Role of notch signal in angiotensin II induced pulmonary vascular remodeling
Pulmonary hypertension (PH) is a fatal and common cardiovascular disease caused by small pulmonary artery obstruction from vascular proliferation and remodeling (1), it is characterized by elevated pulmonary arterial pressure and increase pulmonary vascular resistance, frequently leading to right-sided heart failure and death (2-4), but the mechanisms of its occurrence and development still remain obscure. Pulmonary vascular remodeling has been found among all types of severe PHs, indicating its role in the common pathological process of the pulmonary circulation in response to various pathological stimuli. Hence, pulmonary vascular remodeling is a key to the research into PH (5-7).
As shown in clinical and animal experiments, the activity of Ang II, a potent mitogen for VSMC in lung tissues, significantly increased on the occurrence of PH, which promoted the proliferation, hypertrophy, and migration of pulmonary artery smooth muscle cells. Animal studies found that angiotensin-converting enzyme inhibitors (ACEIs) and AT1 receptor antagonists could reduce the pulmonary artery pressure in PH rats, relieve the extent of right ventricular hypertrophy, and reduce or even reverse pulmonary vascular remodeling, which was a key to containing the development of PH-associated pulmonary vascular remodeling (8-13).
The mammalian Notch family molecules include four Notch receptors, Notch1-4, and five Notch ligands, Jagged1, Jagged2, DLL1, DLL3, and DLL4. Notch activation has been identified as an important signaling pathway in the development of many cell populations (14,15). It regulates multiple aspects of vasculogenesis and angiogenesis, including endothelial cell (EC) proliferation and migration, smooth muscle differentiation, and vascular patterning (16,17). It has been shown that DLL4 expression of the EC was enhanced by vascular endothelial growth factor (VEGF) and basic fibroblast factor (18), and VEGF function was, in a negative feedback manner, inhibited by the upregulated DLL4 through down-regulation of EC VEGF-R2 expression (19).
Latest studies have found that the Notch signaling pathway plays an important role in the vascular remodeling during embryonic development, blood vessel damage repair and tumor growth (20-26). However, there are few reports on the effect of angiotensin II on the Notch signaling pathway. And even few have been done to reveal Notch signaling pathway’s effect on pulmonary vascular remodeling associated with PH. In other words, it still remains unknown whether the Notch signaling pathway is involved in Ang II-induced pulmonary vascular remodeling.
In this study, pulmonary artery strips were cultured and induced with angiotensin II as an in vitro model of PH-associated pulmonary vascular remodeling to investigate the effect of inhibition of the Notch signal and its downstream gene HERPI-2 with γ-secretase inhibitors on pulmonary vascular remodeling.
Materials and methods
Animals
The study was carried out on thirty-six 8-week-old healthy adult Wistar rats (clean grade), weighing 180-210 g, which were provided by the Experimental Animal Center of Sichuan University Huaxi Medical Center.
Reagents
Reagents used in the study included: M199 medium (purchased from GIBCOTM, Invitrogen Corporation, Part No.P1504); γ-secretase inhibitor N[N-(3,5-Difluorophenacetyl)-L-alaryl] S-phenylalycine t-butyl ester(DAPT), C23H26F2N2O4 (purchased from Sigma, D5942, Synonym LY-374973), dissolved in dimethyl sulfoxide (DMSO); Ang II (MW 1047.2), purchased from AnaSpec, Inc. (USA); mouse anti-rat PCNA (proliferating cell nuclear antigen) monoclonal antibodies, and rabbit anti-rat caspase-3 monoclonal antibodies, purchased from Neomarker; and immunohistochemical staining kit SP-9002, purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. Cell lysate (Trizol) was purchased from MRC, U.S.; RevertAidTM First Strand cDNA Synthesis Kit (#K1622), purchased from MBI, Lithuanian; Taq DNA polymerase, purchased from Beijing BioDev Co., Ltd.; dNTP, purchased from Promega Corporation USA; and SYBR Green I, purchased from Roche, Switzerland. Notch1 primers, upstream series: 5'-TCTCAACTGCCAGAACCTTGT-3', downstream series: 5-ATGCCTCGCTTCTGTGCAG-3'(amplified cDNA length 171 bp); Notch2 primers, upstream series: 5-CACTGAGAGCTCCTGTTTCAA-3', downstream series: 5-CAGGCCATCAACACACGTTC-3'(cDNA length 160 bp); Notch3 primers, upstream series: 5-ATGGCAGGCTTCACAGGAAC-3', downstream series: 5-TGCAGCTGAAGCCATTGACT-3' (cDNA length 109 bp); Notch4 primers, upstream series: 5-AGTGTCTCCCAGGCTTTGAA-3', downstream series: 5-GAAGATCAAGGCAGCTGGCT-3' (cDNA length of 96 bp); HERP1 primers, upstream series: 5-GATGCTCCAGGCAACAGG-3', downstream series: 5-GTGGGTCCGAAGGGTCAA-3' (cDNA length 143 bp); HERP2 primers, upstream series: 5'-CCAACCACATCGTCCCA-3', downstream series: 5'-CTAGCTTCGCAGATCCCTG-3' (cDNA length 145 bp); GAPDH primers, upstream series: 5’-CCTCAAGATTGTCAGCAAT-3', downstream Series: 5’-CCATCCACAGTCTTCTGAGT-3' (cDNA length 141 bp), synthesized by TaKaRa Biotechnology (Dalian) Co., Ltd.
