Transient hypothyroidism in the newborn: to treat or not to treat
Introduction
Congenital hypothyroidism (CH) is deficiency of thyroid hormone present at birth and is categorized into permanent and transient forms. Infants with permanent CH has persistent deficiency of thyroid hormone and need life-long supplementation. Infants with transient CH has deficiency of thyroid hormone which is temporary and improve to normal thyroid hormone levels usually in few months (1). Transient hypothyroxinemia of prematurity (THOP) is defined as deficiency of thyroid hormone [low thyroxine (T4)] accompanied by weak or absent TSH surge after birth in preterm infants. Primary hypothyroidism is deficiency in thyroxine due to functional or structural defect in the thyroid gland. Secondary hypothyroidism is due to deficiency of pituitary hormone, TSH and usually associated other hormone deficiencies.
Most newborns with CH at birth have no or few clinical features, so it is impossible to predict who are affected. CH is a common preventable cause of intellectual disability worldwide. Newborn screening (NBS) programs developed 1970s to detect CH, have been largely successful in diagnosis and prevention of intellectual disability in these infants (2).
Epidemiology
The prevalence of CH differs based on the cutoffs used for thyroid levels. The prevalence of recognized CH rose from 1 in 6,500 prior to screening era to approximately 1 in 2,000 to 3,000 with screening (2). The incidence of transient CH in North America is 5% to 10% in children positive for CH on mass screening, or 1/50,000. However, transient CH accounted for 40% of CH-positive cases in mass screening from 1981 to 2002 in France (3), while in a study from the Michigan NBS Program, USA, about 24% to 36% of children diagnosed with CH by NBS were later determined to have transient hypothyroidism (4).
The incidence of CH fluctuates by geographic area and by race. A study from New York Screening Program from 2000 to 2003 showed an incidence of 1:1,601 (5). The incidence was slightly higher in Hispanic (1:1,559) and Asian infants (1:1,016) compared to white (1:1,815) and black infants (1:1,902) (5). Additionally, the incidence was almost twice in twins (1:876) compared to singletons (1:1,765), and even higher in triplets (1:575). Preterm infants (1:1,396) had higher incidence than term infants (1:1,843) did. Moreover the incidence was higher in older mothers (>39 years of age; 1:1,328) than younger mothers (<20 years; 1:1,703) (5).
A review of the Screening Program in the state of Oregon, USA over a 7-year period from 2005 to 2011, involving 331,688 total births, 197 babies were diagnosed with CH, an incidence of 1:1,684. Oregon NBS program follows a primary T4, reflex TSH method for which the first blood sample is collected in first week of life and a second sample is collected at approximately 2–4 weeks of life in all babies. In the infants diagnosed on the first test, 83% were permanent and 17% were transient, while in the infants diagnosed on the second test, 23% were permanent and 77% were transient (6).
Another study of the Scottish Congenital Hypothyroid database over a period of 34 years (1980 to 2014), screened 2,116,132 newborns, of whom 919 infants (1:2,300) were referred with TSH elevation: 606 (65.9%) with definite CH, 18 (2.0%) with probable CH, 51 (5.5%) with status uncertain, and 211 (23.0%) with transient TSH elevation (7).
The reported incidence of CH in South Korea has been increasing over the past decade, from 1:5,449 newborns tested in 2004 to 1:1,231 newborns tested in 2012. In Korea, a total of 13 laboratories perform the NBS TSH, and the cutoff levels of TSH vary from 10.0 to 22.5 mIU/mL according to each laboratory protocol (8). Another recent study from Korea showed that nearly 80% of preterm infants with CH treated for 3 years with thyroxine were later diagnosed with transient hypothyroidism (9). A study from Iran over a period of 7 years, 2006 to 2012 revealed the prevalence of transient CH of 1:628 and permanent CH, 1:581 (10).
A retrospective study of NBS program in Egypt, from 2003 to 2011 showed transient CH in ~18% of positive CH cases. Through NBS program 248 cases were detected initially with CH, 204 (82.3%) infants were diagnosed with permanent CH (prevalence 1/3,587 live birth), and 44 (17.7%) infants were diagnosed with transient CH (prevalence 1/16,667 live birth) (11). Infants with trisomy 21 (Down syndrome) have a higher incidence of hypothyroidism detected by NBS programs, occurring as commonly as 1:50 newborns but they rarely have transient hypothyroidism (12).
The differences between various screening programs can be explained by different time frames when the studies were conducted and the fact that each program used varying diagnostic tests and criteria for diagnosis.
