Pediatric melanoma and aggressive Spitz tumors: a retrospective diagnostic, exposure and outcome analysis
Introduction
Melanoma remains a rare but serious pediatric disease, with incidence ranging from 0.3–2% of all total melanomas diagnosed depending on the study and the precise patient populations examined (prepubertal versus pubertal) (1,2). True pediatric melanomas and melanocytic lesions such as Spitz tumors of undetermined biological significance (S-UBS) are rare and lead to a number of clinical challenges in the diagnosis and treatment of such lesions (3-8). While “classic” adult melanomas follow the standard “ABCDE” (asymmetry, irregular borders, color variety, large diameter, and evolving), pediatric melanomas can often be colorless (9), lending to potential challenges in initial lesion identification in the primary care setting.
While it has been suspected that S-UBS behave differently/less malignantly than true melanomas (4,10,11), there is still much controversy surrounding the aggressiveness of the array of Spitz lesions in children. Sentinel lymph node biopsy of difficult/indeterminate lesions is one adjunct by which to assess a lesion’s aggressive nature (12) and is a standard practice at many institutions. More recently, new molecular studies have revealed that a minority of Spitz tumors have amplifications in chromosome 11p (13) and more commonly have HRAS compared to BRAF or NRAS mutations that are commonly seen in melanoma (4,14). While these may help in guiding diagnostic decision making, they are again only present in a minority of cases.
Given the difficulty in determining the aggressiveness of Spitz lesions/spitzoid melanoma, pediatric patients with positive sentinel node biopsies have been largely managed in the same manner with melanoma of the same stage at the time of this study. The upfront interventions, from surgery/lymph node dissection to interferon therapy, have a range of side effects, some of them long-lasting including mood disturbances and hypothyroidism (15). Interferon alpha therapy has been routinely offered to pediatric patients with stage III/high risk lesions based on data from the adult literature demonstrating a survival advantage in adult melanoma patients receiving interferon (16,17). However, this differs from stage III melanoma therapy in adults where interferon is typically not administered because of the significant side effect profile and limited benefit in that setting. In general, pediatric patients tend to tolerate interferon therapy better when compared to the adult population (9).
With the overall incidence of pediatric melanoma on the rise at about 2% annually (18) and delays in diagnosis potentially leading to disease progression and metastasis requiring aggressive, invasive medical interventions, recognizing these lesions early and accurately is essential. Further, taking steps toward identifying lower risk pediatric patients and minimizing interventions could also lend to decreasing long term morbidity. In this retrospective study, we aimed to determine factors leading to diagnosis delay, side effects and exposures during therapy and overall survival in this patient population. Further, through this analysis we aim to identify points of intervention to be used in future studies to improve the diagnostic process and limit side-effects/exposures in this patient population.
Methods
Study design
The Institutional Review Board (IRB) approved (HUM00050014) University of Michigan Pediatric Oncology “Study of Long Term Outcome of Children and Young Adults with Cancer” database was queried (IRB study approval number HUM00117410) for all cases of pediatric melanoma and high risk atypical melanocytic lesions referred to our clinic for consideration of adjuvant therapy (inclusion criteria included a minimum stage III disease at diagnosis according to the National Comprehensive Cancer Network melanoma guidelines, version 3.2015, or equivalent for spitzoid lesions with positive sentinel lymph node biopsies) over a 13-year time period [2000–2013]. A retrospective chart review of these cases was performed in order to address the specific parameters and outcomes in these patients including presenting features, age, sex, ethnicity, lesion location, time from parental concern noted to presentation to PCP, time from PCP presentation to final diagnosis, histology, genomic data, Clark level, Breslow depth, stage, outside pathological diagnosis, final pathological diagnosis upon expert dermatopathology review at our institution, lymph node status, surgical interventions, presence of lymphedema, presence of scar or keloid, other surgical complications, treatment received, side effects from interferon therapy, use of PJP prophylaxis, other skin lesions biopsied after diagnosis, scans following diagnosis, family history and current patient status. Patient information was de-identified and kept confidential in accordance with the Health Insurance Portability and Accountability Act (HIPAA) standards. Patients referred to our clinic with concerning lesions/lymph node positivity but were found by our multidisciplinary team to have a final diagnosis of pigmented epithelioid melanocytoma (PEM, n=2) were excluded from final survival analyses given the known benign course of these lesions. The PEM patients also did not undergo interferon therapy treatment.
