Zoledronic acid in pediatric metabolic bone disorders
Introduction
Bisphosphonates (BPs) have been used widely since the 1990s and are considered a cornerstone for the treatment of osteoporosis (1). This class of drug has also been used to treat a variety of bone disorders other than osteoporosis in adults, such as Paget’s disease of bone, multiple myeloma, hypercalcemia and bone metastasis (2). Since the landmark report of its successful use in 1998 in a large series of children with osteogenesis imperfecta (OI), BPs have been increasingly used in children suffering from primary and secondary osteoporosis, as well as various skeletal disorders. Pamidronate, a second generation BP is the most widely used intravenous BP. It is given as a 4-hour intravenous infusion for 3 consecutive days in cycles every 2–4 months for an annual dose of 9 mg/kg. It has several beneficial effects in children with OI: increased bone mineral density (BMD), reduced fracture rate, and substantial improvement in functional status. More recently, several studies have demonstrated that zoledronic acid (ZA), a more potent third generation BP, can be used safely and effectively in childhood osteoporosis. ZA treatment offers greater ease and convenience of use, as compared to pamidronate, as it is administered as a single intravenous infusion over a shorter duration of 30 minutes, with a longer interval between infusions. This dosing schedule greatly reduces the need for repeated venous cannulation, and is more cost effective in terms of health care utilization. This makes ZA an attractive and efficient treatment option. This article describes the pharmacologic properties of ZA and summarizes safety and efficacy findings from clinical studies of its use in children with various skeletal disorders.
Mechanism of action and pharmacokinetics
ZA is a member of the nitrogen-containing BPs, a third generation which has higher potency in decreasing osteoclast-mediated bone resorption compared to other drugs in its class (1,2). Structurally, all BPs have the required phosphorus-carbon-phosphorus core and various side chains, with a hydroxyl group at the R1 position (Figure 1A). Substitution of side chain at location R1 and R2 determines the potency of anti-resorption and how avidly the BPs attach to bone (Figure 1). First generation BPs (etidronate and clodronate) have alkyl or halide side chains. Second-generation BPs, such as alendronate and pamidronate, have amino-terminal groups and therefore, are known as aminobisphosphonates. Second-generation BPs have an increased antiresorptive potency of 10–100 times the previous generation. ZA and risedronate, third-generation BPs, have a cyclic side chain. The heterocyclic imidazole group attached to the R2 position makes ZA more potent compared to other BPs. Like other nitrogen-containing BPs, ZA inhibits farnesyl pyrophosphate synthase (FPPS), a major regulatory enzyme, essential for the osteoclast formation and function, thus inhibiting bone resorption and inducing osteoclastic apoptosis (3). In vitro, ZA has the highest affinity for binding hydroxyapatite in mineralized bone, when compared with alendronate, ibandronate, or risedronate (4). The high binding affinity of ZA for mineralized bone accounts for its long duration of action. ZA is 850 times more potent than pamidronate in inhibiting osteoclast-mediated bone resorption and 150 times more potent than pamidronate in increasing trabecular bone mass (3). Indeed, it is the most potent BP (5). Recent evidence indicates that ZA not only inhibits osteoclast activity, but also exerts anabolic activity by stimulation of osteoblast differentiation (6). This may explain the superior efficacy of ZA in increasing bone density compared to other BPs.

After intravenous infusion, plasma concentrations of ZA increase rapidly and rapidly decline to <1% of maximal concentration after 24 hours (7). ZA is not metabolized and does not inhibit CYP enzymes. About 55% of the dose is taken up by bone where it is retained for years and released back into the systemic circulation very slowly. The remaining 45% of a dose is excreted unchanged in urine within 24 hours. As renal excretion is the main route of elimination, ZA dose should be reduced and used with caution when creatinine clearance is <60 mL/min. ZA is contraindicated in patients with creatinine clearance <35 mL/min or evidence of acute renal impairment. Though ZA does not undergo hepatic metabolization, the effects of hepatic impairment on the pharmacokinetics of ZA have not been studied.
Therapeutic uses of ZA in children
Since early 2000s, ZA has been investigated as potential treatment for children and adults with OI (8) and osteoporosis associated with chronic illnesses with report of positive outcome first appeared in 2004 (9). The use of ZA has expanded to many other conditions, including hypercalcemic disorders, osteonecrosis (ON), and malignant bone diseases, all of which are used on a compassionate basis in the US. Most of the studies that report positive outcomes with ZA are uncontrolled studies or case series. Currently, none of BP drug class, including ZA, has been approved by the US Food and Drug Administration (FDA) for the treatment of osteoporosis or any bone and mineral disorders in children. As shown in Table 1, ZA has shown beneficial effects in the treatment of primary and secondary osteoporosis and various skeletal disorders. Common disorders are discussed in this review.

