Hypoglycemia in the preterm neonate: etiopathogenesis, diagnosis, management and long-term outcomes
Introduction
Hypoglycemia [Gk. hypo (below or under) + glykys (sweet) + haima (blood)] meaning a decreased level of sugar in the blood was coined by Harris in the late 19th century (1). Over the last century there has been a large body of literature looking into the effects of low blood glucose, specifically on neonates, however there is little consensus regarding its definition or acceptable range of glucose in various types of neonates. Hypoglycemia has since been measured and defined as a range of clinical manifestations and lab values and approach to this problem has slowly been tailored to the particular infant’s unique physiologic adaptations. The association of hypoglycemia and neurodevelopmental abnormalities in preterm infants was first defined as early as 1937. This led to categorization of hypoglycemia as “mild” between 40 to 50 mg/dL (2.2 to 2.8 mmol/L), “moderate” between 20 and 40 mg/dL (1.1 to 2.2 mmol/L) and “severe” as 20 mg/dL (1.1 mmol/L) (2). One group of investigators suggested 30–35 mg/dL (1.67 to 1.94 mmol/L) as the normal low range of glucose during the first 24 hours of life; 45 mg/dL (2.5 mmol/L) after feeding and 40–50 mg/dL (2.22 to 2.78 mmol/L) after completion of 24 hours of life (3). Since then, many operational thresholds have been described. The American Academy of Pediatrics (AAP) and the Pediatric Endocrine Society (PES) have both tried to come to a consensus for a safe range of glucose in neonates. It is now accepted that plasma glucose values drop down to 30 mg/dL (1.67 mmol/L) in the first 2 hours of life and subsequently rise to a value of at least 45 mg/dL (2.5 mmol/L) before stabilizing around 12–24 hours (4). The AAP has arbitrarily adopted the numerical plasma glucose value of 47 mg/dL (2.6 mmol/L) to define hypoglycemia in neonates (5).
Small for gestational age (GA) neonates and preterm infants
Studying energy requirements specifically glucose, in both small for gestational age (SGA) and appropriate size for gestational age (AGA) preterm infants has been a topic of interest for the last many decades. Initial studies dating back to the early 1970’s confirmed the belief that not only are these infants more susceptible to lower blood glucose values, but they are also at higher risk for slow recovery and poor long-term prognosis (6,7). The estimated incidence of hypoglycemia in SGA infants is around 70% (8). One study showed that 15% of preterm hypoglycemic infants were AGA and the rest were either large for gestational age (LGA) or SGA (9). In 1970’s, Cornblath et al. put forth the possibility of a lower operational threshold of blood sugar for preterm infants at 20 mg/dL based on the rationale that their being symptom free proved that their brains are less susceptible to and better adapted to low glucose values (3). Transabdominal cordocentesis was done to measure fetal blood glucose values and then compared with maternal serum glucose concentration, showed fetal venous glucose values of 72–90 mg/dL (4–5 mmol/L). There was a widening of glucose gradient between maternal and fetal circulation as the infant approached term (10). It has been shown that intra-uterine growth restriction correlated closely with the degree of hypoxia but not hypoglycemia. The hypoglycemia itself is more a result of decreased production of glucose due to small stores of glycogen then excess utilization (11). There is a strong correlation between fetal and maternal glucose values during early gestation. However, approaching the third trimester, with increased fetal glucose utilization, the gradient increases and maternal values are higher than fetal values (12). This suggests that preterm and SGA infants should characteristically have similar serum glucose concentration as their mothers. In order to promote facilitated diffusion in severely growth restricted fetuses, this gradient is widened and is a function of clinical severity, hence, fetal blood glucose values correlate with GA as well as maternal glucose (13).
Physiology of glucose metabolism in the neonate
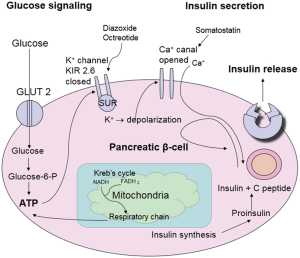
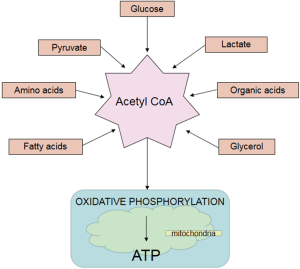
A steady state glucose level is maintained by various processes of gluconeogenesis, metabolism, insulin secretion, and ‘counter regulatory’ hormones in the neonate as shown in Figures 1 and 2. This homeostasis is maintained not only by insulin and glucagon but also by hormones such as catecholamines, growth hormone, and cortisol that determine its uptake and utilization. Ingestion of feed or infusion of glucose in the form of carbohydrate contributes to increasing glucose concentration in the body which in turn activates glucokinase and β cell glycolysis (14,15). This process eventually generates acetyl-coenzyme A (acetyl CoA) which is a common end product not only of glycolysis but also of protein degradation and lipolysis. Acetyl Co-A enters the Kreb’s cycle which then provides the adenosine triphosphate (ATP) and supports all cell functions (15,16).
