteriosus or transposition of the great arteries (TGA) with pulmonary stenosis, the RVOT can also be artificially altered as seen in context of a Ross procedure treating aortic valve diseases. Surgical repair of congenital and acquired RVOT obstructions are performed either with transannular patch technique or by implantation of biological valves such as homograft or xenograft valves (2). In general, the surgical short- and mid-term results are excellent, more than 95% of children undergoing repair can be expected to survive to adulthood (3). However, RVOT surgery remains a palliative approach; almost all repaired RVOTs need one or more follow-up interventions (4).
Surgical enlargement of the RVOT, oftentimes with a transannular patch completely relieves obstructions, on the expense of varying degree of pulmonary regurgitation (PI) even when a self-made mono-cusp valve is used. Progressive dilatation of the RVOT with consecutive dysfunction of the right ventricle increases the risk of right as well as left heart failure (5,6) and in particular the risk of sudden death (7). On the other hand, implanted prosthetic valves and conduits need repetitive operations caused by degeneration and calcification of artificial tissue leading to obstruction, regurgitation or both. Traditionally, patients with biomedical conduits require multiple surgical interventions and life-long follow-up control. Repetitive open-heart surgeries carry a high risk of morbidity and mortality. The indications for re-operations were based on severe obstructions leading to right ventricular pressures (RVP) 2/3 of the systemic or systolic left ventricle (LV) pressure (8). Considering the degree of PI, RV-volume of more than 150 mL/m2 indicates usually re-surgery (9). It is of general consent that exercise intolerance, arrhythmia or heart failure must be avoided an intervention should not be deferred. A significant change in decision making for RVOT treatment came up when PPVI were successful established as a less-invasive alternative to surgery.
Philipp Bonhoeffer developed the so-called Melody® valve and performed the first successful percutaneous pulmonary valve implantation (PPVI) in the beginning of the new millennium (10). Meanwhile, the PPVI technique is worldwide used with an unexpected success rate nowadays of approximately 95% (1). Currently, two certificated valve systems are commercially available. The Melody™ valve (Medtronic Inc., Minneapolis, MN, USA) is made from a bovine jugular vein valve that is sutured within a platinum iridium graft-stent. The Melody valve comes in two sizes (16–20 and 18–22 mm) with a corresponding delivery system.
The Edwards SAPIEN XT (Edwards Life Sciences, Irvine, CA, USA) is a valve created by bovine pericardial tissue containing three equal sized leaflets that are hand sewn to a stainless-steel balloon-expandable stent. The valve is available in three diameters: 23, 26 and 29 mm.
The devices were approved for patients with conduit/bioprosthetic RVOT valve obstructions. This group reflects only about 25% of patients requiring valve implantation. The other 75% of patients in need of a pulmonary valve have a non-conduit reconstructed RVOT. Therefore, over the time, techniques have been developed that may extend the use of PPVI beyond the current indications, that more patients can benefit from this technology.
Here, we report short- and medium-term outcomes after transcatheter pulmonary valve placement in a series of 26 patients with “native” RVOT in whom this technology was used to perform PPVI in various anatomic settings.
Methods
A retrospective study design was conducted to analyze the mid-term outcome of patients who received PPVI for treating a native or patched RVOT in the Hessen Pediatric Heart Center Frankfurt & Giessen between 2007 and 2017. Approval from the local ethics committee and written informed consent from all patients were obtained. Emphasis was placed on the off-label use of the Melody® or Sapien™ stent-valves for the indication of a free, non-conduit RVOT. Patients repaired with valved conduits, homografts or xenografts were excluded. The data contain clinical follow-up results including echocardiography and cardiac magnetic resonance imaging (MRI). Cardiac MRI (Magnetom, 3 Tesla, Siemens Erlangen, Germany) was used to quantify PI and to obtain volumetric measurements of the RV and LV. In echocardiography left and right ventricular dimensions and function were measured; RVOT additionally analyzed by CW- and PW-Doppler technique obtaining mean and peak systolic and diastolic gradients; Color-Doppler was even used for semi-quantification of all cardiac valve functions. Indications and contra-indications as well as technical details of PPVI were published elsewhere (11). Open-cell designed XXL AndraStents® (Andramed GmbH, Reutlingen, Germany) were preferred for RVOT-pre-stenting to reduce the risk of embolization and to achieve stent diameters up-to 33 mm. CP-Stents or CP-Graft-Stents (NuMed, Inc., Hopkinton, NY, USA) were used for RVOT diameters less than 26 mm; several patients needed pre-stenting of the RVOT with one or more AndraStents®. Depending on the required stent-diameter BIB-(balloon-in-balloon) catheters (NuMed, Inc., Hopkinton, NY, USA) or Z-MED II balloons (pfm medical, Köln, Germany) were used. The Russian doll method (12) of overlapping implantation of multiple stents with decremental diameters was utilized to reduce the size of RVOT and to create a sufficient landing zone for the final stent-valve implantation. The choice for a Melody® or Sapien™ valve was usually taken in consideration of anatomy, length and diameter of the RVOT. Following PPVI, patients were usually observed for four days in the hospital. All patients received acetylsalicylic acid (2 mg/kg, maximal dosage of 100 mg) once daily for 6 months; few patients received additionally clopidogrel (0.5–1 mg/kg/day). Follow-up was arranged 1, 3 and 6 months, respectively; at least annually thereafter. Routine follow-up consisted of detailed physical examination, electrocardiogram and transthoracic echocardiography.