Full table
Childhood osteoporosis
Osteoporosis is defined as a systemic skeletal disease characterized by compromised bone strength, and microarchitectural deterioration of bone, leading to fragility fractures. There has been increasing awareness of osteoporosis in children in the past 2 decades. The diagnosis of osteoporosis in children should not be made on the basis of densitometric criteria alone (10). The presence of bone fragility with a history of clinically significant fractures and BMD Z score ≤–2.0 are required for diagnosis of pediatric osteoporosis according to the International Society for Clinical Densitometry (ISCD) (10). A clinically significant fracture history ≥2 long bone fractures occurring by 10 years of age or ≥3 long bone fractures occurring by 19 years of age (10). Vertebral fracture in the absence of high energy trauma or local disease is pathognomonic for osteoporosis and can allow the diagnosis without detection of significantly low bone density by DEXA (10). Screening for vertebral fractures in children with risk factors for osteoporosis is essential. Prior vertebral fractures are a powerful predictor of future fractures and when present are an indication for treatment with BPs (11). Many children with primary and secondary osteoporosis have radiographically detected vertebral fractures (loss of at least 20% of vertebral body height) without any clinical symptoms (12). Surveillance of vertebral fractures in children at risk for osteoporosis, particularly in those treated with chronic glucocorticoids (GCs), is obtained by spinal radiographs, or vertebral fracture assessment using a densitometric lateral spinal imaging. The latter imaging technique has the advantage of being performed at the time of dual energy X-ray absorptiometry (DXA) scan, thus offering convenience for the patient and a substantially lower radiation exposure than a traditional spinal radiograph. While the diagnosis of osteoporosis requires the presence of both a clinically significant fracture history and low bone mineral content (BMC) or BMD, the ISCD emphasizes that children who have fragile bones with increased fracture risk can still have normal BMD (BMD Z score >−2) as assessed by DXA (10). In other words, in children normal BMD does not exclude the possibility of bone fragility. For detailed discussion of management of pediatric osteoporosis with diagnostic approach algorithm, the reader is referred to an excellent recent review (13).
Osteoporosis in children is clinically heterogeneous with a broad range of etiologies. It can be classified into two groups: primary osteoporosis or genetic brittle bone disease, and secondary osteoporosis due to underlying chronic diseases (13). Studies of ZA in children with primary and secondary osteoporosis with BMD and fractures outcome as well as adverse effects are summarized in Table 2. It is important to emphasize the benefits of other conservative measures to optimize bone health in children with osteoporosis before embarking upon ZA treatment. These include treating the underlying condition causing bone fragility ensuring adequate weight-bearing exercise, and adequate vitamin D and calcium intake.
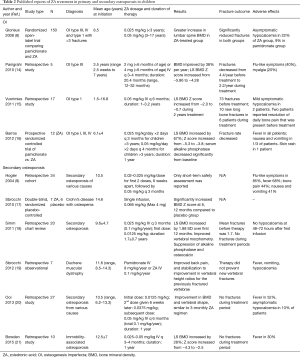
Full table
Primary osteoporosis
Primary osteoporosis occurs due to an intrinsic skeletal defect of genetic origin such as OI, Ehlers-Danlos syndrome, homocystinuria, Marfan’s syndrome, and or juvenile idiopathic osteoporosis. OI is the most common cause of primary osteoporosis and is characterized by bone fragility, increased risk of fracture, skeletal deformity, and disability. It is the most common clinical condition for which BP therapy is used in children. All studies of ZA in children with moderate to severe OI (type III, IV) and/or OI type 1 with multiple fractures have demonstrated improved BMD and reduced fractures (8,14-16). One randomized open-label study showed greater increase in lumbar spine BMD in the ZA treated group compared with the pamidronate group (8).
Fibrous dysplasia (FD) is a benign fibro-osseous bone disease characterized by the replacement of bone with cellular fibrous tissue and this condition may be associated with bone pain and fragility fractures. Treatment with pamidronate in six pediatric patients with FD showed improvement in bone pain and radiographic features of FD (22). ZA treatment at the dose of 0.05 mg/kg resulted in resolution of pain and improvement in bone lesions in a 12-year-old boy with craniofacial FD (23).
Secondary osteoporosis
Secondary osteoporosis is caused by a variety of different underlying chronic illnesses that adversely affect bone mass accrual and/or maintenance (Table 1). The most common risk factors associated with secondary osteoporosis in children are immobility, chronic GC therapy, inflammatory conditions, chemotherapy, hypogonadism, and poor nutrition. Often these risk factors overlap within individual patients.
Steroids-induced osteoporosis
Glucocorticoid-induced osteoporosis (GIO) is a serious consequence of chronic GC therapy (>3 months) leading to fractures in 30–50% of patients (24,25). Bone loss from high dose GCs is biphasic with a rapid initial phase during the first few months, followed by a slower but steady phase with continued use. The early rapid bone loss from GCs is due to its effects on increasing bone resorption while inhibiting bone formation. GCs stimulate osteoclast bone resorption by suppressing synthesis of osteoprotegerin (an inhibitor of osteoclast differentiation) and by stimulating synthesis of the receptor activator of nuclear factor kappa-B (RANK), which activates osteoclasts (26). Suppression of bone formation is the predominant effect of long-term GC use, which is mediated by inhibition of osteoblasts and apoptosis of mature osteoblasts and osteocytes. In addition, GCs also result in reduced intestinal calcium absorption, increased renal calcium excretion, and diminished sex steroid and growth hormone production, all of which contribute to bone loss. GCs have a predilection for trabecular bone, particularly vertebrae, which increase the risk and rate of vertebral compression fractures. BPs are indicated in the prevention and treatment of steroid-induced osteoporosis and to reduce the risk of vertebral fractures in adults (27). Limited published data exist on the use of BPs for GIO in children. GIO is prevalent in children with Duchene muscular dystrophy treated with long term GCs and many studies have reported positive outcome (28,29). It is important to note that there is potential for recovery of bone density with reduced fracture risk to expected for age in children once GCs are discontinued (30,31).