Preparation and culture of pulmonary arteries
After the rats were sacrificed by cervical dislocation and disinfected with 75% alcohol, their chests were opened for isolation and removal of the main pulmonary arteries. The arteries were washed in a dish containing sterile PBS buffer while fat tissues around the vascular adventitia were removed. Cut along the vertical axis (about 4 mm × 5 mm) without damaging the intima, each artery was made into a pulmonary artery strip. The 36 strips collected from 36 rats were randomly divided into six groups with six vessel strips apiece. One group (normal pulmonary arteries) was directly fixed with 4% paraformaldehyde and embedded in paraffin. Strips of the other five groups were cultured in 35 mm petri dishes and embedded in a rat tail collagen gel matrix [700 µL rat tail collagen, 100 µL M199 (10× liquid), 200 µL sterile NaHCO3 (11.76 mg/mL) (1 mL in total) mixed in a dish on ice and spread evenly]. The strips were unfolded on the gel with the endothelial surface on top. The dishes were incubated for about 15 min in a CO2 incubator at 37 °C. As collagen fibers formed, the pulmonary artery strips sank gently and were fixed in the gel. One hour following the addition of 1.5 mL M199 (1× liquid), the medium was replaced with M199 (1× liquid) containing 100 u/mL dual antibiotics (penicillin, streptomycin). The five groups were: (I) control (M199) group, (II) Ang II + M199 group, (III) Ang II + DMSO group, (IV) Ang II + low-dose DAPT group (DAPT concentration in medium: 1 µM) and (V) Ang II + high-dose DAPT group (DAPT concentration in medium: 10 µM). Specifically, after incubation of one day, the following contents were added into the daily replaced 1.5 mL medium of respective groups: (I) 4.5 µL of M199 medium; (II) 1.5 µL of diluted Ang II (to a final concentration of 1×10-7 M) and 3 µL M199; (III) 1.5 µL Ang II (to a final concentration of 1×10-7 M) and 3 µL DMSO; (IV) 1.5 µL Ang II (to a final concentration of 1×10-7) and 3 µL DAPT (to a final concentration of 1 µM); and (V) 1.5 µL Ang II (to a final concentration of 1×10-7 M) and 3 µL DAPT (to a final concentration of 10 µM). It took 7 d for this process to complete.
On day 9, incubated strips were collected and each cut in half. One half was preserved in liquid nitrogen for RT-PCR, and the other fixed in 4% paraformaldehyde at 4 °C for 2 h, conventionally dehydrated, embedded in paraffin and cut into 5 µm thick serial sections, which were subject to hematoxylin-eosin, elastic fiber, PCNA and caspase-3 staining.
Immunohistochemical staining
Instructions on the SP staining kit were followed using PCNA (1:300) and caspase-3 (1:100). PBS buffer (0.01 mol/L, pH 7.4) was used as a negative control in place of the primary antibody. The results were scanned in Image-Pro Plus software. Five random fields of each vessel strip (×400) were observed to calculate the ratios of vessel wall PCNA or caspase-3 positive cells. An average of the five was used to describe the degree of proliferation and apoptosis of vessel wall cells.