Data were collected using Excel spreadsheets. Results are presented as mean ± standard deviation (SD); the median and range are also given when the distribution is non-normal.
Results
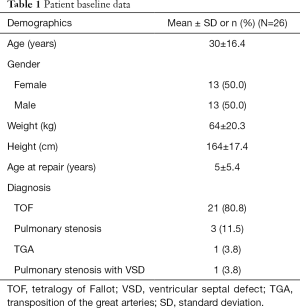
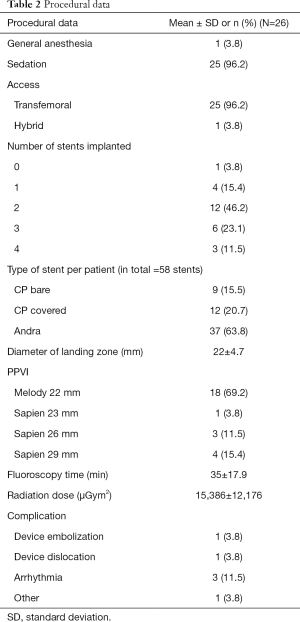
On Table 1 demographic and procedural data are summarized; the mean age of the patients was 30±16.4 years (range, 5–62 years, median 24 years); 50.0% were female. 21 out of 26 patients (80.8%) had TOF as the underlying primary diagnosis; three patients had a congenital pulmonary stenosis (11.5%); one patient a TGA combined with pulmonary stenosis and VSD. Transannular patch was the most frequent surgical technique to enlarge the RVOT (24/26; 92.3%); two patients had a native RVOT following pulmonary balloon valvuloplasty (7.7%). All PPVI patients received RVOT pre-stenting (n=25), two patients 8 weeks before PPVI. Transapical valve implantation was performed without pre-stenting in a 5-year-old patient with a weight of 12 kg (Table 2). Based on right ventricular angiography, the RVOT diameter was measured with 22±4.7 mm in average. In the mean, RVOT pre-stenting was performed with two stents; AndraStents® were used in 23 (88.5%) patients, and 38 AndraStents® were implanted in total. Twelve CP-graft stents were used in 11 patients. Patients with a following Sapien® valve implantation (n=8) received in the mean 1.6 stents; Melody® valve candidates 2.4 stents, respectively. The 22 mm Melody® model was mostly chosen (n=18; 69.2%); Sapien valves were implanted with a size of 29 mm in 15.4% of the patients, a 26 mm Sapien model in 11.5%, only one patient received a 23 mm Sapien valve (Table 2).
Full table
Full table
Functional outcome and midterm data
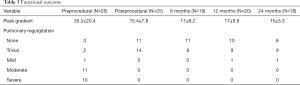
PPVI was successful in 25 (96.2%) patients. An elective surgical approach became necessary because the ensemble of the balloon-mounted Melody® valve could not proceeded through the pre-stented RVOT (13). Valve function remained very stable during the follow-up of 12–24 months without a significant pressure gradient or regurgitation (Table 3). The mean pressure gradient across the manipulated RVOT was 15.4±7.8 immediately after PPVI. In a follow-up of 24 months the mean pressure gradient remained in average 15±5.3 mmHg. The improvement of the pre-interventional PI was mostly striking. The mean degree of PI declined from 3±0.9 (median =3) to 1±0.5 (median =1); the implanted valve remained competent even after 24 months. There was no stent migration or indication for stent fracture during the observation period. X-ray was not routinely performed; therefore, insignificant stent fractures could not be excluded. Endocarditis did also not become evident.