Immobilization osteoporosis associated with neuromuscular disorders
Non-ambulatory children with neuromuscular disorders such as spastic quadriplegic cerebral palsy, spinal muscular dystrophy, and myelomeningocele, are at risk for fragility fractures and low bone density. Collectively immobility-related osteoporosis due to neuromuscular disorders comprises by far the largest number of children with osteoporosis at our pediatric institution and this observation likely holds true at most pediatric referral centers. The prevalence of low bone mass was found in almost all (97%) children >9 years with moderate and severe cerebral palsy (32), while fracture prevalence was 26% in children >10 years (32), and increased with increasing age (33). Multiple risk factors contribute to bone fragility in this population, include immobilization, limited weight-bearing ambulation, lack of muscular forces on bone, decreased exposure to sunlight, nutritional deficiencies with low calcium and vitamin D intake and antiepileptic agents (34). A recent study in this patient population showed that ZA increased lumbar BMD by 28%, and produced a net gain in lumbar BMD Z score by 1.76 after 1 year (21), similar to 27% gain in BMD (35), and 1.5 point gain in Z-score (36) observed in a similar group of patients after 1 year of pamidronate therapy.
Males with Duchenne muscular dystrophy (DMD), an X-linked recessive disorder characterized by progressive muscle weakness, with eventual loss of ambulation, also have significantly increased risks for osteoporosis secondary to multiple factors, including long-term corticosteroid therapy (used to prolong ambulation and maintain muscle strength and pulmonary function), chronic inflammation from lack of dystrophin gene in the muscles, reduced mobility, delayed puberty and poor growth. Prevalence of fragility fractures of long bones and vertebrae range from 25–60% (28). BP therapy has been shown to increase BMD, and IV BP with either pamidronate or ZA showed significant improvement of back pain after vertebral fracture in affected children within a month after starting therapy (19,37-39). Although BPs are currently the most effective therapy for steroid-induced osteoporosis in DMD, long term safety and efficacy remain unclear. Since reduced bone formation with low trabecular volume and low cortical thickness is characteristic in DMD even before BP therapy, and bone turnover declines further with BP treatment (40), newer treatments that stimulate bone formation may be superior options for this and other types of low bone turnover osteoporosis in children.
A study in growing Mdx mice (mouse model of DMD) showed that pamidronate treatment not only increased cortical BMD and bone strength, but also had positive effects on skeletal muscle with decreased serum and muscle creatine kinase and evidence of improved muscle histology and muscle strength (41). These results support the finding from one study in Canada that showed BP treatment improved survival in patients with DMD (42), which may be at least partially explained by the positive effects of BPs on muscles.
Chemotherapy-related ON
ON is a well-recognized complication of chemotherapy in children and adolescents with acute lymphoblastic leukemia (ALL) or lymphoma, resulting in acute and long term disability with chronic pain. It usually develops within 1–2 years following chemotherapy, with hip and knee are the most frequently affected joints. GC use has been implicated as a major etiological factor in the development of ON in this patient group. Other risk factors include age ≥10 years, female gender, Caucasian race, and higher body mass index (43). In a large cohort of 251 pediatric patients with ALL, 18 patients (7.2%) developed ON (44). Pain improved after treatment with intravenous ZA, given at 0.025 mg/kg/dose at 12 weekly interval, for 1–2 years. However, most patients with ON of the hips still demonstrated radiological progression, and 16% of patients required arthroplasty (44) despite ZA use.
Hypercalcemic disorders
BPs are effective treatment for severe hypercalcemia due to their inhibiting effects on osteoclast-mediated release of calcium into the serum. BP use should be reserved for severe, refractory hypercalcemia unresponsive to other measures (saline hydration, loop diuretics, GCs, or calcitonin). ZA has been frequently utilized in hypercalcemia of malignancy in adults. In two randomized, controlled clinical trials in adults, ZA was superior to pamidronate in treatment of hypercalcemia of malignancy, with normalization of serum calcium levels occurring by day 4 in approximately 50% of patients treated with ZA and in only 33.3% of the pamidronate-treated patients (45). Hypercalcemia of malignancy in children is often associated with ALL due to elevated parathyroid hormone-related protein or calcitriol produced by the tumors, or osteolytic metastases with local release of osteoclast-activating factors (46). A few case reports of ZA in hypercalcemia of malignancy in children showed resolution of hypercalcemia within 24–48 hours after a single dose of ZA. The ZA dose used in these reports were 0.025 mg/kg (47), 0.05 mg/kg (48), and 4 mg (49). There is a recent case report of an 8-week old infant with calcitriol-mediated hypercalcemia associated with pneumocystis jiroveci pneumonia who was successfully treated with ZA (cumulative dose of 0.1 mg/kg over 3 consecutive days) (50). Marked hypercalcemia (serum calcium of 24.3 mg/dL) in this case normalized 2 weeks after ZA infusion.