Pathophysiology of transient hypothyroidism
During gestation, the fetal thyroid starts to concentrate Iodine starting around 10 weeks and starts to secrete T4 and triiodothyronine (T3) around 12 weeks of gestation which increases gradually throughout gestation. Placental estrogen and hCG production modulates maternal thyroid function significantly leading to increased TBG, T4 and T3 in the maternal circulation thus increased thyroid function (Figure 1). This does not lead to a significant impact on the growing fetus since the placenta acts as a barrier to transfer of maternal TSH, and majority of maternal T4 is inactivated. Fetal thyroid develops independently of maternal thyroid function, though early in gestation small amounts of maternal thyroid hormone do cross the placenta. The fetus normally remains in a state of anabolism, where there is limited need for thyroid hormones. This is ensured by inactivation of thyroid hormones produced by the fetal thyroid gland by converting these into sulfated moieties by action of monodeiodinases (MDI) especially MDI-3 expressed in the placenta. Maturation of the hypothalamic-pituitary-thyroid (HPT) axis starts around 20 weeks and is complete only close to term (13).
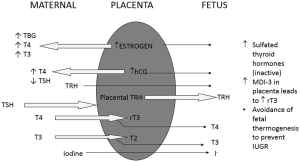
Postnatally the neonatal thyroid gland is called upon to provide a constant supply of thyroid hormones to ensure thermogenesis and support development of other organs like the central nervous system. In the preterm infant with an immature HPT axis and limited capacity to generate bioactive thyroid hormones a dip in thyroid hormones is commonly witnessed around one week of age (14). This decrease is exaggerated in sick preterm neonates where a poorly functioning HPT axis is unable to produce enough TSH in response to low T4 and T3, thus leading to a relative deficiency. This relationship is responsible for the majority of transient CH witnessed in many neonates.
Etiology of transient CH
Iodine deficiency
Worldwide iodine deficiency is the most common cause of transient CH. It is a critical element for thyroid hormone production, containing 59% of T3 and 65% of T4 by weight. Fetal and neonatal iodine stores are essentially dependent on iodine status of the mother during pregnancy and on the iodine content of human milk or formula postnatally. Iodine deficiency is more common in European countries, due to deficiency of iodine in maternal diets (3,15,16). Preterm babies are remarkably susceptible to the effects of iodine deficiency, due to decreased in utero thyroidal iodine stores and immaturity of the HPT axis for thyroid hormone production, and decreased ability to convert T4 to metabolically active T3. Deficiency of other elements, selenium and iron may effect neurologic development and thyroidal response to iodine supplementation (17). Though North America is usually considered iodine-sufficient, recent NHANES (National Health and Nutrition Examination Survey 2005–2008) survey in USA demonstrated that 57% of pregnant women had urinary iodine levels less than 150 mcg/L, which suggests iodine sufficiency (18).
Thyrotropin receptor blocking antibodies (TRBAbs)
These develop in women with autoimmune thyroid disease (Graves, chronic lymphocytic thyroiditis & acquired hypothyroidism, DUOX2 enzyme mutations). The antibodies can cross the placenta and block the TSH receptor in thyroid gland of the newborn. Seen in approximately 1:180,000 newborns, this diagnosis should be considered if more than one infant from the same mother has CH. Transient CH due to TRBAbs can last up to 3 to 6 months from birth as maternal antibody levels drop (19-21).
Intrauterine exposure to antithyroid drugs
Antithyroid drugs like propylthiouracil or methimazole can cross the placenta and cause decrease in thyroid hormone production in the fetus, which lasts for a few days to 2 weeks from birth. These babies may develop airway problems due to enlarged thyroid glands, especially in severe cases.
Fetal iodine exposure
Amiodarone usage during pregnancy may cause transient hypothyroidism in newborns. This resolves around 5 months of age, but can be associated with adverse neurologic outcomes (22,23). Maternal use of Iodine antiseptic compounds or exposure to iodinated contrast agents can cause transient CH. Ingestion of excessive iodine from nutritional supplements during pregnancy is reported to cause transient CH (24). The risk of hypothyroidism may be linked to the type and length of exposure. Recent studies showed normal thyroid functions in the infants of mothers who received iodide contrast during pregnancy (25,26). Amniofetography with iodine containing contrast also induces transient CH (27).
Neonatal iodine exposure
Neonatal exposure to high amounts of iodine can cause hypothyroidism due to Wolff-Chaikoff effect. This can occur especially in premature babies (28,29) and in term infants with congenital heart disease needing cardiac catheterization (30,31). Preterm infants get exposed high doses of iodine with usage of iodine containing antiseptics for umbilical and peripherally inserted central catheter (PICC) lines.