Pancreatic β cells produce insulin and α cells produce glucagon. β cells contain ATP-sensitive potassium channels (also known as KATP channels which contain two subunits: sulfonylurea [sulfonylurea receptors (SUR)] and an inward rectifier potassium channel (Kir6.2) (14,16). ATP produced as a result of the citric acid cycle closes the KATP channels simultaneously creating an inflow of calcium through voltage gated calcium channels that leads to release of insulin (17) (Figure 2). If there is paucity of glucose in the blood; glucagon is secreted, possibly due to a similar mechanism via KATP channels on the α cells.
Facilitated transport of glucose
Glucose is the major source of energy for the brain, fetal and neonatal brains are capable of utilizing ketone bodies, lactate and even amino acids in extreme conditions (3,18). The neonatal brain is one of the most energy efficient organ as it oxidizes almost all of the glucose delivered to it (19). Glucose transport (GLUT) through the blood-brain barrier as well as its permeation through lipid membranes of neurons and glia takes place through a process known as facilitated diffusion. Facilitated diffusion is an energy independent pathway that transports glucose from blood into the cytoplasm and the process is bidirectional. Thus, ongoing glycogenolysis and gluconeogenesis often contribute to efflux of glucose from the cell.
During gestation, in mature placentas, insulin does not alter or assist glucose uptake from the maternal or fetal side (20). The large surface area of choriodecidual villi is directly in contact with maternal blood resulting in the mother-baby dyad sharing a common pool of glucose (19,21). Most of the glycogen deposition in the fetal tissue occurs during the second half of pregnancy. Adequate transport of glucose from mother to baby is mainly determined by the umbilical blood flow since there has been no demonstrable difference in levels of expression of glucose transporter-1 (GLUT-1) protein in placentas (22).
GLUT proteins
Facilitated glucose transfer is mediated by a family of GLUT proteins, nearly 14 of which have been identified so far. Protein and mRNA levels of GLUT1 increase in the placenta with fetal maturation (23). It has been shown to be responsible for transfer of glucose, transporting it from maternal blood into the cytoplasm of the syncytiotrophoblast and then into the extracapillary space in the fetal circulation, by facilitating its exit through the basement membrane (21).
GLUT 1, 3 and 11 are found in the placenta and are important for the growing fetus while GLUT 2 and 4 are insulin responsive glucose transporters (20). GLUT 1 helps transport glucose across the blood brain barrier (BBB) and working closely with GLUT 3 assists in brain cell glucose uptake (24). GLUT’s play a significant role in determining the phenotype of the large and small for GA infants. Gestational diabetes leads to increased glucose in the maternal blood and hence increased transplacental transport into the placenta and the fetus resulting in excess growth mediated through insulin like growth factor-1 (IGF-1). In these pregnancies GLUT 1 levels were found to be elevated while no changes were found in GLUT 3 and GLUT 4, suggesting that fetal hyperglycemia in diabetic pregnancies is a direct correlate of GLUT 1 levels. In animal studies of IUGR pregnancies, there has been an observed relative fetal hypoglycemia which further enhances facilitated diffusion (20,21,25-28).
Physiology of transition from fetal to the neonatal life
Transition from fetal to the neonatal life is the most complex and crucial physiological adaptation in human life (29,30). As the placental supply of glucose ceases, the plasma glucose values hit a nadir in the first 2 hours after birth triggering release of counter regulatory hormones important for gluconeogenesis within the first 6–24 hours of life (31). There is a surge in catecholamines (epinephrine and norepinephrine), which play a crucial role in adaptation to various stressors outside the womb (29,32). Infants born via cesarean section have lower catecholamine levels compared to those born via spontaneous vaginal delivery and therefore are more prone to developing hypoglycemia. Paradoxically some preterm infants have higher cord blood levels of catecholamines compared to term infants (29). Catecholamines assist in maintaining blood pressure, and normothermia by stimulating alpha receptors which then increase blood pressure and helps utilize brown fat. This also helps prevents hypoxia by induction of alveoli to increase surfactant production (29). Epinephrine promotes liver glycogenolysis and gluconeogenesis which helps prevent hypoglycemia (33).
A “cortisol surge” occurs along with catecholamine release, promoting increased β receptor density in blood vessels and increases gluconeogenesis which further raises serum glucose levels (29,34). Preterm infants (33–36 weeks GA) have been shown to have higher cortisol blood levels than term infants (35) but levels in preterm infants 24–36 weeks GA are inversely proportional to the gestation and tend to remain high from day of life 2 through 6. However, infants <28 weeks GA and extremely sick infants do not demonstrate the same response thus making them more vulnerable to hypoglycemia (36).