Malignant bone tumor
ZA has demonstrated efficacy in reducing skeletal-related events in adult patients with metastatic bone disease (51). Furthermore, ZA has beneficial effects beyond bone health, as it may prolong disease-free and/or overall survival in patients with breast cancer (52) and multiple myeloma (52). A retrospective study of 15 pediatric patients (mean age 12.5 years) with primary malignant bone disease or metastatic bone disease from various cancers demonstrated that ZA can improve pain control and stabilize bone disease (53). In this report, ZA was well tolerated even in some patients who received multiple high doses (4 mg in patients >10 years of age). There is recent evidence that ZA has a direct antitumor effect, and synergistically augment the effects of antitumor agents in osteosarcoma cells as well as soft tissue sarcoma cells (54). High doses of ZA are currently being evaluated in phase III clinical trials in Europe for the treatment of malignant pediatric primary bone tumors (55).
Potential novel uses of ZA
ZA has potential for use to prevent sickle bone disease in patients with sickle cell disease. Pain and skeletal complications, which can be acute or chronic, are important causes of morbidity in sickle cell disease (56). The acute bone complications are painful vaso-occlusive crisis, osteomyelitis, or stress fractures, whereas chronic bone complications include vertebral collapse, compression spine deformities, ON, and osteoporosis. The likely mechanism for the development of sickle bone disease is due to recurrent vaso-occlusive crisis with ischemic/reperfusion injury which suppresses osteogenic lineage and activates osteoclasts (57). ZA has been shown to prevent development of sickle cell bone disease and bone loss in sickle cell disease mice by inhibiting osteoclast activity and promoting osteogenic lineage (57). At present, there has been no report of BP treatment in children with sickle bone disease in the literature.
Beyond bone health, ZA may also have therapeutic potential as substrate reduction therapy for Krabbe’s disease, a neurodegenerative disorder caused by accumulation of psychosine, due to deficiency of lysosomal enzyme galactocerebrosidase. ZA has been identified as selective inhibitor of human ceramide galactosyltransferase that may prevent pathological storage of this highly cytotoxic glycolipid substrate in lysosomal storage disorders (58).
Safety and adverse effects of BPs
Acute phase reaction (APR)
APR, manifesting as flu-like symptoms including low-grade fever, nausea, myalgia and bone pain, or fatigue, is the most common adverse effect of ZA and, when it occurs, typically develops within 48 hours after the first infusion. The frequency varies from 19% to 70% in reported series (59,60). Subsequent ZA infusion induces no APR symptoms or much milder degree of APR than at first exposure. Unfortunately, some patients who experience an APR are reluctant to receive further treatment and thus steps to prevent this reaction are clearly warranted. APR is associated with elevated levels of the pro-inflammatory cytokines IL-6, IFN-γ, and TNF-α (61). We recommend pretreatment with non-steroidal anti-inflammatory drugs (ibuprofen) prior to each ZA infusion, as has been reported by our group to reduce the APR associated with intravenous BP therapy (62). Severe systemic inflammatory response following ZA infusion has been recently reported in only one case, a 7-year-old child who developed shock, multiple organ dysfunction syndrome following first ZA infusion (63). This child had underlying complex medical conditions and had previously been treated with four cycles of pamidronate. He received botulinum toxin 1 day before ZA infusion and his home medications included baclofen, clonazepam, gabapentin, levetiracetam, levocarnitine, and levothyroxine. This child recovered after supportive intensive care and corticosteroids treatment. Because of the inflammatory response following ZA, one might consider using pretreatment with GCs to mitigate the reaction. However, a recent randomized controlled study using single-dose dexamethasone 4 mg did not reduce the incidence or severity of APR following first exposure to ZA (64).
Hypocalcemia and hypophosphatemia
In 2004, the first study of ZA use in 34 children reported high rate of flu-like illness (85%), hypocalcemia (74%), and hypophosphatemia (82%), following the first dose of 0.02–0.025 mg/kg (9). Subsequently, a lower initial dose of 0.0125 mg/kg was used which resulted in reduction of the frequency (42%) and intensity of hypocalcemia, but no change in the incidence of flu-like symptoms (60). Though this reduced hypocalcemia rate of 42% is similar to that reported in children with OI treated with intravenous pamidronate, others have reported lower rates of symptomatic hypocalcemia (3% to 21%) in children treated with ZA (59,65). Hypophosphatemia was reported in 25% of 81 children following ZA treatment and was dose-related, that is more common in patients receiving ZA dose >0.025 mg/kg (27).
A low vitamin D level appears to be a strong risk factor for APR and pain following BP infusion (66), as well as for hypocalcemia (13). Therefore, it is important to have vitamin D sufficiency (25-vitamin D level >30 ng/dL) prior to BP treatment. Calcium supplementation for 10 days before and after ZA infusion should be given to reduce the risk of postinfusion hypocalcemia.