Statistical methods
The Wilcoxon rank-sum test was used to compare time to final diagnosis between two groups (first diagnosed as a wart vs excluding wart primary diagnosis). Overall patient survival was defined from the date of diagnosis to death of any cause. Data was censored at the last follow-up for patients who were still alive at the time of analysis. Progression-free survival was defined from the date of diagnosis to the earlier date of either disease progression or death. Survival curves were constructed using the Kaplan-Meier method and survival differences were assessed using the log-rank test. Statistical significance was defined as a P<0.05. All analyses were performed using SAS 9.4 software.
Results
The University of Michigan Division of Pediatric Hematology and Oncology saw 30 pediatric patients from 2000 to 2013 with skin lesions that included the following pathologic diagnoses: aggressive atypical Spitz tumor of uncertain biologic potential (~50%), melanoma (~23%), nodular melanoma (10%), superficial spreading melanoma (~7%), PEM (~7%), and other (~3%). A breakdown of the pathologic subtypes and further patient demographic information is detailed (Figure 1).
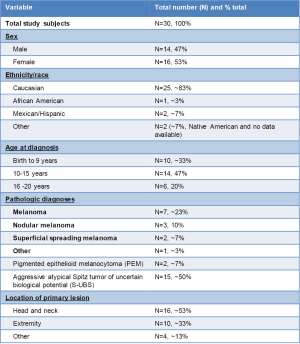
Briefly, there was a near-even distribution of males versus females (1 to 1.14), which is consistent with the SEER (Surveillance, Epidemiology, and End Results) data for pediatric melanomas (18). The majority (approximately 83%) of the patients were Caucasian and the approximate mean age at the time of diagnosis was 11 years. The head and neck (~53%) and extremity (~33%) were the most common location of primary lesions. As about 10% of melanomas are thought to be hereditary (19) and there are reported familial cancer syndromes including melanoma, we also analyzed documented family histories for relevant associations such as other family members with melanoma, pancreatic cancer [given links with CDKN2 mutations (20)], breast cancer and mesothelioma (21,22) (Table 1). Most patients in this study had no significant family history suggestive of a familial cancer syndrome, but one familial syndrome was discovered out of the 30 patients reviewed. This family was referred to genetics for further risk assessment and long-term management. There were no obvious links with the obtained family histories and patient outcomes in this study. Family history data for 29 of 30 pediatric patients included in this study, as the remaining patient was adopted and therefore no family history was available.

Full table
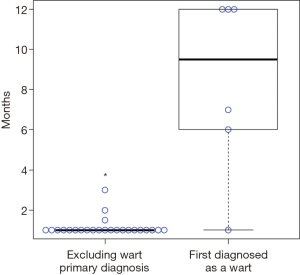
Upon analyzing the initial presenting skin lesion features, we noted that lesion growth, bleeding, and itching were the most common reasons why children were taken to their primary pediatricians for evaluation. In contrast to the “classic” darkly pigmented lesions, melanomas can also present with amelanotic or flesh-colored lesions in both pediatric and adult populations. Six of our thirty patients had such lesions lacking pigment and these six lesions were all initially misdiagnosed as warts. The patients with amelanotic lesions were treated with multiple rounds of wart therapies such as liquid nitrogen prior to a biopsy being performed. In our patient population, initial misdiagnosis led to significant delays in the diagnosis of melanoma or atypical melanotic lesions compared to lesions not labeled as a wart in the primary care setting (P=0.00011, Figure 2). The median time of diagnosis delay in the amelanotic group was greater than 9 months. Of the patients initially clinically diagnosed with warts, four had a pathological diagnosis of a S-UBS and two were diagnosed with melanoma. We next analyzed the pathologic data to look for changes in diagnosis upon expert pathology review. In this series of thirty patients, two (6–7%) had changes in diagnosis. Six other patients were noted to be very challenging cases from a pathology perspective and requested second opinions prior to establishing a final diagnosis.