Hepatic hemangiomas
Congenital large hepatic hemangiomas produce large amounts of enzyme type 3-iodothyronine deiodinase causing a consumptive type of hypothyroidism. Serum TSH and reverse T3 levels are elevated and T4 levels are low. High doses of thyroxine are necessary to keep a euthyroid state. Hypothyroidism resolves as the hemangioma involutes or is surgically treated (32,33).
Loss of function mutations in DUOX2 (THOX2) and DUOXA2 cause transient CH
These genes are needed to produce hydrogen peroxide, required by thyroid peroxidase to synthesize thyroid hormones. DUOX2 mutation is inherited as autosomal recessive trait (34-36).
THOP
Preterm babies are more susceptible to transient hypothyroidism, and the incidence of THOP increases with decreasing gestational age (37). Several studies have demonstrated a correlation of THOP with subsequent low IQ and neurologic sequelae (37-41). However, these were observational studies that did not establish causality. A recent study from the Netherlands showed no association between THOP and neurodevelopmental outcomes in young adulthood (42). Efficacy of thyroid hormone therapy has also not been established.
Clinical features
The classic clinical symptoms and signs of CH are usually absent in the newborn period in vast majority of infants and present gradually over few weeks, even in infants with thyroid agenesis (43). Placental transfer of maternal thyroxine temporarily protects the newborn. In babies with some functional thyroid tissue, the clinical manifestations can be delayed by months to years. CH is significantly and independently associated with reduced variability in fetal heart rate tracing patterns (44).
In parts of the world where NBS programs are not available, infants have clinical manifestations like lethargy, inactivity, hypotonia, periorbital edema, perioral cyanosis, mottled skin, hypothermia, pallor, prolonged icterus, feeding difficulty, poor or hoarse crying, constipation and respiratory distress. Due to myxedema of the airway, few babies may develop respiratory distress with noisy breathing, nasal stuffiness, and intermittent perioral cyanosis. Prolonged physiologic jaundice after the first week of life may be the presentation of hypothyroidism. Primary hypothyroidism presents with indirect hyperbilirubinemia predominantly, whereas central hypothyroidism has both indirect and direct hyperbilirubinemia (45).
Classic features of CH are a relatively narrow forehead, large anterior and posterior fontanelles, depressed nasal bridge, puffy eyelids, large tongue, thick, dry, cold skin, long abundant coarse hair, bradycardia, hypotension with narrow pulse pressure, anemia abdominal distention, umbilical hernia and hyporeflexia (45). Few infants with thyroid dyshormonogenesis present with a palpable goiter.
Associated congenital anomalies
CH is associated with increased risk of other anomalies affecting the heart, kidneys, urinary tract, gastrointestinal, and skeletal systems (46-48). In a population-based study of 1,420 infants with CH, the prevalence of cardiac malformations was fourfold higher (8.4%) than in the control infant population (1% to 2%) (46). A study from New York State Congenital Malformation Registry (980 children), showed an increased risk of renal and urologic abnormalities [odds ratio (OR) 13.2] (47). A loss-of-function mutation in transcription factor, TTF-2 presented with CH, athyreosis and cleft palate (48).
NBS
One hundred thirty million infants are born annually worldwide, only 37 million infants (28.4%) get NBS and about 12,000 infants with hypothyroidism are detected annually. About 71% of babies worldwide are born in areas without an established NBS program, despite the existence of screening for over 50 years. It is a huge public health challenge as the majority of babies with CH worldwide are not detected and treated early, leading to the economic burden of mental retardation (49). The goal of NBS is to detect neonates with primary CH; those programs that employ a primary T4-reflex TSH or combined T4 and TSH tests strategy have the potential to detect infants with secondary CH (estimated incidence about 1:25,000) (49).
Management
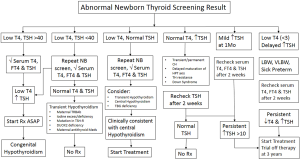
In newborns with abnormal newborn screen results, thyroid function tests should be done including TSH and free T4. The clinician should be aware that during the first 6 h of life after birth, a physiologic TSH surge occurs, and the concentration of TSH may remain above 10 mU/L during the first 24 to 48 h of life. Babies with newborn screen specimens collected before 24 h of life and have mildly elevated TSH values very likely will have normal thyroid function tests on repeated testing (50).