Atypical femoral fractures (AFF)
In adults with osteoporosis, AFF were first reported in 2005 as a rare adverse event associated with long term BP therapy (67). The absolute risk of AFFs in adult patients on BPs ranges from 3.2 to 50 cases per 100,000 person-years (68). Fractures are ‘atypical’ in that they involved the strongest part of the femur, from the subtrochanteric to supracondylar regions. They are pathologic fractures associated with low energy or no trauma with characteristic radiographic features of non-comminuted transverse or short oblique fracture lines (68). Delayed fracture healing is sometimes associated with AFFs. Severe suppression of bone remodeling associated with long-term BP therapy, with accumulation of micro-fractures and increased bone stiffness, are the likely mechanisms of AFFs. There have been reports of AFFs in seven children with OI on long-term pamidronate therapy (5 to 11 years), 6 of whom had preexisting intramedullary rods (69,70). Another young adult male with OI treated with pamidronate initially, followed by oral alendronate from age 10 to 18 years developed AFF at age 21, 3 years after stopping BP therapy (71). As BP therapy suppresses bone turnover markers in children with bone fragility (72), monitoring bone turnover markers during the course of ZA therapy is essential to avoid over suppressed bone remodeling that may result in AFFs. Whether other approaches such as low-frequency and/or low-dose long-term BP therapy or drug holidays may prevent development of AFF should be explored.
Dental development and risk of ON of the jaw
A recent study compared BP-treated OI children with BP naïve children and found that BP treatment in OI had normal rate of dental development indistinguishable from normal (73). This is due to the fact that OI patients naïve to BPs had advanced dental age by 0.63 years from chronological age because BP naïve OI patients have faster resorption of the deciduous teeth than the treated ones. Therefore, the net effect is normal timing of secondary dentition in BP-treated OI patients. ONJ represents a severe dento-alveolar bone defect related to poor bone healing. Although the association of increased ONJ rates in adults treated with high dose BPs has been fully established, to date, there has been no reported case of ONJ in children treated with BPs (74).
ZA dosage and administration
Though various dosages of ZA for osteoporosis have been utilized in published studies (Table 2), the dose of 0.1 mg/kg/year divided into 2 doses per year given as 0.05 mg/kg/dose every 6 months (20,75) has had the widest acceptance. The lower initial dose at 0.0125 mg/kg reduces the likelihood and severity of hypocalcemia (60). In our institution, all patients are hospitalized for one night (<24 hours) during the first infusion. Subsequent infusions are given at the infusion clinic or given at home by a home health nurse. We recommend pretreatment with acetaminophen or ibuprofen prior to and 6 hours after intravenous ZA infusion to minimize APR and then as needed if fever, myalgia or bone pain is a continuing concern (62). APR usually does not occur with subsequent doses of ZA. When used for primary or secondary osteoporosis, we require a DXA for BMD assessment obtained at baseline and yearly to guide adjustment of therapy with a goal of achieving and maintaining the lumbar BMD Z score near 0 or in the 0 to +1 range. This is usually accomplished through decreasing the doses and frequency after 2 years of initial ZA therapy. This approach results in significant increase in lumbar spine BMD Z score in the first 3 years and then plateauing of the BMD for age and size after 3–4 years of therapy (21). The recommended dosage based on age, including dose and frequency reduction according to lumbar spine areal BMD Z score proposed by Rauch (75) and the administration guidelines are summarized in Table 3. For children with underlying disorders that continue to adversely affect bone health (e.g., OI or long term GC therapy), the therapy should be continued until completion of linear growth to avoid metaphyseal fractures at the junction of treated dense bone and the new (untreated) thinner bone after BP discontinuation (77). A recent report of a child with OI, while on ZA every 6 months for 3 years, who had a fracture at a sclerotic metaphyseal line (76), raises the question whether children with high bone turnover, such as in OI, may benefit from more a frequent low dose treatment regimen. On the other hand, children with secondary or low bone turnover osteoporosis (such as Duchene muscular dystrophy on chronic GCs) may benefit from a different dosing frequency. Studies in adults suggest that the efficacy of BPs (including ZA) on fracture prevention reaches a plateau after 4–5 years (78). Whether this phenomenon is the same in children will require longitudinal studies to assess long term safety and anti-fracture efficacy of BPs. Well-constructed studies with large number of subjects will certainly be needed to identify effective dosing regimen and optimal length of therapy for children with all forms of osteoporosis and bone disorders. Given the paucity of long term safety and efficacy data of ZA in children, ZA should be used judiciously and prescribed only by physicians with experience and expertise in treating metabolic disease in children.

Full table
Conclusions
ZA is a highly potent anti-resorptive BP with superior efficacy in fracture reduction compared to other BPs. Its use in children has expanded from primary and secondary osteoporosis to various disorders, including hypercalcemia of malignancy, FD, chemotherapy-related ON, as well as bone neoplasms with positive outcomes. Long term studies are needed to determine optimal dosing regimen and length of therapy that will result in lasting positive results for children with these conditions.
Acknowledgements
None.
Footnote
Conflicts of Interest: SA Bowden is a member of the advisory board for Novartis. JD Mahan has no conflicts of interest to declare.