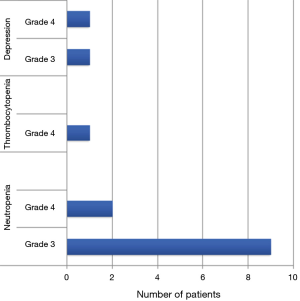
All patients were considered for interferon alpha therapy, a common practice in pediatric oncology during this study period based on adult literature demonstrating a survival advantage (16). Twenty-six patients received interferon alpha therapy. The four patients that did not receive interferon included patients with the final diagnosis of PEM [reported to be a very benign behaving lesion with excellent clinical outcomes despite frequent lymph node involvement (23,24)], a patient that was non-compliant with follow-up and one patient with rapid disease progression. Those patients receiving interferon alpha therapy were planned to received 4 weeks of high dose IV interferon (20 million units/m2, 5 days weekly) followed by 48 weeks of low dose subcutaneous interferon (10 million units/m2, 3 days weekly). Noted side effects documented from interferon therapy included but were not limited to fevers, chills, myalgias, headache, nausea, fatigue, weight loss, transaminitis, hair loss, mood disturbances, Bell’s Palsy and pain. Interferon dose adjustments or discontinuation in our patient population were implemented for side effects including neutropenia, thrombocytopenia, and depression/anxiety (mood disturbances). The number of patients experiencing and grades of severity (based on Common Terminology Criteria for Adverse Events, CTCAE) of these side effects are in Figure 3. The majority of dose reductions occurred during high dose interferon therapy (the first four weeks). Five patients experienced abnormal thyroid studies but none required dose modifications. As noted in Figure 4A, 7 of 13 patients with the diagnosis of melanoma and 14 of 15 patients with S-UBS completed 52 weeks of interferon therapy without dose reduction or drug discontinuation.
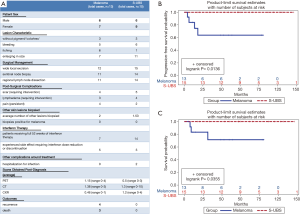
We then examined both the side effect burden (taking into consideration all aspects of therapy including surgical management (25,26), surgical complications, interferon therapy, radiation exposure due to scans, etc.) and the overall survival of patients with S-UBS versus melanoma patients in our population. Patients with PEM were excluded from the analyses, given the benign nature of these lesions and the fact that these patients did not receive interferon therapy (as discussed previously). Figure 4A compares the melanoma versus S-UBS groups for differences in variables including patient sex, lesion characteristics, surgical management, post-surgical complications, other skin lesions biopsied, interferon therapy, other treatment complications, surveillance scans, and outcomes. There was an even distribution of male and female cases in each group. Regarding lesion characteristics, itching was reported more commonly in patients diagnosed with melanoma versus S-UBS (6 vs. 1 patient, respectively). Surgical complications including lymphedema, scar formation and pain were comparable between the two groups. Both groups had similar numbers of subsequent lesions biopsied, none revealing melanoma. Seven of 13 patients (~54%) of melanoma patients and 14 of 15 (~93%) of S-UBS completed 52 weeks of interferon therapy without interruption (dose reduction or drug discontinuation). Two S-UBS patients were hospitalized for infections while PICC lines were in place (around the time of high dose interferon therapy). Disease monitoring with scans revealed great variability both in the number and type of scans ordered. This appeared to be due to individual physician preference. On scans performed at the end of interferon therapy, one melanoma patient was noted to have new lung lesions, later confirmed to be new metastatic disease. None of the scans obtained in the S-UBS patients were positive for disease/new lesions.
Lastly, we examined progression free and overall survival based on diagnosis. A significant difference in progression-free (Figure 4B) and overall survival (Figure 4C) was noted, with the S-UBS group having no patients demonstrating progression (P=0.0136) or death (P=0.0355), while the melanoma group had four patients progress, with three of these eventually passing away from disease. None of the patients with delayed diagnoses due to a “wart-like” appearance of their lesions progressed or passed away (again, this group included both melanoma and S-UBS patients). One of the patients whose pathological diagnosis was notably challenging upfront (and required expert pathology review to establish a final diagnosis) was one of the three that passed away.