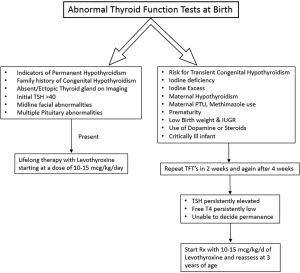
Figures 2 and 3 provide an algorithm of suggested approach to treatment of CH to differentiate permanent and transient CH. High TSH and low free T4 concentrations on serum testing confirm the diagnosis of primary hypothyroidism. Treatment with levothyroxine is indicated immediately, with initial dose of 10–15 µg/kg/day. High TSH and normal free T4 or total T4 concentrations suggest subclinical hypothyroidism. In cases with marginally elevated TSH (6 to 10 mIU/L), monitor carefully, repeating a serum TSH and free T4 in a week. However, if the serum TSH does not normalize by four to six weeks of age, thyroxine treatment is recommended. Low or normal TSH and low free T4 concentrations suggest central hypothyroidism. As TSH is not elevated, this form of hypothyroidism is not detected by programs that screen with TSH alone. These infants should be treated with thyroxine.
Infants with euthyroid sick syndrome or non-thyroidal illness also may have normal TSH and low free T4 serum concentrations. Thyroid function in preterm infants is characterized by decreased TSH and T4 responses to labor, low serum total T4 and TSH levels and variable free T4 concentrations during the first 2–4 postnatal weeks of life which improves when infants are corrected to term gestational age (37).
Additional testing
Thyroid imaging (thyroid ultrasonography or radionuclide uptake scan)
These studies provide information about the underlying etiology, e.g., thyroid dysgenesis or dyshormonogenesis. In transient hypothyroidism due to iodine deficiency, imaging shows increased radionuclide uptake in normally located thyroid tissue. Cases of transient CH due to maternal TRB-Ab typically do not have radionuclide uptake on scanning, but on ultrasonography, a normal thyroid gland may be visualized. There is decreased radionuclide uptake in iodine excess and increased uptake in iodine deficiency. There is some argument regarding the risk benefit ratio of early thyroid scanning of babies with suspected CH.
X-ray
In about half of the infants with primary hypothyroidism, retardation of bone maturation is seen. Absence of distal femoral or proximal tibial epiphyses in a newborn suggest intrauterine thyroid hormone deficiency.
Thyroid autoantibodies
Useful for diagnosis in babies born to women with known autoimmune thyroid disease, and in families with a previous sibling detected with CH.
Serum thyroglobulin concentration
This test is useful in further delineating the causes of CH after thyroid imaging since it may be useful in diagnosis of subclinical hypothyroidism.
Urinary iodine concentration
Useful in infants who had intra uterine iodine exposure or during the neonatal period to confirm an iodine excess state. It is also useful in diagnosis of infants born in iodine deficient endemic areas.
Management of transient CH
Transient CH should be treated since there are severe long term consequences for untreated infants with long term morbidities and due to ack of availability of imaging modalities (US scan expertise, T99 scan and I123 scan) and problems in their interpretation, and all centers are not able to diagnose the cause of transient CH. There is also lack of availability of genetic tests required to differentiate permanent CH and transient CH in most centers. The cost of treatment for up to 3 years and cost of laboratory follow up tests are minimal compared to the high cost of caring for affected infants. A population specific approach will be helpful where there is a higher incidence of autosomal recessive diseases or a lack of Iodine supplementation or lack of prenatal care and testing. If FT4 is low or TSH stays greater than 5 mU/L on serial testing within the first month after birth, the infant should be started on thyroxine until 3 years of age to protect brain development. Persistent TSH elevation (beyond 4 weeks of age) is detrimental for brain development. We cannot confirm whether the case of CH was transient or permanent until later. Thus, most clinicians err on the side of caution and elect to treat infants with low free T4 and refrain from treatment when free T4 remains within the normal range while repeating TSH and free T4 periodically.
The goal of therapy is to ensure normal growth and development by keeping the serum total T4 or FT4 concentration in the upper half of the reference range during the infancy, with a serum TSH in the reference range (optimally 0.5–2.0 mU/L) (1).
Evidence not to treat THOP
Cochrane review does not support the use of prophylactic thyroid hormones in preterm infants to reduce neonatal mortality, neonatal morbidity or improve neurodevelopmental outcomes (51). A randomized controlled trial of thyroxine supplementation, TIPIT (thyroxine trial in preterm infants) trial targeting extremely premature infants done in the UK showed that supplementing all babies below 28 weeks’ gestation with LT4 had no apparent effect on brain size. These results do not support routine supplementation with LT4 for all babies born below 28 weeks’ gestation (52). A recent multi-center randomized clinical trial from Japan showed that thyroxine supplementation in VLBW infants with THOP demonstrated no beneficial effect at 3 years of age (53).