Etiopathogenesis of hypoglycemia
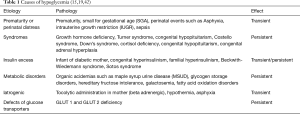
Hypoglycemia essentially results from either decreased production or excessive utilization of glucose reserves. Thus, hypoglycemia occurs in a neonate who is born with low glycogen and fat stores with limited capacity to generate glucose via the gluconeogenesis pathway or excessive peripheral tissue utilization of glucose like in an infant of a mother with insulin dependent diabetes (37-39). Euglycemia after birth is maintained by a combination of finely controlled metabolic adjustments and hormone secretions. Extremely low-birth weight (ELBW) preterm neonates are born with low stores of glycogen and adipose tissues. This situation is further complicated by the fact that several enzymes involved in gluconeogenesis viz. Phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase, fructose-1,6-diphosphatase, and Pyruvate caroboxylase are expressed at very low levels limiting their capacity for gluconeogenesis. Preterm infants and those with IUGR are highly likely to become hypoglycemic in the immediate neonatal period (40). Various congenital disorders such as Beckwith-Wiedemann syndrome, Turner syndrome, Down syndrome, Costello syndrome, congenital hypopituitarism, and congenital adrenal hyperplasia also predispose the infant to hypoglycemia. Inborn errors of metabolism like maple syrup urine disease, glycogen storage disorders, fructose intolerance, and fatty acid enzyme deficiencies can also result in persistent hypoglycemia.
Neonates have a poorly developed counterregulatory mechanisms to counter hypoglycemia which makes them highly vulnerable. The hypoglycemic neonate defends itself by decreasing insulin secretion and increasing glucagon, epinephrine, growth hormone, and cortisol secretion which leads to glucose production & mobilization of fatty acids from adipose tissues. The increase in glucose production comes initially from the breakdown of glycogen (up to 1–2 h) and later there is protein breakdown with increasing levels of cortisol. This process is evident by increased plasma levels of gluconeogenic amino acids, alanine, and glutamine. Hypoglycemia occurs when there is excessive production of insulin (overutilization), poor gluconeogenesis (underproduction), or failure of counterregulatory mechanisms (pituitary or adrenal failure).
Approach to diagnosis and management of hypoglycemia in the preterm infant
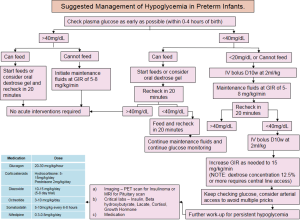
The diagnosis and management of hypoglycemia depends mostly on the cause and severity of hypoglycemia, the clinical presentation and the underlying etiology. Thus, the treatment plan should be individualized for each infant (Figure 3). The Whipple’s triad of clinical signs, low glucose value, and resolution of signs on treatment (38,41) was utilized in the past but lately we have moved more towards a proper definitive biochemical diagnosis. The key to diagnosis is to determine whether the hypoglycemia is likely to be transient or persistent (Table 1). Based on that determination, one should refer an infant with persistent hypoglycemia to a tertiary center and order sophisticated investigation as outlined below.
The infant can present with either neurogenic or neuroglycopenic signs and symptoms of disease. Neurogenic refers to an active catecholamine based response involving, tachycardia, vomiting, sweating, tremors, vomiting. Neuroglycopenic signs manifest as a result of neuronal deprivation of glucose presenting as hypotonia, apnea, seizures with coma being the worst outcome (43). Biochemical estimation of blood glucose values is more accurate than clinical assessment alone. Plasma glucose values are higher than whole blood values by 10–18% (4). The Pediatric Endocrine Society (PES) and the American Academy of Pediatrics (AAP) recommend a glucose level of ≥40 mg/dL (mmol/L) in the first 4 hours; ≥45 mg/dL (mmol/L) after feeding at 4–24 hours and treat values of ≤40 mg/dL (mmol/L) parenterally (15,19,44). The PES recommends a similar strategy suggesting levels of >50 mg/dL (mmol/L) in first 48 hours and >60 mg/dL (mmol/L) thereafter (19).
Persistent hypoglycemia is a medical emergency and calls for a fast action where a delay will lead to complications and blood glucose needs to be maintained at >70 mg/dL (mmol/L) (19). Normal term infants should be fed where possible resulting in increase in blood glucose by 30 mg/dL (1.67 mmol/L) per 30–60 mL of standard infant formula. Blood glucose should continue to be evaluated before and after feeds for at least 12–24 hours. If normoglycemia is not attained, the next step is administration of parenteral dextrose, starting with the “mini bolus” approach of 200 mg/kg of 10% dextrose in water (2 mL/kg D10W) followed by constant IV infusion and a recheck of blood sugar after 30 minutes later and then hourly (15).