Discussion
Pediatric patients with aggressive S-UBS and melanoma continue to be a rare and very challenging patient population. Given the possibility of familial cancer syndromes in such patients (one identified in this study), a detailed cancer history should be obtained. With the presence of clear side effects from both the medical and surgical interventions surrounding the diagnosis and treatment of pediatric melanomas and S-UBS, it is of critical importance to prevent diagnosis delays as much as possible in order to minimize the amount of interventions required and to accurately triage these patients upfront. This study revealed that in our pediatric patient population, lesions being incorrectly labeled and treated as warts lead to a significant delay in diagnosis. While this is a difficult visual diagnosis, typically warts have thrombosed capillaries and interrupt skin lines while more concerning lesions often have “red flag” features such as itching. This “wart-like” appearance does not discriminate between melanomas and S-UBS. While none of these patients misdiagnosed as warts died, it is possible that if diagnosed earlier, the disease may have not been staged as high and these patients could have been spared some interventions (further lymph node dissection, interferon treatment, or exposure to radiation for scans for disease detection or monitoring). We feel that continued education of primary care providers about such lesions could be a key intervention to prevent delays. As warts are far more common than melanoma in pediatrics, we propose that if warts have corresponding lack of response to therapy, lesion growth, bleeding, or itching, that these patients be promptly biopsied prior to continuing wart therapy.
A second issue noted was the initial pathological misdiagnosis or diagnostic uncertainty surrounding these cases. Eight of the thirty cases (~27%) were documented to be challenging pathology cases and did result in some changes in diagnosis. This fact highlights the point that it is essential upfront for expert case review to be sought out in these pediatric cases.
A patient management point that was discovered during our analysis is the high variability in the types and frequency of scans for monitoring this cohort of patients. While some patients only received scans upfront, half-way and the end of therapy, other patients had scans at more frequent intervals. Patients were monitored with chest X-rays, CT scans, PET scans or some combination of these. Only one patient with a diagnosis of melanoma had asymptomatic new metastatic lesions that were noted on end of therapy scan only. There is a lack of consensus in the pediatric literature pertaining to the type and frequency of scans in these patients. Given that in general, limiting radiation exposure in children is a priority and the fact that S-UBS patients do well and likely would not need scans for monitoring at any point, forming consensus recommendations for imaging in this patient population will be a future priority.
With the controversy surrounding the aggressiveness of spitzoid lesions, the survival data obtained from our single center study suggests that even patients with histologically aggressive/lymph node positive S-UBS have very favorable outcomes and the role of adjuvant therapy as well as imaging for surveillance in these patients needs to be examined in the future in a prospective multi-institutional study. On the other hand, true pediatric melanomas showed aggressive clinical behavior and mortality similar to their adult counterparts despite interferon therapy (27). New investigational approaches are desperately needed and agents recently approved for adult melanoma, including B-RAF inhibitors, MEK inhibitors, CTLA-4 inhibitors and PD-1 inhibitors (28-30) need to be tested either as a single agent or as combination therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board (IRB) approved (HUM00050014) University of Michigan Pediatric Oncology “Study of Long Term Outcome of Children and Young Adults with Cancer” database was queried (IRB study approval number HUM00117410) for all cases of pediatric melanoma and high risk atypical melanocytic lesions referred to our clinic for consideration of adjuvant therapy (inclusion criteria included a minimum stage III disease at diagnosis according to the National Comprehensive Cancer Network melanoma guidelines, version 3.2015, or equivalent for spitzoid lesions with positive sentinel lymph node biopsies) over a 13-year time period [2000–2013].