References
- Chen JS, Sambrook PN. Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol 2011;8:81-91. [Crossref] [PubMed]
- Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008;83:1032-45. [Crossref] [PubMed]
- Green JR. Zoledronic acid: pharmacologic profile of a potent bisphosphonate. J Organomet Chem 2005;690:2439-48. [Crossref]
- Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 2006;38:617-27. [Crossref] [PubMed]
- Green JR, Rogers MJ. Pharmacologic profile of zoledronic acid: A highly potent inhibitor of bone resorption. Drug Dev Res 2002;55:210-24. [Crossref]
- Dalle Carbonare L, Mottes M, Malerba G, et al. Enhanced Osteogenic Differentiation in Zoledronate-Treated Osteoporotic Patients. Int J Mol Sci 2017;18:E1261. [Crossref] [PubMed]
- Dhillon S. Zoledronic Acid (Reclast(®), Aclasta(®)): A Review in Osteoporosis. Drugs 2016;76:1683-97. [Crossref] [PubMed]
- Glorieux F, Bishop N, Bober M, et al. Intravenous zoledronic acid (zol) compared to IV pamidronate (PAM) in children with severe osteogenesis imperfecta (OI). Calcif Tissue Int 2008;82:S85.
- Högler W, Yap F, Little D, et al. Short-term safety assessment in the use of intravenous zoledronic acid in children. J Pediatr 2004;145:701-4. [Crossref] [PubMed]
- Bishop N, Arundel P, Clark E, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom 2014;17:275-80. [Crossref] [PubMed]
- Cummings EA, Ma J, Fernandez CV, et al. Incident Vertebral Fractures in Children With Leukemia During the Four Years Following Diagnosis. J Clin Endocrinol Metab 2015;100:3408-17. [Crossref] [PubMed]
- Mäkitie O, Doria AS, Henriques F, et al. Radiographic vertebral morphology: a diagnostic tool in pediatric osteoporosis. J Pediatr 2005;146:395-401. [Crossref] [PubMed]
- Rosen CJ, Brown S. Severe hypocalcemia after intravenous bisphosphonate therapy in occult vitamin D deficiency. N Engl J Med 2003;348:1503-4. [Crossref] [PubMed]
- Panigrahi I, Das RR, Sharda S, et al. Response to zolendronic acid in children with type III osteogenesis imperfecta. J Bone Miner Metab 2010;28:451-5. [Crossref] [PubMed]
- Vuorimies I, Toiviainen-Salo S, Hero M, et al. Zoledronic acid treatment in children with osteogenesis imperfecta. Horm Res Paediatr 2011;75:346-53. [Crossref] [PubMed]
- Barros ER, Saraiva GL, de Oliveira TP, et al. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab 2012;25:485-91. [Crossref] [PubMed]
- Sbrocchi AM, Forget S, Laforte D, et al. Zoledronic acid for the treatment of osteopenia in pediatric Crohn’s disease. Pediatr Int 2010;52:754-61. [Crossref] [PubMed]
- Simm PJ, Johannesen J, Briody J, et al. Zoledronic acid improves bone mineral density, reduces bone turnover and improves skeletal architecture over 2 years of treatment in children with secondary osteoporosis. Bone 2011;49:939-43. [Crossref] [PubMed]
- Sbrocchi AM, Rauch F, Jacob P, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int 2012;23:2703-11. [Crossref] [PubMed]
- Ooi HL, Briody J, McQuade M, et al. Zoledronic acid improves bone mineral density in pediatric spinal cord injury. J Bone Miner Res 2012;27:1536-40. [Crossref] [PubMed]
- Bowden S, Jessup A, Akusoba C, et al. Zoledronic Acid in Non-Ambulatory Children and Young Adults with Fragility Fractures and Low Bone Mass Associated with Spastic Quadriplegic Cerebral Palsy and Other Neuromuscular Disorders. J Endocrinol Diabetes Mellit 2015;3:35-41. [Crossref]
- Kos M, Luczak K, Godzinski J, et al. Treatment of monostotic fibrous dysplasia with pamidronate. J Craniomaxillofac Surg 2004;32:10-5. [Crossref] [PubMed]
- Di Pede C, Congedi S, Rossin S, et al. Use of Zoledronic Acid in Paediatric Craniofacial Fibrous Dysplasia. Case Rep Pediatr 2016;2016:2329483. [PubMed]
- Fraser LA, Adachi JD. Glucocorticoid-induced osteoporosis: treatment update and review. Ther Adv Musculoskelet Dis 2009;1:71-85. [Crossref] [PubMed]
- Hansen KE, Kleker B, Safdar N, et al. A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children. Semin Arthritis Rheum 2014;44:47-54. [Crossref] [PubMed]
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007;18:1319-28. [Crossref] [PubMed]
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid‐Induced Osteoporosis. Arthritis Rheumatol 2017;69:1521-37. [Crossref] [PubMed]