The goal is to provide a continuous glucose infusion rate (GIR) of 6–8 mg/kg/min (3), this is particularly important in preterm and low birth weight infants since it is equivalent to the sugar that would have been provided by the liver via gluconeogenesis (3,10,45). A lower dextrose infusion rate of 3–5 mg/kg/min may be used for infants born to mothers with diabetes to provide minimal stimulation to their pancreas to secrete insulin (19). With these interventions, if an infant does not attain normoglycemia it is prudent to go up on the GIRs to 8, 10, 12 and then 15 mg/kg/min over a period of 24 hours (3). A dextrose concentration of higher than 12.5% calls for central venous access (46). Obtaining arterial access may also be prudent in these infants to avoid repeated needle pricks to the fingers, toes and heels of these extremely preterm babies.
Continuous glucose monitoring (CGM), although still experimental, is emerging as the new standard of care for monitoring blood glucose in tiny babies. The CGM device measures and updates blood glucose values every 5 minutes, providing real time data (42,47). Once a reliable means of evaluating the infant’s blood glucose level has been established it is important to titrate dextrose infusion accordingly. With effective therapy, most infants attain euglycemia in 2–4 days. A period of 5–7 days of hypoglycemia points toward a diagnosis of persistent neonatal hypoglycemia and necessitates alternative therapies (46). The PES suggests consideration of persistent hypoglycemia after a period of 48 hours and recommends further work up. This includes laboratory tests like insulin level, a metabolic profile, genetic studies and imaging to look for pathology in the pancreas, adrenal and the pituitary glands (19).
Corticosteroids such as hydrocortisone at 5–15 mg/kg/day or prednisone at 2 mg/kg per day decreases peripheral utilization of glucose, thus remain the second line of treatment after starting glucose infusion for persistent hypoglycemia (3,48-51). Glucagon, produced by the α cells in the pancreas, is a counter regulatory hormone and initiates gluconeogenesis and glycogenolysis during hypoglycemia (52), is helpful in raising blood glucose when infant has adequate glycogen stores. However, it’s effectivity is limited in infants of mothers who were on beta blockers such as atenolol or metoprolol (53). Glucagon administered at 30 mcg/kg or 300 mcg/kg/min infusion in infants with adequate glycogen stores promotes glycogenolysis and gluconeogenesis (16). Glucagon is especially helpful for term infants, infant of diabetic mothers when short-term treatment is desirable like during transport of critically ill infants.
Somatostatin which inhibits insulin and growth hormone release is usually reserved as a last line of treatment when other therapies fail to raise and maintain blood sugar (48). Octreotide, a long acting analogue of endogenously occurring somatostatin which acts directly on the voltage gated calcium channels has an inhibitory effect on insulin release is used where diazoxide (14,54). It is used as a constant infusion at 3–10 mcg/kg/day (14), however, it is not currently FDA approved and there are concerns that it impedes neonatal growth.
Hyperinsulinism related to genetic defects of SUR where there is unchecked production of insulin usually responds well to treatment with diazoxide (16,48). Diazoxide, a high affinity KATP channel opener acts by stabilizing these channels, and it blocks insulin secretion (55) thereby helping in the management of persistent hyperinsulinism. If hyperinsulinism is secondary to abnormalities in the SUR and Kir 6.2 subunits of the glucose channels, an impressive response to diazoxide may or may not be seen. Diazoxide is commonly used in the dose range of 10–15 mg/kg/day where responders show effect within 2–4 days (56).
Nifedipine is another off-label drug for neonatal use in cases where a response to diazoxide and octreotide are not seen (57). Nifedipine has been used in babies at a dose of 0.3–0.8 mg/kg/day by Bas et al. (58) and was effective when other therapies failed, but this medication is not the mainstay of treatment due to its cardiovascular side effects.
Newer approaches to management of neonatal hypoglycemia
Recently oral dextrose gel administration has been a very promising new intervention for management of neonatal hypoglycemia especially in the term, near term, or late preterm infants. Several studies have been conducted using dextrose gel and are summarized in Table 2. In the Sugar Babies trial, Harris et al. (64) were able to effectively show improvement in hypoglycemia within the first 48 hours; promote bonding, and prevent separation of mothers from the babies. The follow-up study conducted by the same group questioned the previous promising results by showing that it did not impact neurodevelopmental outcomes (63). Several studies have concluded that oral dextrose gel is suitable to treat hypoglycemia in stable infants, who can be fed (59-62). Dextrose gel is being favored not only for prevention and treatment of hypoglycemia but for promoting breast feeding and bonding of mothers and babies.