If permanent CH has not been confirmed by 2 to 3 years of age both the European Society for Paediatric Endocrinology (ESPE) and American Academy of Pediatrics (AAP) recommend a thirty-day trial off thyroxine therapy (1,54). If thyroid function tests show elevated TSH and low serum T4 or free T4, the diagnosis of permanent CH is confirmed and the patient should be restarted on thyroxine. If the serum TSH and T4 or free T4 remain normal, the presumed diagnosis is transient CH and the patient no longer needs thyroxine supplementation. Yet, they must be followed closely and monitored for hypothyroidism signs and symptoms.
Recent studies have advocated that the course of CH is transient in many premature babies where discontinuing thyroxine supplementation is possible before 3 years of age (55,56). A recent study showed that infants with CH requiring lower L-thyroxine doses (<3.25 µg/kg) are likely to have transient CH, and thus might be re-evaluated at 12 or 24 months rather than 3 years of age (57). There is a critical necessity for specific guidelines regarding the follow-up and reevaluation of transient CH, especially in preterm babies.
Prognosis
NBS programs, which have been in existence over the last few decades in most developed nations, have led to early diagnosis and treatment of babies with CH and have eradicated the severe neurodevelopmental impairments resulting from late detection. Studies on cognitive function in children with CH treated soon after birth have shown that normal development can be accomplished in most of them, although some may have subtle neurocognitive impairments (2). The major determinant of neurodevelopmental outcome is the timing of starting thyroxine supplementation. Review of a number of studies comparing thyroxine supplementation at earlier time periods (12–30 days of life) versus later time periods (>30 days of life) found that infants treated earlier be around 15.7 IQ points higher (58).
Conclusions
We have presented the evidence whether to treat or not to treat transient hypothyroidism, especially in preterm infants. Our current AAP and European guidelines are not specific for preterm babies with CH; but with the current guidelines majority of preterm infants in whom hypothyroidism is most likely transient, are treated for 3 years. Specific guidelines for the diagnosis, treatment and follow-up for transient hypothyroidism are critically needed.
Appendix
European Society for Paediatric Endocrinology Guidelines for management of Congenital Hypothyroidism (54):
- Introduce newborn screening for CH worldwide, with initial priority to detect all forms of CH: mild, moderate, and severe. The most sensitive test for detecting primary CH is TSH determination. Second screening should be considered for low birth weight (LBW) & very low-birth weight (VLBW) infants, ill and preterm newborns admitted to neonatal intensive care units (NICU).
- If capillary TSH on neonatal screening is ≥40 mU/L whole blood, recommend starting treatment as soon as a good venous sample can be obtained, without waiting for the venous blood test result. If capillary TSH concentration is <40 mU/L of whole blood, the clinician may wait for the results of venous TFT, provided that these results are available on the following day.
- If venous free T4 (FT4) concentration is below norms for age, treatment should be started immediately. If venous TSH concentration is >20 mU/L, treatment should be started, even if FT4 concentration is normal.
- If venous TSH concentration is between 6 to 20 mU/L beyond 21 days in a well-baby with a FT4 concentration within the reference for age, we suggest (I) investigation, which should include diagnostic imaging, for a definitive diagnosis; (II) consider discussion with the family, of either initiating thyroxine supplementation immediately and retesting, off treatment, at a later stage; or withholding treatment but retesting 2 weeks later.
- X-ray of the knee to assess the severity of intrauterine hypothyroidism by the presence or absence of femoral and tibial epiphyses in term infants. Thyroid imaging should never be allowed to delay the initiation of treatment.
- L-thyroxine (L-T4) is recommended as the medication of choice for treating CH. Thyroxine treatment should be initiated as soon as possible and no later than 2 weeks after birth or immediately after confirmatory serum test results in infants in whom CH is detected by a second routine screening test. An initial L-T4 dose of 10–15 µg/kg per day should be given. Infants with severe disease, as defined by a very low pretreatment TT 4 or FT4 concentration, should be treated with the highest initial dose.
- L-T4 should be administered orally; if intravenous treatment is necessary the dose should be no more than 80% of the oral dose. Serum FT4 (or TT4) and TSH concentrations should be determined at least 4 h after the last L-T4 administration. TSH concentration should be maintained in the age specific reference range; TT4 or FT4 concentration should be maintained in the upper half of the age-specific reference range. Any reduction of L-T4 dose should not be based on a single increase in FT4 level during treatment.