References
- Livestro DP, Kaine EM, Michaelson JS, et al. Melanoma in the young: differences and similarities with adult melanoma: a case-matched controlled analysis. Cancer 2007;110:614-24. [Crossref] [PubMed]
- Wong JR, Harris JK, Rodriguez-Galindo C, et al. Incidence of childhood and adolescent melanoma in the United States: 1973-2009. Pediatrics 2013;131:846-54. [Crossref] [PubMed]
- Kirkwood JM, Jukic DM, Averbook BJ, et al. Melanoma in pediatric, adolescent, and young adult patients. Semin Oncol 2009;36:419-31. [Crossref] [PubMed]
- Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions part I. Background and diagnoses. J Am Acad Dermatol 2011;65:1073-84. [Crossref] [PubMed]
- Paradela S, Fonseca E, Prieto VG. Melanoma in children. Arch Pathol Lab Med 2011;135:307-16. [PubMed]
- Aldrink JH, Selim MA, Diesen DL, et al. Pediatric melanoma: a single-institution experience of 150 patients. J Pediatr Surg 2009;44:1514-21. [Crossref] [PubMed]
- Cerroni L, Barnhill R, Elder D, et al. Melanocytic tumors of uncertain malignant potential: results of a tutorial held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. Am J Surg Pathol 2010;34:314-26. [Crossref] [PubMed]
- Neier M, Pappo A, Navid F. Management of melanomas in children and young adults. J Pediatr Hematol Oncol 2012;34 Suppl 2:S51-4. [Crossref] [PubMed]
- Reed D, Kudchadkar R, Zager JS, et al. Controversies in the evaluation and management of atypical melanocytic proliferations in children, adolescents, and young adults. J Natl Compr Canc Netw 2013;11:679-86. [Crossref] [PubMed]
- Dika E, Fanti PA, Fiorentino M, et al. Spitzoid tumors in children and adults: a comparative clinical, pathological, and cytogenetic analysis. Melanoma Res 2015;25:295-301. [Crossref] [PubMed]
- Ludgate MW, Fullen DR, Lee J, et al. The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer 2009;115:631-41. [Crossref] [PubMed]
- Su LD, Fullen DR, Sondak VK, et al. Sentinel lymph node biopsy for patients with problematic spitzoid melanocytic lesions: a report on 18 patients. Cancer 2003;97:499-507. [Crossref] [PubMed]
- Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol 2000;157:967-72. [Crossref] [PubMed]
- van Dijk MC, Bernsen MR, Ruiter DJ. Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and spitzoid melanoma. Am J Surg Pathol 2005;29:1145-51. [Crossref] [PubMed]
- Navid F, Furman WL, Fleming M, et al. The feasibility of adjuvant interferon alpha-2b in children with high-risk melanoma. Cancer 2005;103:780-7. [Crossref] [PubMed]
- Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17. [Crossref] [PubMed]
- Anaya DA, Xing Y, Feng L, et al. Adjuvant high-dose interferon for cutaneous melanoma is most beneficial for patients with early stage III disease. Cancer 2008;112:2030-7. [Crossref] [PubMed]
- Slade AD, Austin MT. Childhood melanoma: an increasingly important health problem in the USA. Curr Opin Pediatr 2014;26:356-61. [Crossref] [PubMed]
- Ransohoff KJ, Jaju PD, Tang JY, et al. Familial skin cancer syndromes: Increased melanoma risk. J Am Acad Dermatol 2016;74:423-34. [Crossref] [PubMed]
- Whelan AJ, Bartsch D, Goodfellow PJ. Brief report: a familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N Engl J Med 1995;333:975-7. [Crossref] [PubMed]
- Carbone M, Ferris LK, Baumann F, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med 2012;10:179. [Crossref] [PubMed]
- Debniak T. Familial malignant melanoma - overview. Hered Cancer Clin Pract 2004;2:123-9. [Crossref] [PubMed]
- Mandal RV, Murali R, Lundquist KF, et al. Pigmented epithelioid melanocytoma: favorable outcome after 5-year follow-up. Am J Surg Pathol 2009;33:1778-82. [Crossref] [PubMed]
- Zembowicz A, Carney JA, Mihm MC. Pigmented epithelioid melanocytoma: a low-grade melanocytic tumor with metastatic potential indistinguishable from animal-type melanoma and epithelioid blue nevus. Am J Surg Pathol 2004;28:31-40. [Crossref] [PubMed]
- Kayton ML, La Quaglia MP. Sentinel node biopsy for melanocytic tumors in children. Semin Diagn Pathol 2008;25:95-9. [Crossref] [PubMed]
- Lohmann CM, Coit DG, Brady MS, et al. Sentinel lymph node biopsy in patients with diagnostically controversial spitzoid melanocytic tumors. Am J Surg Pathol 2002;26:47-55. [Crossref] [PubMed]
- Lewis KG. Trends in pediatric melanoma mortality in the United States, 1968 through 2004. Dermatol Surg 2008;34:152-9. [Crossref] [PubMed]
- Foletto MC, Haas SE. Cutaneous melanoma: new advances in treatment. An Bras Dermatol 2014;89:301-10. [Crossref] [PubMed]
- Jazirehi AR, Lim A, Dinh T. PD-1 inhibition and treatment of advanced melanoma-role of pembrolizumab. Am J Cancer Res 2016;6:2117-28. [PubMed]
- Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275-87. [Crossref] [PubMed]