Measurement of vascular wall thickness
Verhoeff’s iron hematoxylin staining was used. The results were scanned in Image-Pro Plus software. Four random fields of each vessel strip (×400) were observed and the distance between the internal and external elastic plates in each field was measured for three times. The wall thickness was identified by the average of those measurements.
mRNA expression of Notch 1,2,3,4 receptors and their downstream effector HES-related inhibitor proteins 1, 2 (HERP1, 2)
SYBR dyes were used and the mRNA of test samples was subject to quantitative analysis with the 2-△△Ct method. The total RNA was extracted according to Trizol instructions. cDNA synthesis was performed as per the RevertAidTM First Strand cDNA Synthesis Kit instructions. RT-PCR of the target genes was carried out with the FTC2000 fluorescence quantitative PCR (Maple Ridge Co., Ltd., Canada). The reaction conditions were as follows: initial denaturation at 94 °C for 2 min, followed by 45 cycles of denaturation 94 °C for 20 s, annealing at 52 °C for 30 s, and extension at 72 °C for 40 s. A blank tube containing no cDNA sample was used as negative control. The increase in fluorescence intensity (DRn) of each reaction tube during each cycle was recorded to generate the reaction kinetic curve of each tube to determine the cycle number (Ct) at which the fluorescence generated in it a reaction crossed the threshold fluorescence intensity. △△Ct = [Ct GI (test sample)–Ct GAPDH (test sample)] [Ct GI (calibrator)–Ct GAPDH (calibrator)]. The quantity of the target genes relative to the internal reference was calculated by 2-△△Ct.
Statistical analysis
Data were processed in SPSS 12.0 software package. Measurement data were expressed as mean ± standard deviation (x±s). The t-test was used to compare the difference between two groups, and ANOVA or repeated measures analysis of variance to compare multiple groups, with a P<0.05 being considered statistically significant.
Results
Changes in pulmonary arteries induced by Ang II
As a stable cell cycle-related nuclear protein, PCNA directly participated in DNA synthesis. Thus, the ratio of PCNA-positive cells would reflect the intensity of cell proliferation. On the other hand, the expression of caspase-3 indicated both the existence of factors that initiated apoptosis and the occurrence of apoptosis, so the ratio of caspase-3 positive cells reflected the degree of cell apoptosis.
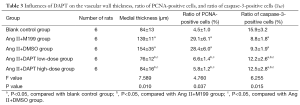
As shown in this study, slightly increased medial thickness of the vessels and higher ratios of vascular wall PCNA-positive cells and caspase-3 positive cells (P<0.05) were shown in the control group cultured in serum-free media for 8 d, compared with normal, uncultured pulmonary arteries, suggesting that these rat vessels could survive in serum-free media for research purposes. With the addition of Ang II, vascular medial thickness was significantly increased by nearly 50% (P<0.05) accompanied by increased ratio of vascular wall PCNA-positive cells (P<0.05) and reduced ratio of caspase-3 positive cells (P<0.05) compared with the control group, indicating that Ang II prompted VSMC proliferation, inhibited its apoptosis, and induced pulmonary vascular remodeling (Table 1, Figures 1,2).
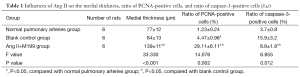
Full table
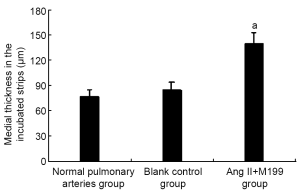
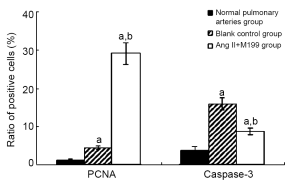
Effect of DAPT on Notch signaling of pulmonary arteries
After the addition of γ-secretase inhibitor DAPT, no change was observed in the mRNA level of Notch 1-4 receptors in either low- or high-dose groups (P>0.05), though the level reduced in transcription factors HERP1, 2 downstream of the Notch signaling pathway (P<0.05), suggesting that DAPT inhibited Notch signals by inhibiting the activation of Notch receptors and thereby inhibiting the transcription of downstream genes HERP1, 2, without compromising the expression of Notch receptors (Table 2).
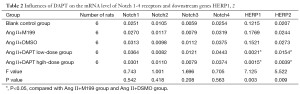
Full table
Effect of DAPT on pulmonary vascular changes
Ang II increased the medial thickness of cultured pulmonary arteries by nearly 50% while significantly increasing the ratio of vascular wall PCNA-positive cells and reducing that of caspase-3 positive cells. These effects of Ang II were offset by the introduction of DAPT to inhibit Notch signaling (Table 3, Figures 3-5).