- The first follow-up examination should take place 1–2 weeks after the start of L-T4 treatment. Subsequent evaluation should take place every 2 weeks until a complete normalization of TSH concentration is reached; then every 1 to 3 months thereafter until the age of 12 months. Between the ages of 1 and 3 years, children should undergo clinical and laboratory evaluations every 2 to 4 months. Thereafter, evaluations should be carried out every 3 to 12 months until growth is completed. More frequent evaluations should be carried out if compliance is questioned or abnormal values are obtained.
- Re-evaluation of the thyroid axis is indicated when no diagnostic assessment was carried out in infancy, and particularly when the infant was preterm/sick at the time of referral. For a precise diagnosis, L-T4 treatment should be phased out over a 4- to 6-week period, and a full reevaluation should be carried out, with both biochemical testing and thyroid imaging if hypothyroidism is confirmed.
- If the presence or absence of permanent CH is being assessed, decrease the dose of L-T4 by 30% for 2–3 weeks and then rechecking thyroid function. If an increase in TSH concentrations to ≥10 mU/L is demonstrated, permanent CH is confirmed. Otherwise the dose can be reduced further, with retesting after another 2–3 weeks.
- Psychomotor development and school progression should be monitored in all children with CH. A personalized educational plan is required if school progress is affected in cases of severe CH. Memory deficits may be corrected by targeted training. Repeated hearing tests should be carried out before school age and, as required. Screen for visual processing problems and speech delay is recommended.
- Genetic counseling should involve explaining the risk of recurrence of CH in an affected family, based on family history and thyroid morphology. Any syndromic association should be studied genetically. The presence of familial cases of dysgenesis in siblings or parents should lead to a search for TSH receptor or PAX8 mutations, respectively.
- Antenatal diagnosis is recommended when goiter is fortuitously discovered during fetal ultrasound, with a family history of dyshormonogenesis and with known defects of genes involved in thyroid function or development. The therapeutic management of affected fetuses should comply with the laws of the country concerned. Only multidisciplinary specialist teams should perform interventions such as intra-amniotic L-T4 injection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Academy of Pediatrics. Rose SR, Section on Endocrinology and Committee on Genetics, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006;117:2290-303. [Crossref] [PubMed]
- Grosse SD, Van Vliet G. Prevention of intellectual disability through screening for congenital hypothyroidism: how much and at what level? Arch Dis Child 2011;96:374-9. [Crossref] [PubMed]
- Gaudino R, Garel C, Czernichow P, et al. Proportion of various types of thyroid disorders among newborns with congenital hypothyroidism and normally located gland: a regional cohort study. Clin Endocrinol (Oxf) 2005;62:444-8. [Crossref] [PubMed]
- Korzeniewski SJ, Grigorescu V, Kleyn M, et al. Transient hypothyroidism at 3-year follow-up among cases of congenital hypothyroidism detected by newborn screening. J Pediatr 2013;162:177-82. [Crossref] [PubMed]
- Harris KB, Pass KA. Increase in congenital hypothyroidism in New York State and in the United States. Mol Genet Metab 2007;91:268-77. [Crossref] [PubMed]
- Ford GA, Denniston S, Sesser D, et al. Transient versus Permanent Congenital Hypothyroidism after the Age of 3 Years in Infants Detected on the First versus Second Newborn Screening Test in Oregon, USA. Horm Res Paediatr 2016;86:169-77. [Crossref] [PubMed]
- Mansour C, Ouarezki Y, Jones J, et al. Trends in Scottish newborn screening programme for congenital hypothyroidism 1980-2014: strategies for reducing age at notification after initial and repeat sampling. Arch Dis Child 2017;102:936-41. [Crossref] [PubMed]
- Kang MJ, Chung HR, Oh YJ, et al. Three-year follow-up of children with abnormal newborn screening results for congenital hypothyroidism. Pediatr Neonatol 2017;58:442-8. [Crossref] [PubMed]
- Chung ML. Incidence and Risk Factor of Permanent Hypothyroidism in Preterm Infants. J Neonatal Biol 2017;6:2. [Crossref]
- Dorreh F, Chaijan PY, Javaheri J, et al. Epidemiology of congenital hypothyroidism in Markazi Province, Iran. J Clin Res Pediatr Endocrinol 2014;6:105-10. [Crossref] [PubMed]
- Bekhit OE, Yousef RM. Permanent and transient congenital hypothyroidism in Fayoum, Egypt: a descriptive retrospective study. PLoS One 2013;8:e68048. [Crossref] [PubMed]
- Bas VN, Ozgelen S, Cetinkaya S, et al. Diseases accompanying congenital hypothyroidism. J Pediatr Endocrinol Metab 2014;27:485-9. [Crossref] [PubMed]
- Polin RA, Fox WW, Abman SH. Fetal and Neonatal Physiology. Fourth Edition. Philadelphia: Elsevier Saunders, 2011.