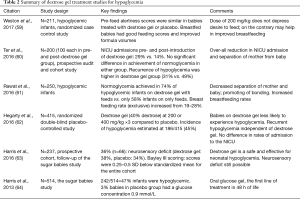
Full table
Neurodevelopmental outcomes after hypoglycemia
Neuroradiological studies to investigate the anatomical location of brain injury due to hypoglycemia specifically using various MRI techniques demonstrated cortical abnormalities in the posterior cerebral cortex with or without subcortical or periventricular injury (65). There is sparing of the parietal and occipital lobes (66,67). In rare circumstances there can be thalamic involvement or injury to the basal ganglia as well. Therefore, follow-up MRI studies are indicated when there is history of prolonged and severe hypoglycemia (65). Hypoglycemic brain injury does not necessarily track the vascular supply, thus it may be possible at times to differentiate hypoglycemic from hypoxic brain injury (68). Neurodevelopmental outcomes in infants with hypoglycemia have been extensively reviewed and some of these studies are summarized in Table 3 (8,69-78). Hypoglycemic brain injury may cause a long-term effect on the developing nervous system and may be the reason for developmental delay, cerebral palsy and other neuropsychiatric deficits noted in these children.
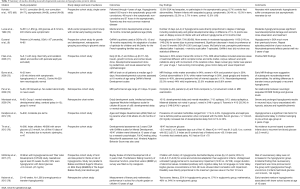
Full table
The Babies and Blood Sugars study (BABIES) studied influence of hypoglycemia on EEG using bed-side amplitude integrated electroencephalography (aEEG) to assess real-time neuronal injury due to hypoglycemia (79). Levels of glucose as well as non-glucose cerebral fuels such as lactate, beta hydroxybutyrate and glycerol were measured. These studies showed no significant EEG abnormality even during periods of low glucose and use of alternate cerebral fuels hence demonstrating that aEEG was not a useful tool (79).
Normal brain obtains more than 50% of its energy needs from glucose oxidation (44). In SGA infants, there is a strong association between hypoglycemia and small head circumference measured at 12 months, 18 months, and 5 years corrected age (8). It is known that glial proliferation takes place during the third trimester of fetal life and continues after birth (80). Hypoglycemia delays astrocyte proliferation in preterm infants and up until about 4–5 weeks of age the sensorimotor cortex, thalamus, midbrain, brainstem and cerebellar vermis are the most sensitive to hypoglycemic injury besides the occipital cortex (18,19,81). Hypoglycemic brain injury is associate with a smaller head circumference and poor cognitive abilities as shown in a cohort of 249 very low birth weight infants (82). Twelve percent of these infants (30/249) had subnormal or −2 SD age adjusted head circumference values at birth, 23% (57/249) at term corrected GA, and 13% (33/249) at 8 corrected months of age. These children had lower intelligence quotient (IQ) along with lower scores for receptive language (82). In summary, the duration, severity as well as the number hypoglycemia events closely correlate with the outcome of hypoglycemia. This may manifest as early as a few hours of life in the form of seizures or coma, or may manifest later in childhood with delayed milestones, developmental delays, poor Bayley scores, motricity and perception scores, or poor test proficiency by 4th or 5th grade.
Conclusions
While we continue to strive for a precise definition of hypoglycemia, it remains one of the most common causes of morbidity in 30–60% of preterm neonates with some of them suffering serious long-term complications (75,83,84). The extent, severity, and duration of hypoglycemia is directly proportional to the poor outcomes if not treated in a timely manner. Extreme preterm infants are susceptible to multiple comorbidities due to perinatal risk factors. all these effects are exacerbated by accompanying hypoglycemia. Late preterm or near-term infants are also at similar high risk if immediate measures to prevent hypoglycemia are not put in place. Each case should be clinically evaluated and categorized into transient or persistent hypoglycemia and all infants with persistent hypoglycemia need to be referred to a tertiary care center where advanced diagnostic and therapeutic interventions may be available. Glucose gel is a promising new tool in management of near term or late preterm neonates. A long-term neurodevelopmental follow up should be obtained for all infants who had severe and prolonged hypoglycemia and this workup should include quality neuroimaging such as MRI scans.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Harris S. Hyperinsulinism and dysinsulinism. JAMA 1924;83:729-33. [Crossref]