Full table
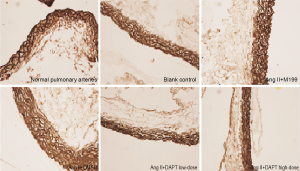
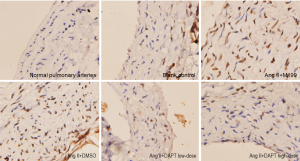
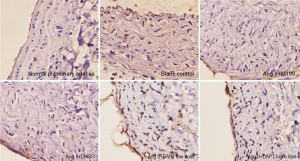
Discussion
Angiotensin II (Ang II) is a potent mitogen for vascular smooth muscle cells (VSMCs) and key factor in the development of PH-associated pulmonary vascular remodeling (8-13). The Ang II type1 receptor (AT1) has been linked to the development of PH (27). Local production of Ang II by ACE stimulates the growth of smooth muscle cells in the arterial wall to cause maladaptive changes and function of the pulmonary vasculature, which can result in the development of PH (28). Our current study found that Ang II caused medial thickening of pulmonary arteries in vitro and induced similar pulmonary vascular remodeling.
Highly conserved in evolution, the Notch signaling pathway played a critical role in the process of vascular remodeling associated with physiological functions as well as a variety of diseases. At present, four Notch receptors had been cloned in mammals (Notch 1-4). With the presence of common transmembrane domains in both receptors and ligands, Notch signal transduction was only possible through local cell contact and interactions. Two enzymolyses of Notch receptors following the interaction between ligands and receptors resulted in the release of the Notch intracellular domain (NICD) through the cell membrane. Upon direct entry into the nucleus, NICD activated the transcription repressor factors, HES (hairy enhancer of split)-1, 5, 7 and HERP-1, 2, 3, to exert their biological effects. Thus, intercellular interactions and environmental conditions became key links during the transduction of Notch signals because of the need for local contact and interaction (20-22).
In this study, rat tail collagen-based three-dimensional gel matrix was used for culturing the pulmonary arteries to retain the endothelium, smooth muscles, fibroblasts and extracellular matrix, maximizing the consistency of pulmonary artery smooth muscle cells with the in vivo environment for Notch signaling.
DAPT was a specific small molecule γ-secretase inhibitor of Notch signaling. As shown in cell culture and Drosophila studies, it inhibited γ-secretase required in the second enzymolysis of Notch receptors. Recent studies also found that it was a potent inhibitor that inhibited both Notch proteolysis and NICD nuclear translocation (13,29,30). In this study, the addition of DAPT to cultured vessel strips did not significantly affect the mRNA level in receptors, though that of downstream target genes HERP1 and HERP2 was evidently reduced, which suggested that the inhibitory effect of DAPT on Notch signaling was irrelevant to the expression of Notch receptors.
There were three major receptors (Notch-1, 3, 4) and three downstream effectors (HERP-1, 2, 3) in the vascular Notch signaling system, mostly expressed in arteries (20-22). As few reports were available on the mechanism employed by Notch signaling to induce vascular remodeling (especially smooth muscle cells), a possible process based on the current data might involve: promoting VSMC proliferation through Notch 1 and Notch 3 receptors, thus eliminating the VSMC growth inhibition and cell cycle arrest; inhibiting VSMC apoptosis; promoting VSMC migration and aggregation; and stimulating the transformation of endothelial cells and fibroblasts into smooth muscle cells (20-26).