- Mercado M, Yu VY, Francis I, et al. Thyroid function in very preterm infants. Early Hum Dev 1988;16:131-41. [Crossref] [PubMed]
- Delange F, Dalhem A, Bourdoux P, et al. Increased risk of primary hypothyroidism in preterm infants. J Pediatr 1984;105:462-9. [Crossref] [PubMed]
- Delange F. Neonatal screening for congenital hypothyroidism: results and perspectives. Horm Res 1997;48:51-61. [Crossref] [PubMed]
- Delange F, Burgi H, Chen ZP, et al. World status of monitoring iodine deficiency disorders control programs. Thyroid 2002;12:915-24. [Crossref] [PubMed]
- Caldwell KL, Makhmudov A, Ely E, et al. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005-2006 and 2007-2008. Thyroid 2011;21:419-27. [Crossref] [PubMed]
- Zakarija M, McKenzie JM, Eidson MS. Transient neonatal hypothyroidism: characterization of maternal antibodies to the thyrotropin receptor. J Clin Endocrinol Metab 1990;70:1239-46. [Crossref] [PubMed]
- Brown RS, Bellisario RL, Mitchell E, et al. Detection of thyrotropin binding inhibitory activity in neonatal blood spots. J Clin Endocrinol Metab 1993;77:1005-8. [PubMed]
- Pacaud D, Huot C, Gattereau A, et al. Outcome in three siblings with antibody-mediated transient congenital hypothyroidism. J Pediatr 1995;127:275-7. [Crossref] [PubMed]
- Bartalena L, Bogazzi F, Braverman LE, et al. Effects of amiodarone administration during pregnancy on neonatal thyroid function and subsequent neurodevelopment. J Endocrinol Invest 2001;24:116-30. [Crossref] [PubMed]
- Lomenick JP, Jackson WA, Backeljauw PF. Amiodarone-induced neonatal hypothyroidism: a unique form of transient early-onset hypothyroidism. J Perinatol 2004;24:397-9. [Crossref] [PubMed]
- Connelly KJ, Boston BA, Pearce EN, et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr 2012;161:760-2. [Crossref] [PubMed]
- Atwell TD, Lteif AN, Brown DL, et al. Neonatal thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. AJR Am J Roentgenol 2008;191:268-71. [Crossref] [PubMed]
- Bourjeily G, Chalhoub M, Phornphutkul C, et al. Neonatal thyroid function: effect of a single exposure to iodinated contrast medium in utero. Radiology 2010;256:744-50. [Crossref] [PubMed]
- Rodesch F, Camus M, Ermans AM, et al. Adverse effect of amniofetography on fetal thyroid function. Am J Obstet Gynecol 1976;126:723-6. [Crossref] [PubMed]
- Linder N, Davidovitch N, Reichman B, et al. Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants. J Pediatr 1997;131:434-9. [Crossref] [PubMed]
- Aitken J, Williams FL. A systematic review of thyroid dysfunction in preterm neonates exposed to topical iodine. Arch Dis Child Fetal Neonatal Ed 2014;99:F21-8. [Crossref] [PubMed]
- Thaker VV, Leung AM, Braverman LE, et al. Iodine-induced hypothyroidism in full-term infants with congenital heart disease: more common than currently appreciated? J Clin Endocrinol Metab 2014;99:3521-6. [Crossref] [PubMed]
- Linder N, Sela B, German B, et al. Iodine and hypothyroidism in neonates with congenital heart disease. Arch Dis Child Fetal Neonatal Ed 1997;77:F239-40. [Crossref] [PubMed]
- Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 2000;343:185-9. [Crossref] [PubMed]
- Mouat F, Evans HM, Cutfield WS, et al. Massive hepatic hemangioendothelioma and consumptive hypothyroidism. J Pediatr Endocrinol Metab 2008;21:701-3. [Crossref] [PubMed]
- Moreno JC, Bikker H, Kempers MJ, et al. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med 2002;347:95-102. [Crossref] [PubMed]
- Zamproni I, Grasberger H, Cortinovis F, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab 2008;93:605-10. [Crossref] [PubMed]
- Maruo Y, Takahashi H, Soeda I, et al. Transient congenital hypothyroidism caused by biallelic mutations of the dual oxidase 2 gene in Japanese patients detected by a neonatal screening program. J Clin Endocrinol Metab 2008;93:4261-7. [Crossref] [PubMed]