- Hypoglycemia Hartmann A. J Pediatr 1998;132:1-3. [Crossref] [PubMed]
- Cornblath M, Ichord R. Hypoglycemia in the neonate. Semin Perinatol 2000;24:136-49. [Crossref] [PubMed]
- Heck LJ, Erenberg A. Serum glucose levels in term neonates during the first 48 hours of life. J Pediatr 1987;110:119-22. [Crossref] [PubMed]
- Committee on Fetus and Newborn. Adamkin DH. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011;127:575-9. [Crossref] [PubMed]
- Lubchenco LO, Bard H. Incidence of hypoglycemia in newborn infants classified by birth weight and gestational age. Pediatrics 1971;47:831-8. [PubMed]
- Parmelee AH Jr, Minkowski A, Saint-Anne Dargassies S, et al. Neurological evaluation of the premature infant. A follow-up study. Biol Neonate 1970;15:65-78. [Crossref] [PubMed]
- Duvanel CB, Fawer CL, Cotting J, et al. Long-term effects of neonatal hypoglycemia on brain growth and psychomotor development in small-for-gestational-age preterm infants. J Pediatr 1999;134:492-8. [Crossref] [PubMed]
- Gutberlet RL, Cornblath M. Neonatal hypoglycemia revisited, 1975. Pediatrics 1976;58:10-7. [PubMed]
- Kalhan S, Peter-Wohl S. Hypoglycemia: what is it for the neonate? Am J Perinatol 2000;17:11-8. [Crossref] [PubMed]
- Economides DL, Nicolaides KH. Blood glucose and oxygen tension levels in small-for-gestational-age fetuses. Am J Obstet Gynecol 1989;160:385-9. [Crossref] [PubMed]
- Bozzetti P, Ferrari MM, Marconi AM, et al. The relationship of maternal and fetal glucose concentrations in the human from midgestation until term. Metabolism 1988;37:358-63. [Crossref] [PubMed]
- Marconi AM, Paolini C, Buscaglia M, et al. The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet Gynecol 1996;87:937-42. [Crossref] [PubMed]
- Sweet CB, Grayson S, Polak M. Management strategies for neonatal hypoglycemia. J Pediatr Pharmacol Ther 2013;18:199-208. [Crossref] [PubMed]
- Thompson-Branch A, Havranek T. Neonatal Hypoglycemia. Pediatr Rev 2017;38:147-57. [Crossref] [PubMed]
- de Lonlay P, Giurgea I, Touati G, et al. Neonatal hypoglycaemia: aetiologies. Semin Neonatol 2004;9:49-58. [Crossref] [PubMed]
- Schwitzgebel VM, Gitelman SE. Neonatal hyperinsulinism. Clin Perinatol 1998;25:1015-38. [PubMed]
- Vannucci RC, Vannucci SJ. Glucose metabolism in the developing brain. Semin Perinatol 2000;24:107-15. [Crossref] [PubMed]
- Thornton PS, Stanley CA, De Leon DD, et al. Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. J Pediatr 2015;167:238-45. [Crossref] [PubMed]
- Hay WW Jr. Placental-fetal glucose exchange and fetal glucose metabolism. Trans Am Clin Climatol Assoc 2006;117:321-39. [PubMed]
- Simmons RA. Cell glucose transport and glucose handling during fetal and neonatal development. In: Polin RA, Fox WW, Abman SH. editors. Fetal and Neonatal Physiology. 4th Ed. Philadelphia: Elsevier (Saunders), 2011:560-6.
- Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab 1993;77:1554-62. [PubMed]
- Arnott G, Coghill G, McArdle HJ, et al. Immunolocalization of GLUT1 and GLUT3 glucose transporters in human placenta. Biochem Soc Trans 1994;22:272S. [Crossref] [PubMed]
- Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab 2010;298:E141-5. [Crossref] [PubMed]
- Gaither K, Quraishi AN, Illsley NP. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J Clin Endocrinol Metab 1999;84:695-701. [PubMed]
- Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol 1999;180:163-8. [Crossref] [PubMed]
- Kainulainen H, Jarvinen T, Heinonen PK. Placental glucose transporters in fetal intrauterine growth retardation and macrosomia. Gynecol Obstet Invest 1997;44:89-92. [Crossref] [PubMed]
- Xing AY, Challier JC, Lepercq J, et al. Unexpected expression of glucose transporter 4 in villous stromal cells of human placenta. J Clin Endocrinol Metab 1998;83:4097-101. [PubMed]
- Hillman NH, Kallapur SG, Jobe AH. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol 2012;39:769-83. [Crossref] [PubMed]
- Ward Platt M, Deshpande S. Metabolic adaptation at birth. Semin Fetal Neonatal Med 2005;10:341-50. [Crossref] [PubMed]
- Srinivasan G, Pildes RS, Cattamanchi G, et al. Plasma glucose values in normal neonates: a new look. J Pediatr 1986;109:114-7. [Crossref] [PubMed]
- Hume R, Burchell A, Williams FL, et al. Glucose homeostasis in the newborn. Early Hum Dev 2005;81:95-101. [Crossref] [PubMed]
- Hume R, Pazouki S, Hallas A, et al. The ontogeny of the glucose-6-phosphatase enzyme in human embryonic and fetal red blood cells. Early Hum Dev 1995;42:85-95. [Crossref] [PubMed]