With continued stimulation by Ang II for 7 days, pulmonary artery strips showed remarkable increase in the medial thickness, as well as increased vascular cell proliferation (higher ratio of PCNA-positive cells) and reduce apoptosis (higher ratio of caspase-3-positive cells). After adding DAPT to inhibit Notch signaling, an evident decrease in the medial thickness was seen, and the degree of vascular wall cell proliferation was reduced and apoptosis increased compared to strips added with Ang II only, indicating that once the Notch signaling was inhibited, vascular remodeling was suppressed even in the presence of potent mitogen. Hence, inhibiting the Notch signaling pathway could be a powerful measure for anti-remodeling. The underlying mechanism was still unclear, though there was a possible link with the effect of Notch signals on the biological behavior of smooth muscle cells, which in turn affected the vascular remodeling. In conclusion, as it could relieve pulmonary vascular remodeling, inhibition of the Notch signal pathway might be a novel strategy for the treatment of pulmonary hypertension.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:13S-24S. [PubMed]
- Fukumoto Y, Tawara S, Shimokawa H. Recent progress in the treatment of pulmonary arterial hypertension: expectation for rho-kinase inhibitors. Tohoku J Exp Med 2007;211:309-20. [PubMed]
- Miura Y, Fukumoto Y, Sugimura K, et al. Identification of new prognostic factors of pulmonary hypertension. Circ J 2010;74:1965-71. [PubMed]
- Fukumoto Y, Shimokawa H. Recent progress in the management of pulmonary hypertension. Circ J 2011;75:1801-10. [PubMed]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006;7:678-89. [PubMed]
- Ehebauer M, Hayward P, Arias AM. Notch, a universal arbiter of cell fate decisions. Science 2006;314:1414-5. [PubMed]
- Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol 2003;23:543-53. [PubMed]
- Li JL, Harris AL. Notch signaling from tumor cells: a new mechanism of angiogenesis. Cancer Cell 2005;8:1-3. [PubMed]
- Patel NS, Li JL, Generali D, et al. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res 2005;65:8690-7. [PubMed]
- Williams CK, Li JL, Murga M, et al. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood 2006;107:931-9. [PubMed]
- Chung WK, Deng L, Carroll JS, et al. Polymorphism in the angiotensin II type 1 receptor (AGTR1) is associated with age at diagnosis in pulmonary arterial hypertension. J Heart Lung Transplant 2009;28:373-9. [PubMed]
- Orte C, Polak JM, Haworth SG, et al. Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary plexiform pulmonary hypertension. J Pathol 2000;192:379-84. [PubMed]
- Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 2006;99:675-91. [PubMed]
- Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 2008;44:14-30. [PubMed]
- Wei L, Liu T, Liu B, et al. Effect of triptolide on the expression of matrix metalloproteinases 2 and 9 in lungs of experimental pulmonary hypertension. Zhongguo Dang Dai Er Ke Za Zhi 2007;9:479-83. [PubMed]
- Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res 2007;100:1556-68. [PubMed]
- Ehebauer M, Hayward P, Arias AM. Notch, a universal arbiter of cell fate decisions. Science 2006;314:1414-5. [PubMed]
- Morrow D, Guha S, Sweeney C, et al. Notch and vascular smooth muscle cell phenotype. Circ Res 2008;103:1370-82. [PubMed]
- Kurpinski K, Lam H, Chu J, et al. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 2010;28:734-42. [PubMed]
- Kennard S, Liu H, Lilly B. Transforming growth factor-beta (TGF-1) down-regulates Notch3 in fibroblasts to promote smooth muscle gene expression. J Biol Chem 2008;283:1324-33. [PubMed]
- Hellström M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007;445:776-80. [PubMed]
- Morrow D, Sweeney C, Birney YA, et al. Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. Am J Physiol Cell Physiol 2007;292:C488-96. [PubMed]
- Wang XM, Zhou TF, Liu B, et al. Changes of MMP-2,9 and TIMP-1 expressions in rats with pulmonary arterial hypertension after captopril and losartan interventions. Sichuan Da Xue Xue Bao Yi Xue Ban 2009;40:255-9. [PubMed]
- Wang XM, Liu B, Wei L, et al. Effect of Captopril and Losartan on Cell Apoptosis of Lung Tissues in Rats with Pulmonary Artery Hypertension. Shi Yong Er Ke Lin Chuang Za Zhi 2009;24:999-1003.
- McKie PM, Cataliotti A, Boerrigter G, et al. A novel atrial natriuretic peptide based therapeutic in experimental angiotensin II mediated acute hypertension. Hypertension 2010;56:1152-9. [PubMed]
- Bradford CN, Ely DR, Raizada MK. Targeting the vasoprotective axis of the renin-angiotensin system: a novel strategic approach to pulmonary hypertensive therapy. Curr Hypertens Rep 2010;12:212-9. [PubMed]
- Shenoy V, Ferreira AJ, Qi Y, et al. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 2010;182:1065-72. [PubMed]
- Yang D, Xie Y. Angiotensin Converting Enzyme Inhibitor Can Protect Hypoxic Pulmonary Hypertension of Rats through Regulate the Apoptosis of Pulmonary Artery Wall Cell. Zhong Guo Lin Chuang Yi Xue 2008;15:471-3.
- Morohashi Y, Kan T, Tominari Y, et al. C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester). J Biol Chem 2006;281:14670-6. [PubMed]
- Nelson BR, Hartman BH, Georgi SA, et al. Transient inactivation of Notch signaling synchronizes differentiation of neural progenitor cells. Dev Biol 2007;304:479-98. [PubMed]