- Fisher DA. Thyroid function and dysfunction in premature infants. Pediatr Endocrinol Rev 2007;4:317-28. [PubMed]
- Fisher DA. Thyroid system immaturities in very low birth weight premature infants. Semin Perinatol 2008;32:387-97. [Crossref] [PubMed]
- Meijer WJ, Verloove-Vanhorick SP, Brand R, et al. Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Arch Dis Child 1992;67:944-7. [Crossref] [PubMed]
- Den Ouden AL, Kok JH, Verkerk PH, et al. The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatr Res 1996;39:142-5. [Crossref] [PubMed]
- Reuss ML, Paneth N, Pinto-Martin JA, et al. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med 1996;334:821-7. [Crossref] [PubMed]
- Hollanders JJ, Israels J, van der Pal SM, et al. No Association Between Transient Hypothyroxinemia of Prematurity and Neurodevelopmental Outcome in Young Adulthood. J Clin Endocrinol Metab 2015;100:4648-53. [Crossref] [PubMed]
- Alm J, Hagenfeldt L, Larsson A, et al. Incidence of congenital hypothyroidism: retrospective study of neonatal laboratory screening versus clinical symptoms as indicators leading to diagnosis. Br Med J (Clin Res Ed) 1984;289:1171-5. [Crossref] [PubMed]
- Shoham I, Aricha-Tamir B, Weintraub AY, et al. Fetal heart rate tracing patterns associated with congenital hypothyroidism. Am J Obstet Gynecol 2009;201:48. e1-4.
- LaFranchi SH, Murphey WH, Foley TP Jr, et al. Neonatal hypothyroidism detected by the Northwest Regional Screening Program. Pediatrics 1979;63:180-91. [PubMed]
- Olivieri A, Stazi MA, Mastroiacovo P, et al. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991-1998). J Clin Endocrinol Metab 2002;87:557-62. [PubMed]
- Kumar J, Gordillo R, Kaskel FJ, et al. Increased prevalence of renal and urinary tract anomalies in children with congenital hypothyroidism. J Pediatr 2009;154:263-6. [Crossref] [PubMed]
- Castanet M, Park SM, Smith A, et al. A novel loss-of-function mutation in TTF-2 is associated with congenital hypothyroidism, thyroid agenesis and cleft palate. Hum Mol Genet 2002;11:2051-9. [Crossref] [PubMed]
- Ford G, LaFranchi SH. Screening for congenital hypothyroidism: a worldwide view of strategies. Best Pract Res Clin Endocrinol Metab 2014;28:175-87. [Crossref] [PubMed]
- Slaughter JL, Meinzen-Derr J, Rose SR, et al. The effects of gestational age and birth weight on false-positive newborn-screening rates. Pediatrics 2010;126:910-6. [Crossref] [PubMed]
- Osborn DA, Hunt RW. Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2007.CD005948. [PubMed]
- Ng SM, Turner MA, Gamble C, et al. An explanatory randomised placebo controlled trial of levothyroxine supplementation for babies born <28 weeks' gestation: results of the TIPIT trial. Trials 2013;14:211. [Crossref] [PubMed]
- Uchiyama A, Kushima R, Watanabe T, et al. Effect of L-thyroxine supplementation on very low birth weight infants with transient hypothyroxinemia of prematurity at 3 years of age. J Perinatol 2017;37:602-5. [Crossref] [PubMed]
- Leger J, Olivieri A, Donaldson M, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr 2014;81:80-103. [Crossref] [PubMed]
- Lim G, Lee YK, Han HS. Early discontinuation of thyroxine therapy is possible in most very low-birthweight infants with hypothyroidism detected by screening. Acta Paediatr 2014;103:e123-9. [Crossref] [PubMed]
- Jung JM, Jin HY, Chung ML. Feasibility of an Early Discontinuation of Thyroid Hormone Treatment in Very-Low-Birth-Weight Infants at Risk for Transient or Permanent Congenital Hypothyroidism. Horm Res Paediatr 2016;85:131-9. [Crossref] [PubMed]
- Cho MS, Cho GS, Park SH, et al. Earlier re-evaluation may be possible in pediatric patients with eutopic congenital hypothyroidism requiring lower L-thyroxine doses. Ann Pediatr Endocrinol Metab 2014;19:141-5. [Crossref] [PubMed]
- LaFranchi SH, Austin J. How should we be treating children with congenital hypothyroidism? J Pediatr Endocrinol Metab 2007;20:559-78. [Crossref] [PubMed]