Full table
All patients had surgery related right bundle branch block before PPVI. The pre-interventional QRS duration was 139±33 milliseconds in the mean; there was no QRS change after PPVI (Figure 1), whether immediate (133±31 milliseconds) nor during medium‐term follow‐up of 24 months (135±29 milliseconds). Documented arrhythmias occurred in 3 patients (11.5%) after PPVI. Two of them had premature ventricular contractions (PVC) already observed before PPVI. The arrhythmias after PPVI were nonsustained ventricular tachycardia (NSVT) in 1 patient (3.8%) and frequent PVCs in 2 patients (7.6%). The axis of the QRS indicated an RVOT origin in all 3 patients. Arrhythmias were treated with metoprolol succinate.

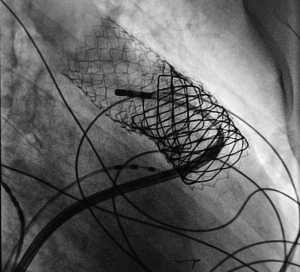
PVCs of two patients could be resolved by a mono-therapy of metoprolol. Discontinuation of metoprolol after a follow-up of 6 months caused recurrence of PVCs; re-treatment became necessary in both patients. In the patient with NSVT, the antiarrhythmic treatment had to be switched to a high-dose propranolol which also remained unsatisfied. A transcatheter ablation was performed but unsuccessful since the origin of the ventricular ectopy was just behind the stent-covered RVOT (Figure 2). Nevertheless, by switching the treatment to sotalol, the NSVTs were permanently eliminated.
Discussion
Transcatheter pulmonary valve replacement is an effective and encouraging technique. Considering the current approved PPV approach based on balloon expandable stent technique, the labelled indication is limited to patients with a need to treat failing RV to PA connecting conduits. Therefore, if the official selection criteria are strictly applied, only 25% of patients with postoperative RVOT dysfunction are qualified for PPVI. Several centres have reported their approach to reconfigure the RVOT to acquire more possible candidates for the transcatheter pulmonary valve therapy (14-17). Recently we published our technical experience remodelling the native or patch-repaired RVOT as an off-label approach (13). Considering, our here reported mid-term results after these and further off-labelled treated patients; the transcatheter approach offers hemodynamic improvements with a low technical and follow-up complication rate despite the wide range of different anatomies with a high variability of RVOT diameters. Twenty-four months after PPVI, the most beneficial effect was achieved by correcting the pre-existing PI without inducing a significant systolic pressure gradient. RV remodelling could be observed. No PPV endocarditis is seen so far; latest also related to a significant residual RVOT obstruction (18). The here post PPVI observed increased Doppler flow velocity, equivalent to a systolic pressure gradient in the mean of 15 mmHg, is probably related to the reduced RVOT compliance in consequence of the stents than based on a true obstruction. Further, stent migration or even embolization could be avoided, despite valve treatment in extreme wide RVOT. Whether treatment of a wide, regurgitating RVOT avoids also stent fractures was not systematically re-examined. However, severely obstructed RVOT conduits (19) are at higher risk for stent fractures with their possible consequences. Data from Melody® multicentre studies showed even a lower incidence of stent fracture among multiple pre-stented patients (20); therefore, the multiplicity of stents for optimal preparing the RVOT has a further impact on the low incidence for re-interventions: the radial load on the implanted stents and valve is reduced and consecutively the incidence of stent fractures. Up-to-now, re-interventions became not necessary in our PPV-treated patients. Unfortunately, our series is too small for discriminating the mid- or even long-term stability and function of both PPV systems, the Sapien-Edwards pericardial valve versus the Melody® bovine jugular vein valve.