- Midgley PC, Russell K, Oates N, et al. Activity of the adrenal fetal zone in preterm infants continues to term. Endocr Res 1996;22:729-33. [Crossref] [PubMed]
- Doerr HG, Sippell WG, Versmold HT, et al. Plasma mineralocorticoids, glucocorticoids, and progestins in premature infants: longitudinal study during the first week of life. Pediatr Res 1988;23:525-9. [Crossref] [PubMed]
- Scott SM, Watterberg KL. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr Res 1995;37:112-6. [Crossref] [PubMed]
- De León DD, Stanley CA. Mechanisms of Disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab 2007;3:57-68. [Crossref] [PubMed]
- Cornblath M, Hawdon JM, Williams AF, et al. Controversies Regarding Definition of Neonatal Hypoglycemia: Suggested Operational Thresholds. Pediatrics 2000;105:1141-5. [Crossref] [PubMed]
- Carmen S. Neonatal hypoglycemia in response to maternal glucose infusion before delivery. J Obstet Gynecol Neonatal Nurs 1986;15:319-23. [Crossref] [PubMed]
- Collins JE, Leonard JV. Hyperinsulinism in asphyxiated and small-for-dates infants with hypoglycaemia. Lancet 1984;2:311-3. [Crossref] [PubMed]
- Whipple AO, Frantz VK. Adenoma of Islet Cells with Hyperinsulinism: A Review. Ann Surg 1935;101:1299-335. [Crossref] [PubMed]
- Wackernagel D, Dube M, Blennow M, et al. Continuous subcutaneous glucose monitoring is accurate in term and near-term infants at risk of hypoglycaemia. Acta Paediatr 2016;105:917-23. [Crossref] [PubMed]
- Sprague JE, Arbelaez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev 2011;9:463-73. [PubMed]
- Adamkin DH. Neonatal hypoglycemia. Curr Opin Pediatr 2016;28:150-5. [Crossref] [PubMed]
- Rozance PJ, Hay WW Jr. New approaches to management of neonatal hypoglycemia. Matern Health Neonatol Perinatol 2016;2:3. [Crossref] [PubMed]
- Marles SL, Casiro OG. Persistent neonatal hypoglycemia: Diagnosis and management. Paediatr Child Health 1998;3:16-9. [Crossref] [PubMed]
- Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Validation of the continuous glucose monitoring sensor in preterm infants. Arch Dis Child Fetal Neonatal Ed 2013;98:F136-40. [Crossref] [PubMed]
- McGowan JE, Perlman JM. Glucose management during and after intensive delivery room resuscitation. Clin Perinatol 2006;33:183-96. [Crossref] [PubMed]
- Belik J, Musey J, Trussell RA. Continuous infusion of glucagon induces severe hyponatremia and thrombocytopenia in a premature neonate. Pediatrics 2001;107:595-7. [Crossref] [PubMed]
- Bhowmick SK, Lewandowski C. Prolonged hyperinsulinism and hypoglycemia. In an asphyxiated, small for gestation infant. Case management and literature review. Clin Pediatr (Phila) 1989;28:575-8. [Crossref] [PubMed]
- Lindley KJ, Dunne MJ, Kane C, et al. Ionic control of beta cell function in nesidioblastosis. A possible therapeutic role for calcium channel blockade. Arch Dis Child 1996;74:373-8. [Crossref] [PubMed]
- Wasserman DH, Spalding JA, Lacy DB, et al. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am J Physiol 1989;257:E108-17. [PubMed]
- Carter PE, Lloyd DJ, Duffty P. Glucagon for hypoglycaemia in infants small for gestational age. Arch Dis Child 1988;63:1264-6. [Crossref] [PubMed]
- McMahon AW, Wharton GT, Thornton P, et al. Octreotide use and safety in infants with hyperinsulinism. Pharmacoepidemiol Drug Saf 2017;26:26-31. [Crossref] [PubMed]
- Virgili N, Mancera P, Wappenhans B, et al. K(ATP) channel opener diazoxide prevents neurodegeneration: a new mechanism of action via antioxidative pathway activation. PLoS One 2013;8:e75189. [Crossref] [PubMed]
- Touati G, Poggi-Travert F, Ogier de Baulny H, et al. Long-term treatment of persistent hyperinsulinaemic hypoglycaemia of infancy with diazoxide: a retrospective review of 77 cases and analysis of efficacy-predicting criteria. Eur J Pediatr 1998;157:628-33. [Crossref] [PubMed]
- Durmaz E, Flanagan SE, Parlak M, et al. A combination of nifedipine and octreotide treatment in an hyperinsulinemic hypoglycemic infant. J Clin Res Pediatr Endocrinol 2014;6:119-21. [Crossref] [PubMed]
- Baş F, Darendeliler F, Demirkol D, et al. Successful therapy with calcium channel blocker (nifedipine) in persistent neonatal hyperinsulinemic hypoglycemia of infancy. J Pediatr Endocrinol Metab 1999;12:873-8. [Crossref] [PubMed]
- Weston PJ, Harris DL, Harding JE. Dextrose gel treatment does not impair subsequent feeding. Arch Dis Child Fetal Neonatal Ed 2017. [Epub ahead of print].