- Buckner JL, Bowden SA, Mahan JD. Optimizing Bone Health in Duchenne Muscular Dystrophy. Int J Endocrinol 2015;2015:928385. [PubMed]
- Bell JM, Shields MD, Watters J, et al. Interventions to prevent and treat corticosteroid-induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. Cochrane Database Syst Rev 2017;1:CD010899. [PubMed]
- van Staa TP, Cooper C, Leufkens HG, et al. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res 2003;18:913-8. [Crossref] [PubMed]
- Bianchi ML. Glucorticoids and bone: some general remarks and some special observations in pediatric patients. Calcif Tissue Int 2002;70:384-90. [Crossref] [PubMed]
- Henderson RC, Lark RK, Gurka MJ, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics 2002;110:e5. [Crossref] [PubMed]
- King W, Levin R, Schmidt R, et al. Prevalence of reduced bone mass in children and adults with spastic quadriplegia. Dev Med Child Neurol 2003;45:12-6. [Crossref] [PubMed]
- Mergler S, Evenhuis HM, Boot AM, et al. Epidemiology of low bone mineral density and fractures in children with severe cerebral palsy: a systematic review. Dev Med Child Neurol 2009;51:773-8. [Crossref] [PubMed]
- Allington N, Vivegnis D, Gerard P. Cyclic administration of pamidronate to treat osteoporosis in children with cerebral palsy or a neuromuscular disorder: a clinical study. Acta Orthop Belg 2005;71:91-7. [PubMed]
- Plotkin H, Coughlin S, Kreikemeier R, et al. Low doses of pamidronate to treat osteopenia in children with severe cerebral palsy: a pilot study. Dev Med Child Neurol 2006;48:709-12. [Crossref] [PubMed]
- Houston C, Mathews K, Shibli-Rahhal A. Bone density and alendronate effects in Duchenne muscular dystrophy patients. Muscle Nerve 2014;49:506-11. [Crossref] [PubMed]
- Palomo Atance E, Ballester Herrera M, Márquez de La Plata M, et al. Alendronato en el tratamiento de la osteoporosis secundaria a la distrofia muscular de Duchenne. Anales de Pediatría; 2011: Elsevier.
- Hawker GA, Ridout R, Harris VA, et al. Alendronate in the treatment of low bone mass in steroid-treated boys with Duchennes muscular dystrophy. Arch Phys Med Rehabil 2005;86:284-8. [Crossref] [PubMed]
- Misof BM, Roschger P, McMillan HJ, et al. Histomorphometry and Bone Matrix Mineralization Before and After Bisphosphonate Treatment in Boys With Duchenne Muscular Dystrophy: A Paired Transiliac Biopsy Study. J Bone Miner Res 2016;31:1060-9. [Crossref] [PubMed]
- Yoon SH, Sugamori KS, Grynpas MD, et al. Positive effects of bisphosphonates on bone and muscle in a mouse model of Duchenne muscular dystrophy. Neuromuscul Disord 2016;26:73-84. [Crossref] [PubMed]
- Gordon KE, Dooley JM, Sheppard KM, et al. Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics 2011;127:e353-8. [Crossref] [PubMed]
- te Winkel ML, Pieters R, Hop WC, et al. Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia. J Clin Oncol 2011;29:4143-50. [Crossref] [PubMed]
- Padhye B, Dalla-Pozza L, Little D, et al. Incidence and outcome of osteonecrosis in children and adolescents after intensive therapy for acute lymphoblastic leukemia (ALL). Cancer Med 2016;5:960-7. [Crossref] [PubMed]
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001;19:558-67. [Crossref] [PubMed]
- Trehan A, Cheetham T, Bailey S. Hypercalcemia in acute lymphoblastic leukemia: an overview. J Pediatr Hematol Oncol 2009;31:424-7. [Crossref] [PubMed]
- Lokadasan R, Prem S, Koshy SM, et al. Hypercalcaemia with disseminated osteolytic lesions: a rare presentation of childhood acute lymphoblastic leukaemia. Ecancermedicalscience 2015;9:542. [Crossref] [PubMed]
- Kolyva S, Efthymiadou A, Gkentzi D, et al. Hypercalcemia and osteolytic lesions as presenting symptoms of acute lymphoblastic leukemia in childhood. The use of zoledronic acid and review of the literature. J Pediatr Endocrinol Metab 2014;27:349-54. [Crossref] [PubMed]
- Park HJ, Choi EJ, Kim JK. A successful treatment of hypercalcemia with zoledronic acid in a 15-year-old boy with acute lymphoblastic leukemia. Ann Pediatr Endocrinol Metab 2016;21:99-104. [Crossref] [PubMed]
- VanSickle JS, Srivastava T, Alon US. Life-Threatening Hypercalcemia During Prodrome of Pneumocystis jiroveci Pneumonia in an Immunocompetent Infant. Glob Pediatr Health 2017;4:2333794X17705955.
- Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 2001;91:1191-200. [Crossref] [PubMed]
- Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol 2008;9:840-9. [Crossref] [PubMed]
- August KJ, Dalton A, Katzenstein HM, et al. The use of zoledronic acid in pediatric cancer patients. Pediatr Blood Cancer 2011;56:610-4. [Crossref] [PubMed]
- Koto K, Murata H, Kimura S, et al. Zoledronic acid inhibits proliferation of human fibrosarcoma cells with induction of apoptosis, and shows combined effects with other anticancer agents. Oncol Rep 2010;24:233-9. [PubMed]
- Lézot F, Chesneau J, Battaglia S, et al. Preclinical evidence of potential craniofacial adverse effect of zoledronic acid in pediatric patients with bone malignancies. Bone 2014;68:146-52. [Crossref] [PubMed]
- Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol 2005;129:482-90. [Crossref] [PubMed]
- Dalle Carbonare L, Matte’ A, Valenti MT, et al. Hypoxia-reperfusion affects osteogenic lineage and promotes sickle cell bone disease. Blood 2015;126:2320-8. [Crossref] [PubMed]
- Pannuzzo G, Graziano AC, Pannuzzo M, et al. Zoledronate derivatives as potential inhibitors of uridine diphosphate-galactose ceramide galactosyltransferase 8: A combined molecular docking and dynamic study. J Neurosci Res 2016;94:1318-26. [Crossref] [PubMed]
- George S, Weber DR, Kaplan P, et al. Short-Term Safety of Zoledronic Acid in Young Patients With Bone Disorders: An Extensive Institutional Experience. J Clin Endocrinol Metab 2015;100:4163-71. [Crossref] [PubMed]
- Munns CF, Rajab MH, Hong J, et al. Acute phase response and mineral status following low dose intravenous zoledronic acid in children. Bone 2007;41:366-70. [Crossref] [PubMed]
- Dicuonzo G, Vincenzi B, Santini D, et al. Fever after zoledronic acid administration is due to increase in TNF-α and IL-6. J Interferon Cytokine Res 2003;23:649-54. [Crossref] [PubMed]
- Robinson RE, Nahata MC, Hayes JR, et al. Effectiveness of pretreatment in decreasing adverse events associated with pamidronate in children and adolescents. Pharmacotherapy 2004;24:195-7. [Crossref] [PubMed]
- Trivedi S, Al-Nofal A, Kumar S, et al. Severe non-infective systemic inflammatory response syndrome, shock, and end-organ dysfunction after zoledronic acid administration in a child. Osteoporos Int 2016;27:2379-82. [Crossref] [PubMed]
- Billington EO, Horne A, Gamble GD, et al. Effect of single-dose dexamethasone on acute phase response following zoledronic acid: a randomized controlled trial. Osteoporos Int 2017;28:1867-74. [Crossref] [PubMed]
- Kumar C, Panigrahi I, Somasekhara Aradhya A, et al. Zoledronate for Osteogenesis imperfecta: evaluation of safety profile in children. J Pediatr Endocrinol Metab 2016;29:947-52. [Crossref] [PubMed]
- Srivastava T, Dai H, Haney CJ, et al. Serum 25-hydroxyvitamin D level and acute-phase reaction following initial intravenous bisphosphonate. J Bone Miner Res 2011;26:437-8. [Crossref] [PubMed]
- Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011;364:1728-37. [Crossref] [PubMed]
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014;29:1-23. [Crossref] [PubMed]
- Hegazy A, Kenawey M, Sochett E, et al. Unusual Femur Stress Fractures in Children With Osteogenesis Imperfecta and Intramedullary Rods on Long-term Intravenous Pamidronate Therapy. J Pediatr Orthop 2016;36:757-61. [Crossref] [PubMed]
- Vasanwala RF, Sanghrajka A, Bishop NJ, et al. Recurrent Proximal Femur Fractures in a Teenager With Osteogenesis Imperfecta on Continuous Bisphosphonate Therapy: Are We Overtreating? J Bone Miner Res 2016;31:1449-54. [Crossref] [PubMed]
- Etxebarria-Foronda I, Carpintero P. An atypical fracture in male patient with osteogenesis imperfecta. Clin Cases Miner Bone Metab 2015;12:278-81. [PubMed]
- Bowden SA, Akusoba CI, Hayes JR, et al. Biochemical markers of bone turnover in children with clinical bone fragility. J Pediatr Endocrinol Metab 2016;29:715-22. [Crossref] [PubMed]
- Vuorimies I, Arponen H, Valta H, et al. Timing of dental development in osteogenesis imperfecta patients with and without bisphosphonate treatment. Bone 2017;94:29-33. [Crossref] [PubMed]
- Hernandez M, Phulpin B, Mansuy L, et al. Use of new targeted cancer therapies in children: effects on dental development and risk of jaw osteonecrosis: a review. J Oral Pathol Med 2017;46:321-6. [Crossref] [PubMed]
- Trejo P, Rauch F. Osteogenesis imperfecta in children and adolescents-new developments in diagnosis and treatment. Osteoporos Int 2016;27:3427-37. [Crossref] [PubMed]
- Biggin A, Briody JN, Ormshaw E, et al. Fracture during intravenous bisphosphonate treatment in a child with osteogenesis imperfecta: an argument for a more frequent, low-dose treatment regimen. Horm Res Paediatr 2014;81:204-10. [Crossref] [PubMed]
- Rauch F, Munns C, Land C, et al. Pamidronate in children and adolescents with osteogenesis imperfecta: effect of treatment discontinuation. J Clin Endocrinol Metab 2006;91:1268-74. [Crossref] [PubMed]
- Fukumoto S, Matsumoto T. Recent advances in the management of osteoporosis. F1000Res 2017;6:625. [Crossref] [PubMed]