However, one question remains currently and even in the future un-answered: if valves with a self-expandable stent system become available; does an enlarged RVOT need a surgically reconstruction or is the implantation of a well-functional valve itself sufficient to resolve the pre-existing and in particular arrhythmic problems during the follow-up. QRS duration is a marker to assess the risk of sudden arrhythmic death in patients with RVOT dysfunction and congenital heart disease (6). Patients with PR who do not undergo pulmonary valve replacement or PPVI have an increased right ventricular end-diastolic volume, which is directly associated with an increase in QRS duration of 2 to 4 milliseconds per year (21,22). Our PPVI study has not shown a significant shortening of QRS duration in the observation period in particular in the cohort of patients with predominant PI. However, a QRS progression could even not be observed, if this can be implied as a PPVI success, remains un-answered, too. However, it has not to be neglected that arrhythmias can even be triggered by PPVI; in our series, frequent isolated PVCs were seen in 2 patients and NSVT in one. Considering the QRS-axis, the etiology of the arrhythmias seemed to be associated to the manipulated RVOT. Significant incidence of new PVCs and NSVT immediately post-PPVI is already described by other groups (23,24). Most of these new arrhythmias resolved after a certain time after the procedure. Nguyen et al. (23) attribute these changes to the irritability caused by the placed stents within the RVOT; The real cause remains speculative, but the contraction-excitation feedback by myocardial stretching during repetitive ballooning of the RVOT and placement of stents and valve is a possible reason. In our arrhythmic patients, some degree of stent protrusion into their RVOT was observed (Figure 2); enhancing the suspicion that the etiology of the ventricular arrhythmia is caused by the manipulated RVOT.
Despite the encouraging results, PPVI based on balloon-expandable stents has unavoidable limitations in a native RVOT with variable geometry. Almost all patients treated surgically for TOF or pulmonary atresia with VSD in the Western hemisphere, suffer from a dysfunctional RVOT afterwards (25). Therefore, as long as this initial surgical technique is not changed, the need for several PPV techniques is evident.
Several transcatheter valves based on a self-expandable stent technique are in evaluation with encouraging initial results (26-29). However, in term of cost-benefit evaluations it remains open, if there is a need to develop several, high cost related valve types with variable diameters or should the research be focused on techniques for transcatheter reconstruction adapting to the multiplicity of the RVOT morphology.
Conclusions
Balloon-expandable stent-valves can be successfully implanted by percutaneous transcatheter techniques also in a non-conduit reconstructed, wide-open RVOT. However, preparation of the RVOT remains challenging. The midterm follow-up of successfully implanted stent-valves is convincing, the hemodynamic results are excellent, the complication rate low. The long-term results need further investigation in a larger patient cohort.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval from the local ethics committee (Ethik-Kommission des Fachbereichs Medizin der Universitätsklinik Gießen/Marburg) and written informed consent from all patients were obtained. Registration number of the local ethics committee in Giessen: 137-14.
References
- Ansari MM, Cardoso R, Garcia D, et al. Percutaneous pulmonary valve implantation: Present status and evolving future. JACC 2015;66:2246-55. [Crossref] [PubMed]
- Al Habib Hamad F. Contemporary Patterns of Management of Tetralogy of Fallot: Data From The Society of Thoracic Surgeons Database. Ann Thorac Surg 2010;90:813-9. [Crossref] [PubMed]
- Jones MI, Qureshi SA. Recent advances in transcatheter management of pulmonary regurgitation after surgical repair of tetralogy of Fallot. F1000Res 2018.7. [PubMed]
- Carminati M, Pluchinotta FR, Piazza L, et al. Echocardiographic assessment after surgical repair of tetralogy of Fallot. Front Pediatr 2015;3:3. [Crossref] [PubMed]
- Therrien J, Marx GR, Gatzoulis MA. Late problems in tetralogy of Fallot: recognition, management and prevention. Cardiol Clin 2002;20:395-404. [Crossref] [PubMed]
- Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 2000;356:975-81. [Crossref] [PubMed]
- Babu-Narayan SV, Diller GP, Gheta RR, et al. Clinical outcomes of surgical pulmonary valve replacement after repair of tetralogy of Fallot and potential prognostic value of preoperative cardiopulmonary exercise testing. Circulation 2014;129:18-27. [Crossref] [PubMed]