- Ter M, Halibullah I, Leung L, et al. Implementation of dextrose gel in the management of neonatal hypoglycaemia. J Paediatr Child Health 2017;53:408-11. [Crossref] [PubMed]
- Rawat M, Chandrasekharan P, Turkovich S, et al. Oral Dextrose Gel Reduces the Need for Intravenous Dextrose Therapy in Neonatal Hypoglycemia. Biomed Hub 2016;1. pii: 448511.
- Hegarty JE, Harding JE, Gamble GD, et al. Prophylactic Oral Dextrose Gel for Newborn Babies at Risk of Neonatal Hypoglycaemia: A Randomised Controlled Dose-Finding Trial (the Pre-hPOD Study). PLoS Med 2016;13:e1002155. [Crossref] [PubMed]
- Harris DL, Alsweiler JM, Ansell JM, et al. Outcome at 2 Years after Dextrose Gel Treatment for Neonatal Hypoglycemia: Follow-Up of a Randomized Trial. J Pediatr 2016;170:54-9.e1. [Crossref] [PubMed]
- Harris DL, Weston PJ, Signal M, et al. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): a randomised, double-blind, placebo-controlled trial. Lancet 2013;382:2077-83. [Crossref] [PubMed]
- Vannucci RC, Vannucci SJ. Hypoglycemic brain injury. Semin Neonatol 2001;6:147-55. [Crossref] [PubMed]
- Anderson LR, Bass AR. Some effects of victory or defeat upon perception of political candidates. J Soc Psychol 1967;73:227-40. [Crossref] [PubMed]
- Volpe JJ. Hypoglycemia and brain injury. In: Volpe JJ. editor. Neurology of the Newborn. Philadelphia: Saunders, 2001:97-520.
- Auer RN. Hypoglycemic brain damage. Forensic Sci Int 2004;146:105-10. [Crossref] [PubMed]
- Koivisto M, Blanco-Sequeiros M, Krause U. Neonatal symptomatic and asymptomatic hypoglycaemia: a follow-up study of 151 children. Dev Med Child Neurol 1972;14:603-14. [Crossref] [PubMed]
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ 1988;297:1304-8. [Crossref] [PubMed]
- Filan PM, Inder TE, Cameron FJ, et al. Neonatal hypoglycemia and occipital cerebral injury. J Pediatr 2006;148:552-5. [Crossref] [PubMed]
- Burns CM, Rutherford MA, Boardman JP, et al. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 2008;122:65-74. [Crossref] [PubMed]
- Per H, Kumandas S, Coskun A, et al. Neurologic sequelae of neonatal hypoglycemia in Kayseri, Turkey. J Child Neurol 2008;23:1406-12. [Crossref] [PubMed]
- Montassir H, Maegaki Y, Ogura K, et al. Associated factors in neonatal hypoglycemic brain injury. Brain Dev 2009;31:649-56. [Crossref] [PubMed]
- Kerstjens JM, Bocca-Tjeertes IF, de Winter AF, et al. Neonatal Morbidities and Developmental Delay in Moderately Preterm-Born Children. Pediatrics 2012;130:e265-72. [Crossref] [PubMed]
- Tin W, Brunskill G, Kelly T, et al. 15-year follow-up of recurrent “hypoglycemia” in preterm infants. Pediatrics 2012;130:e1497-503. [Crossref] [PubMed]
- McKinlay CJ, Alsweiler JM, Ansell JM, et al. Neonatal Glycemia and Neurodevelopmental Outcomes at 2 Years. N Engl J Med 2015;373:1507-18. [Crossref] [PubMed]
- Kaiser JR, Bai S, Gibson N, et al. Association Between Transient Newborn Hypoglycemia and Fourth-Grade Achievement Test Proficiency: A Population-Based Study. JAMA Pediatr 2015;169:913-21. [Crossref] [PubMed]
- Harris DL, Weston PJ, Williams CE, et al. Cot-side electroencephalography monitoring is not clinically useful in the detection of mild neonatal hypoglycemia. J Pediatr 2011;159:755-60.e1. [Crossref] [PubMed]
- Cummins CJ, Lust WD, Passonneau JV. Regulation of glycogenolysis in transformed astrocytes in vitro. J Neurochem 1983;40:137-44. [Crossref] [PubMed]
- Edmond J, Robbins RA, Bergstrom JD, et al. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 1987;18:551-61. [Crossref] [PubMed]
- Hack M, Breslau N, Weissman B, et al. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. N Engl J Med 1991;325:231-7. [Crossref] [PubMed]
- Sexson WR. Incidence of neonatal hypoglycemia: a matter of definition. J Pediatr 1984;105:149-50. [Crossref] [PubMed]
- Williams AF. Hypoglycaemia of the newborn: a review. Bull World Health Organ 1997;75:261-90. [PubMed]