- ESC Guidelines for the management of grown-up congenital heart disease (new version 2010): The Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2915-57. [Crossref]
- Suradi HS, Hijazi ZM. Percutaneous pulmonary valve implantation. Glob Cardiol Sci Pract 2015;2015:23. [Crossref] [PubMed]
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000;356:1403-5. [Crossref] [PubMed]
- Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. Eur Heart J 2011;32:1260-5. [Crossref] [PubMed]
- Boudjemline Y, Brugada G, Van-Aerschot I, et al. Outcomes and safety of transcatheter pulmonary valve replacement in patients with large patched right ventricular outflow tracts. Arch Cardiovasc Dis 2012;105:404-13. [Crossref] [PubMed]
- Esmaeili A, Bollmann S, Khalil M, et al. Percutaneous pulmonary valve implantation for reconstruction of a patch-repaired right ventricular outflow tract. J Interv Cardiol 2018;31:106-11. [Crossref] [PubMed]
- Malekzadeh-Milani S, Ladouceur M, Cohen S, et al. Results of transcatheter pulmonary valvulation in native or patched right ventricular outflow tracts. Arch Cardiovasc Dis 2014;107:592-8. [Crossref] [PubMed]
- Cools B, Brown SC, Heying R, et al. Percutaneous pulmonary valve implantation for free pulmonary regurgitation following conduit-free surgery of the right ventricular outflow tract. Int J Cardiol 2015;186:129-35. [Crossref] [PubMed]
- Haas NA, Carere RG, Oliver Kretschmar O, et al. Early outcomes of percutaneous pulmonary valve implantation using the Edwards SAPIEN XT transcatheter heart valve system. Int J Cardiol 2018;250:86-91. [Crossref] [PubMed]
- Boudjemline Y. A new one-step procedure for pulmonary valve implantation of the melody valve: Simultaneous prestenting and valve implantation. Catheter Cardiovasc Interv 2018;91:64-70. [Crossref] [PubMed]
- Patel M, Malekzadeh-Milani S, Ladouceur M, et al. Percutaneous pulmonary valve endocarditis: Incidence, prevention and management. Arch Cardiovasc Dis 2014;107:615-24. [Crossref] [PubMed]
- McElhinney DB, Cheatham JP, Jones TK, et al. Stent Fracture, Valve Dysfunction, and Right Ventricular Outflow Tract Reintervention After Transcatheter Pulmonary Valve Implantation: Patient-Related and Procedural Risk Factors in the US Melody Valve Trial. Circ Cardiovasc Interv 2011;4:602-14. [Crossref] [PubMed]
- Cabalka AK, Hellenbrand WE, Eicken A, et al. Relationships among conduit type, pre-Stenting, and outcomes in patients undergoing transcatheter pulmonary valve replacement in the prospective North American and European melody valve trials. JACC Cardiovasc Interv 2017;10:1746-59. [Crossref] [PubMed]
- Abd El Rahman MY, Abdul-Khaliq H, Vogel M, et al. Relation between right ventricular enlargement, QRS duration, and right ventricular function in patients with tetralogy of Fallot and pulmonary regurgitation after surgical repair. Heart 2000;84:416-20. [Crossref] [PubMed]
- Neffke JG, Tulevski II, van der Wall EE, et al. ECG determinants in adult patients with chronic right ventricular pressure overload caused by congenital heart disease: relation with plasma neurohormones and MRI parameters. Heart 2002;88:266-70. [Crossref] [PubMed]
- Nguyen HH, Shahanavaz S, Van Hare GF, et al. Percutaneous Pulmonary Valve Implantation Alters Electrophysiologic Substrate. J Am Heart Assoc 2016;5. [Crossref] [PubMed]
- Loar RW, Qureshi AM, Miyake CY, et al. Percutaneous Pulmonary Valve Implantation-Associated Ventricular Tachycardia in Congenital Heart Disease. J Interv Cardiol 2016;29:639-45. [Crossref] [PubMed]
- Schievano S, Coats L, Migliavacca F, et al. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson 2007;9:687-95. [Crossref] [PubMed]
- Cao QL, Kenny D, Zhou D, et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv 2014;84:1131-7. [Crossref] [PubMed]
- Bergersen L, Benson LN, Gillespie MJ, et al. Harmony feasibility trial: acute and short-Term outcomes with a self-Expanding transcatheter pulmonary valve. JACC Cardiovasc Interv 2017;10:1763-73. [Crossref] [PubMed]
- Kim GB, Song MK, Bae EJ, et al. Successful Feasibility Human Trial of a New Self-Expandable Percutaneous Pulmonary Valve (Pulsta Valve) Implantation Using Knitted Nitinol Wire Backbone and Trileaflet α-Gal-Free Porcine Pericardial Valve in the Native Right Ventricular Outflow Tract. Circ Cardiovasc Interv 2018;11:e006494. [Crossref] [PubMed]
- Zahn EM, Chang JC, Armer D, et al. First human implant of the Alterra Adaptive Prestent™:A new self-expanding device designed to remodel the right ventricular outflow tract. Catheter Cardiovasc Interv 2018;91:1125-9. [Crossref] [PubMed]


